A Feature of the Horizontal Directional Solidification (HDS) Method Affects the Microstructure of Al2O3/YAG Eutectic Ceramics
Abstract
1. Introduction
2. Materials and Methods
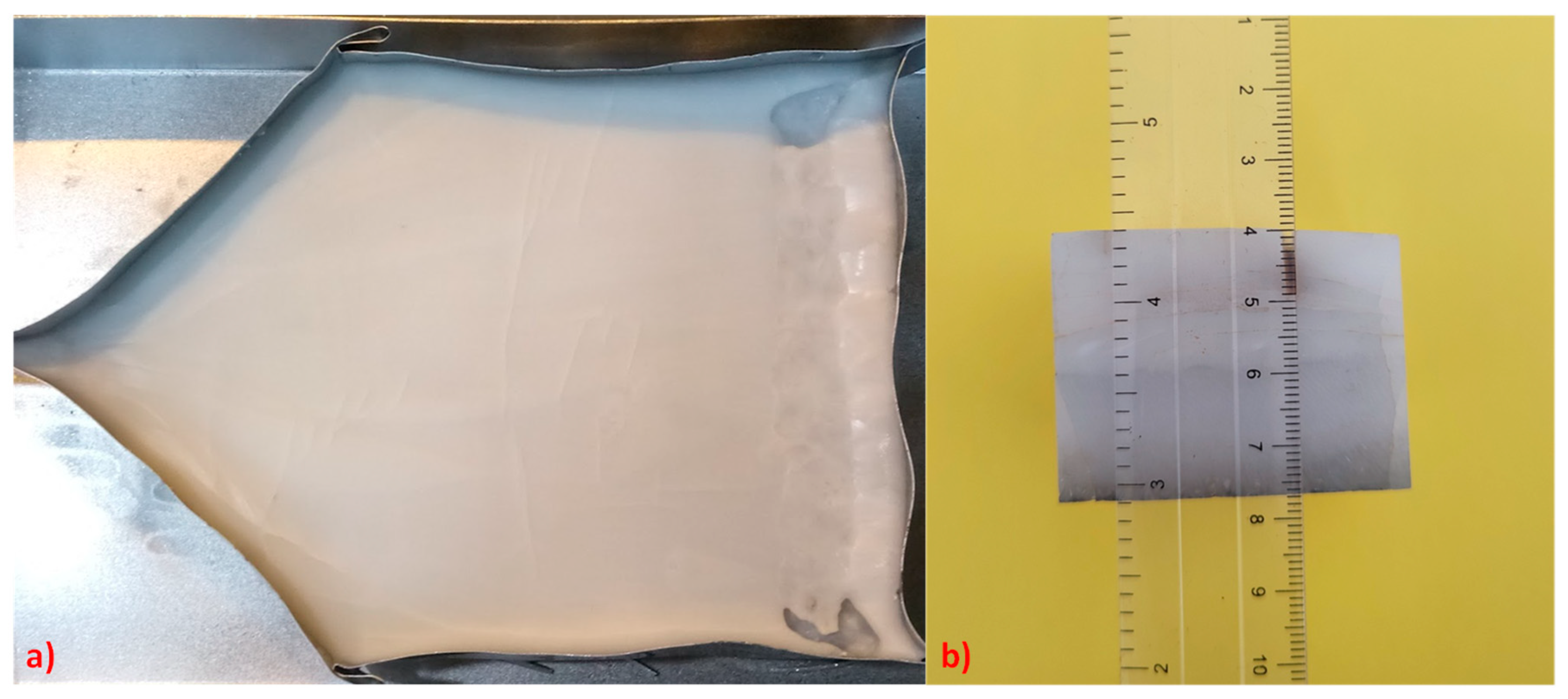
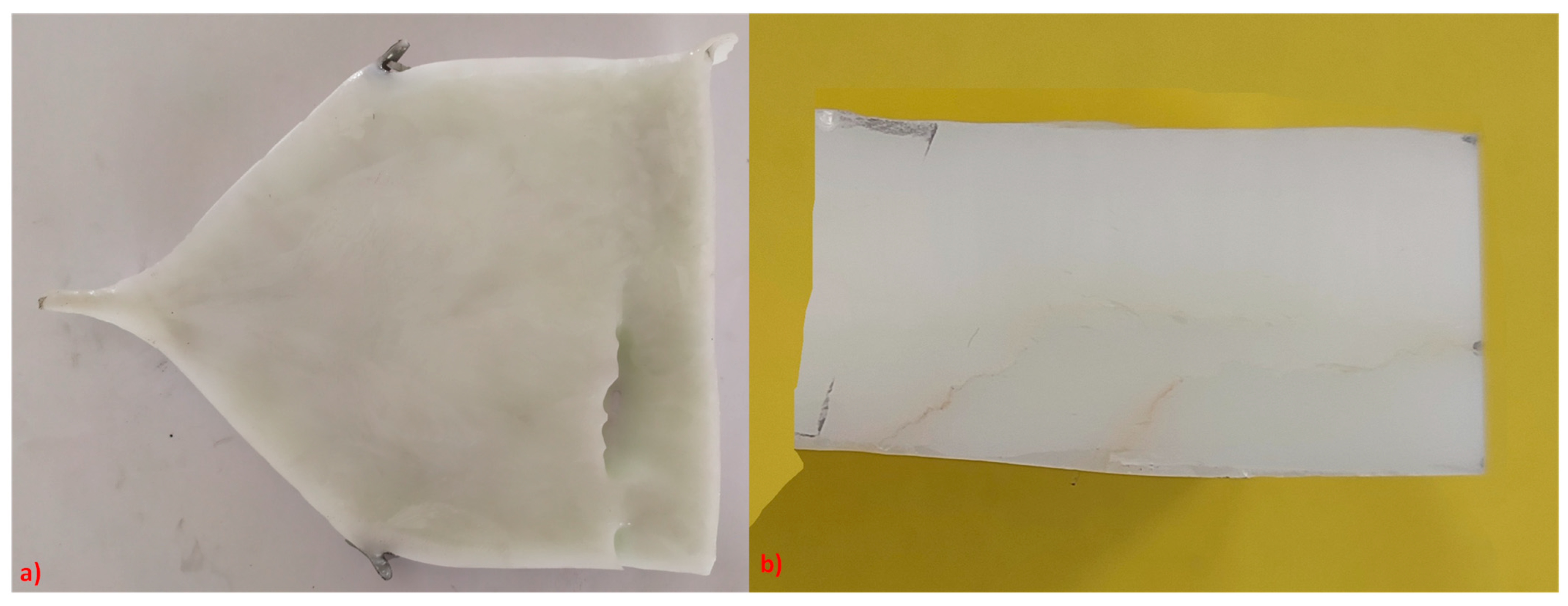
2.1. Al2O3/YAG Eutectic Ceramics Processing
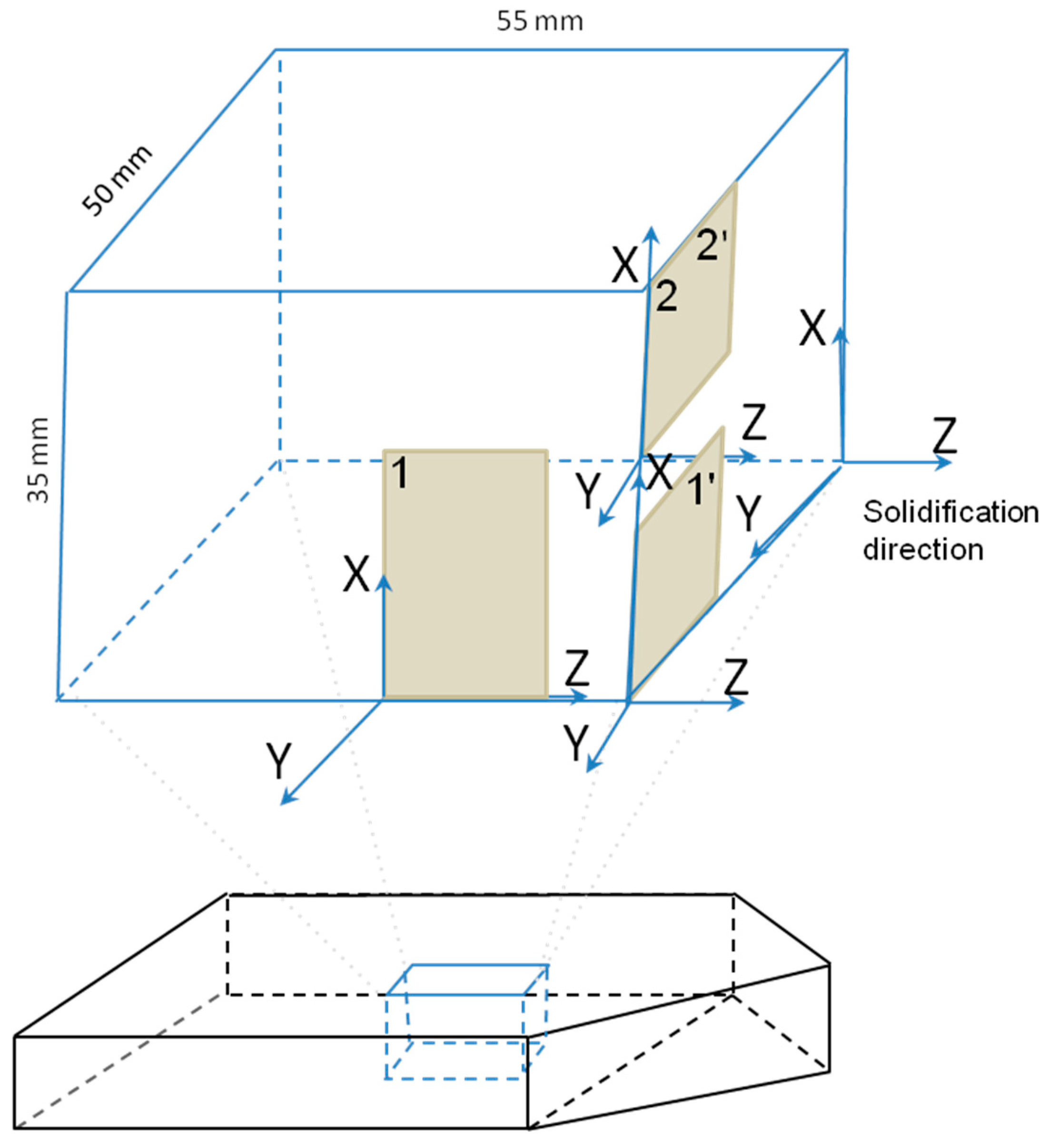
2.2. Characterization
3. Results and Discussion
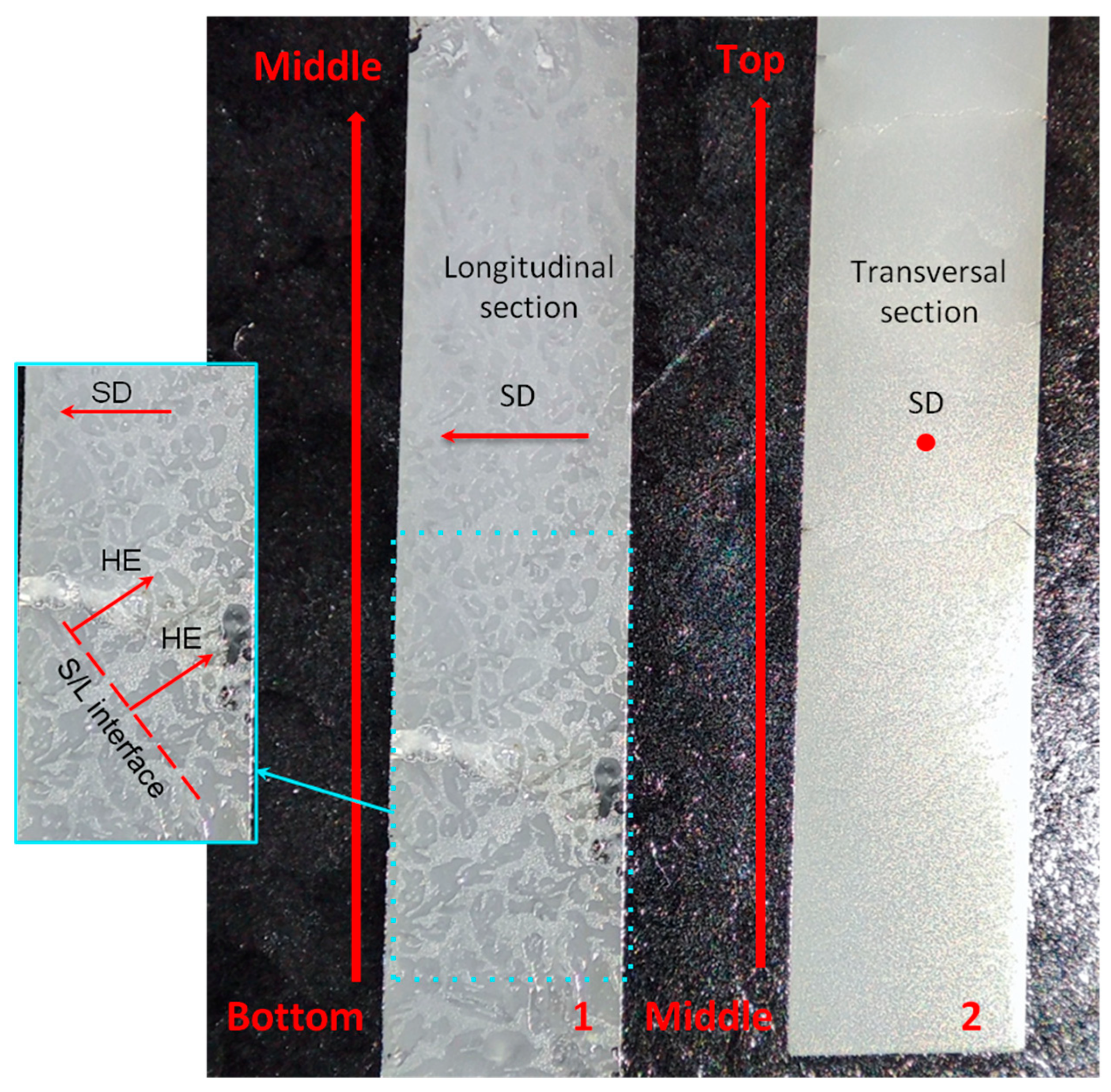
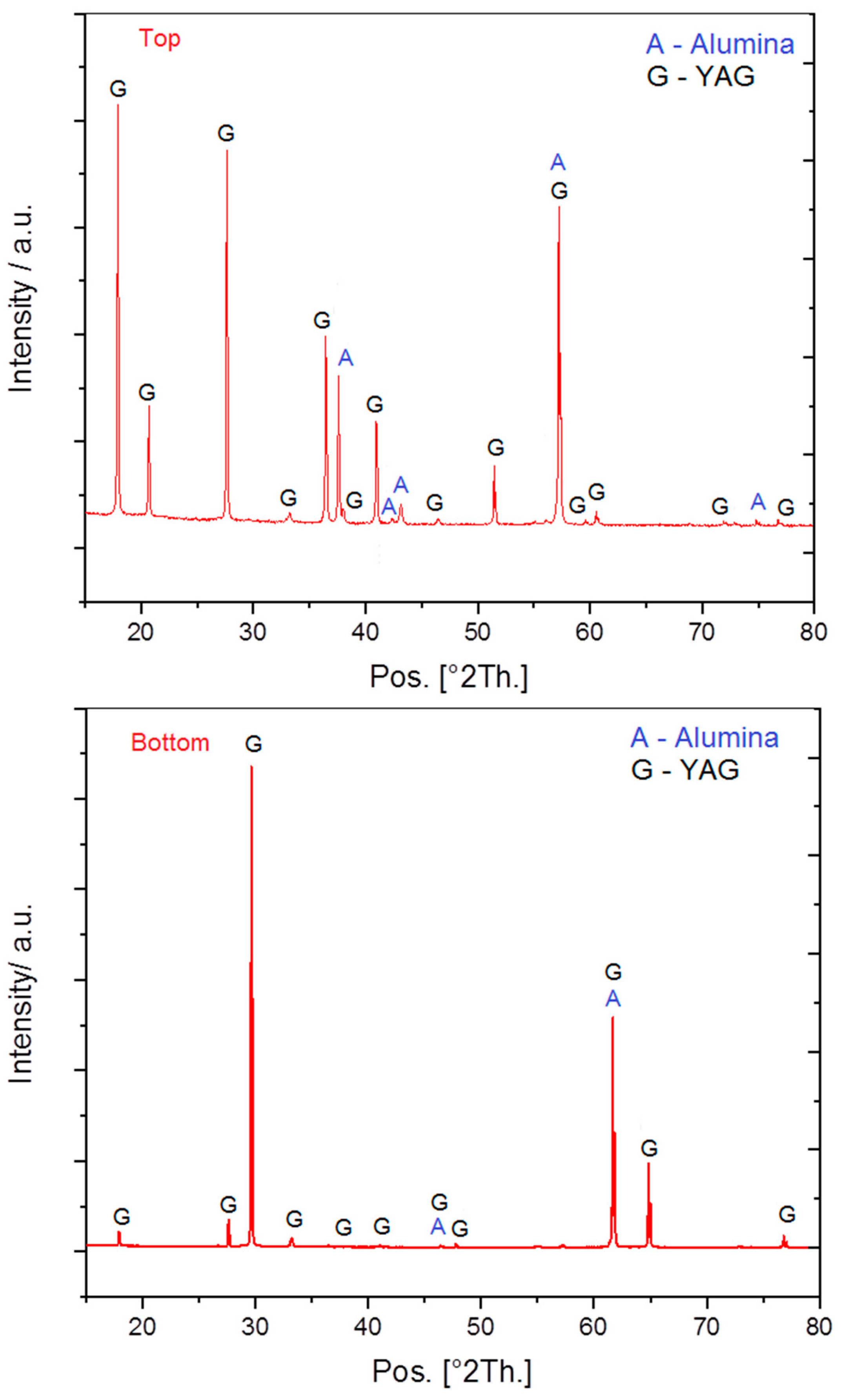
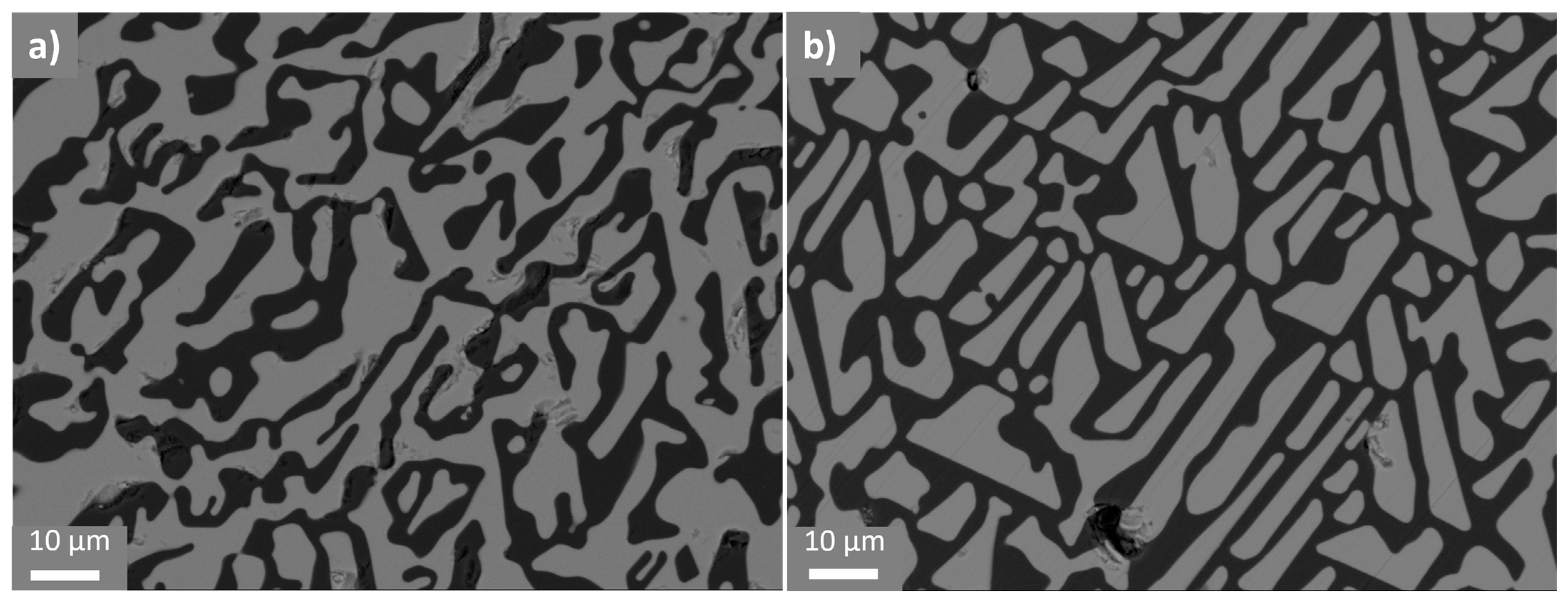
3.1. Al2O3/YAG Eutectic Ceramic Ingot No. 1 (78.5 mol% Al2O3–21.5 mol% Y2O3 Composition)
3.2. Al2O3/YAG Eutectic Ceramic Ingot No. 2 (81.5 mol% Al2O3–18.5 mol% Y2O3 Composition)
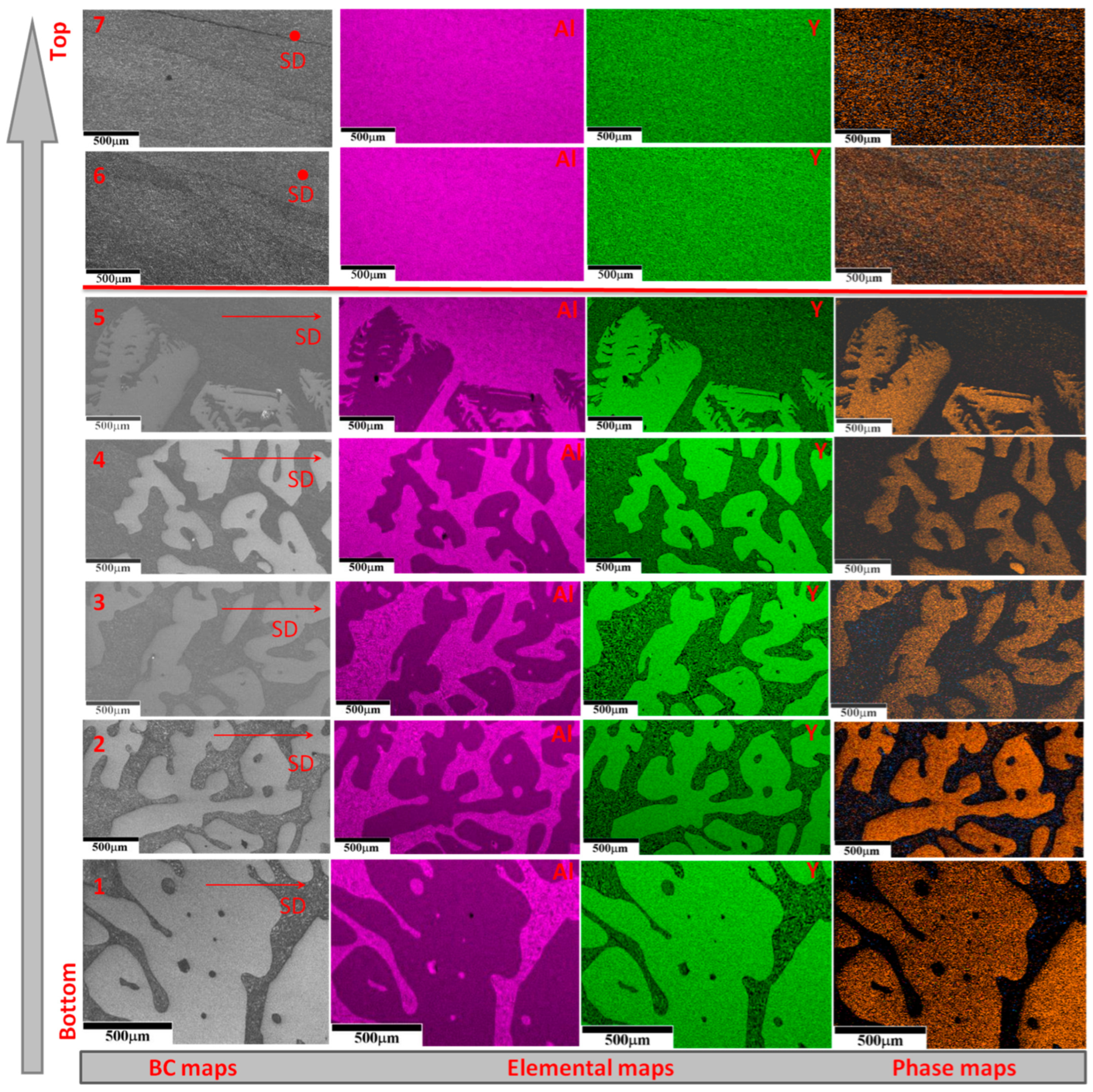
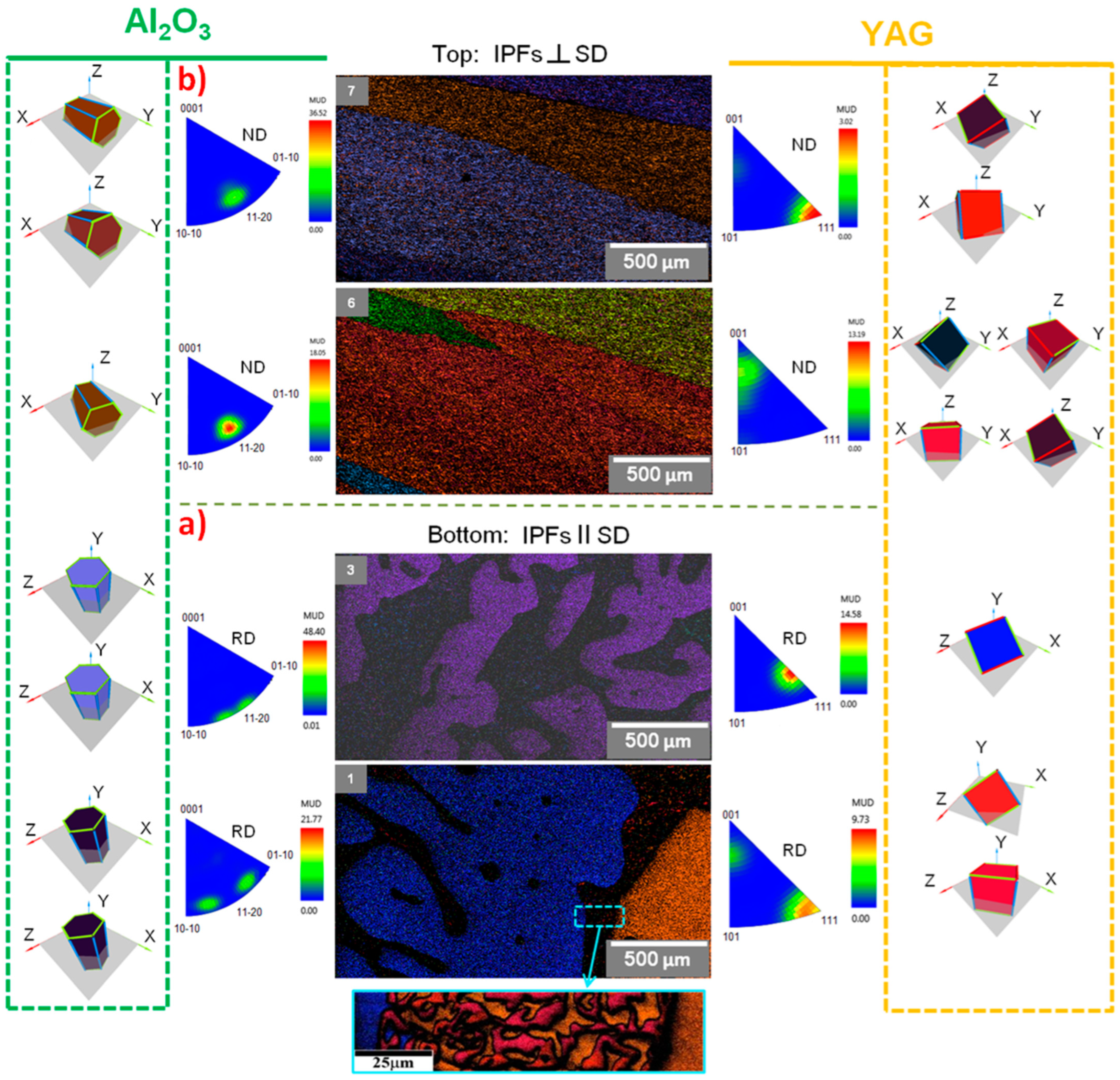
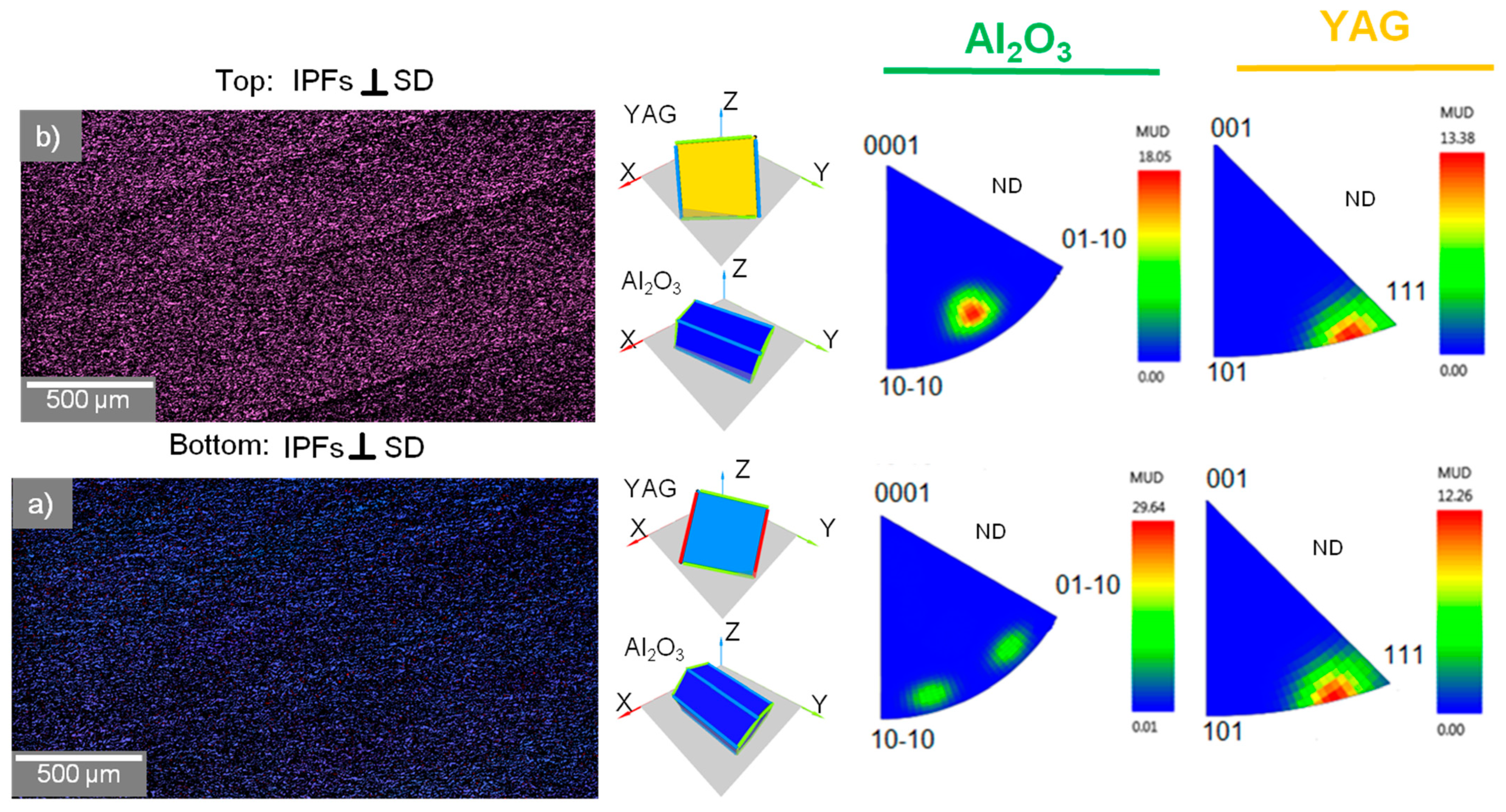
3.3. Characterization of Crystallographic Texture and Microstructure of Al2O3/YAG Eutectic Ceramics No. 1 and No. 2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parlier, M.; Valle, R.; Perrière, L.; Lartigue-Korinek, S.; Mazerolles, L. Potential of Directionally Solidified Eutectic Ceramics for High Temperature Applications. AerospaceLab 2011, 3, 1–13. [Google Scholar]
- Hirano, K. Application of eutectic composites to gas turbine system and fundamental fracture properties up to 1700 °C. J. Eur. Ceram. Soc. 2005, 25, 1191–1199. [Google Scholar] [CrossRef]
- Waku, Y.; Ohtsubo, H.; Nakagawa, N.; Kohtoku, Y. Sapphire Matrix Composites Reinforced with Single Crystal YAG Phases. J. Mater. Sci. 1996, 31, 4663–4670. [Google Scholar] [CrossRef]
- Waku, Y.; Nakagawa, N.; Ohtsubo, H.; Mitani, A.; Shimizu, K. Fracture and Deformation Behavior of Melt Growth Composites at very High Temperatures. J. Mater. Sci. 2001, 36, 1585–1594. [Google Scholar] [CrossRef]
- Stubican, V.S.; Bradt, R.C. Eutectic Solidification in Ceramic Systems. Ann. Rev. Mater. Sci. 1981, 11, 267–297. [Google Scholar] [CrossRef]
- Flemings, M.C. Solidification Processing. Metall. Trans. 1974, 5, 2121–2134. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Dendrite growth in eutectic alloys. Int. Met. Rev. 1979, 24, 177–204. [Google Scholar] [CrossRef]
- Burden, M.H.; Hunt, J.D. The extent of the eutectic range. J. Cryst. Growth. 1974, 22, 328–330. [Google Scholar] [CrossRef]
- Viechnicki, D.; Schmid, F. Eutectic Solidification in the System Al2O3/Y3Al5O12. J. Mater. Sci. 1969, 4, 84–88. [Google Scholar] [CrossRef]
- Epelbaum, B.M.; Yoshikawa, A.; Shimamura, K.; Fukuda, T.; Suzuki, K.; Waku, Y. Microstructure of Al2O3/Y3Al5O12 eutectic fibers grown by μ-PD method. J. Cryst. Growth. 1999, 198–199, 471–475. [Google Scholar] [CrossRef]
- Yoshikawa, A.; Hasegawa, K.; Fukuda, T.; Suzuki, K.; Waku, Y. Growth and diameter control of Al2O3/Y3Al5O12 eutectic fiber by micro-pulling-down method and its high temperature strength and thermal stability. Jpn. J. Appl. Phys. 1999, 20, 275–282. [Google Scholar]
- Waku, Y.; Nakagawa, N.; Wakamoto, T.; Ohtsubo, H.; Shimizu, K.; Waku, Y. High-temperature strength and thermal stbility of a unidirectionally solidified Al2O3/YAG eutectic composite. J. Mater. Sci. 1998, 33, 1217–1225. [Google Scholar] [CrossRef]
- Parthasarathy, T.A.; Mah, T.; Matson, L.E. Processing, structure and properties of Alumina-YAG eutectic composites. J. Ceram. Process. Res. 2004, 5, 380–390. [Google Scholar]
- Liu, Y.; Su, H.; Tan, X.; Shen, Z.; Li, X.; Jiang, H.; Zhao, D.; Guo, Y.; Zhang, Z.; Guo, M. Stability of crystallographic texture and mechanical anisotropy toward Al2O3/YAG eutectic ceramic composite using single crystalline seeds. Compos. B Eng. 2024, 274, 111263. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Xian, Q.; Shen, J.; Zhang, G.; Wang, D.; Wang, J.; Lou, L.; Zhang, J. Preparation and microstructure of large-sized directionally solidified Al2O3/Y3Al5O12 eutectics with the seeding technique. J. Eur. Ceram Soc. 2018, 38, 5625–5631. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Z.; Zhang, W.; Zhong, Y.; Xian, Q.; Zhang, J.; Wang, J. Mechanical properties of directionally solidified Al2O3/Y3Al5O12 eutectic ceramic prepared by optical floating zone technique. J. Eur. Ceram. Soc. 2018, 38, 3610–3617. [Google Scholar] [CrossRef]
- Sai, Q.; Zhao, Z.; Xia, C.; Xu, X.; Wu, F.; Di, J.; Wang, L. Ce-doped Al2O3−YAG eutectic and its application for white LEDs. Opt. Mater. 2013, 35, 2155–2159. [Google Scholar] [CrossRef]
- Waku, Y.; Nakagawa, N.; Wakamoto, T.; Ohtsubo, H.; Shimizu, K.; Kohtoku, Y. A ductile ceramic eutectic composite with high strength at 1873 K. Nature 1997, 389, 49–52. [Google Scholar] [CrossRef]
- Oliete, P.B.; Peña, J.I.; Larrea, A.; Orera, V.M.; LLorca, J.; Pastor, J.Y.; Martín, A.; Segurado, J. Ultra-High-Strength Nanofibrillar Al2O3–YAG–YSZ Eutectics. Adv. Mater. 2007, 19, 2313–2318. [Google Scholar] [CrossRef]
- Borodin, V.A.; Reznikov, A.G.; Starostin, M.Y.; Steriopolo, T.A.; Tatarchenko, V.A.; Chernyshova, L.I.; Yalovets, T.N. Growth of Al2O3−ZrO2(Y2O3) eutectic composite by Stepanov technique. J. Cryst. Growth. 1987, 82, 177–181. [Google Scholar] [CrossRef]
- Su, H.; Zhang, J.; Ma, W.; Wei, K.; Liu, L.; Fu, H.; Feng, S.-P.; Soh, A.K. In situ fabrication of highly-dense Al2O3/YAG nanoeutectic composite ceramics by a modified laser surface processing. J. Eur. Ceram. Soc. 2014, 34, 739–744. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoshikawa, A.; Fukuda, T. Growth of MgAl2O4/MgO eutectic crystals by the micro-pulling-down method and its characterization. J. Eur. Ceram. Soc. 2005, 25, 1351–1354. [Google Scholar] [CrossRef]
- Liu, Y.; Su, H.; Shen, Z.; Zhao, D.; Guo, Y.; Li, S.; Guo, M.; Liu, L.; Fu, H. Effect of seed orientations on crystallographic texture control in faceted Al2O3/YAG eutectic ceramic during directional solidification. J. Mater. Sci. Technol. 2023, 146, 86–101. [Google Scholar] [CrossRef]
- Murayama, Y.; Hanada, S.; Waku, Y. Microstructure and High-Temperature Strength of Directionally Solidified Al2O3/YAG Eutectic Composite. Mater. Trans. 2003, 44, 1690–1693. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, M.; Liu, Y.; Zhao, Y. Microstructure and mechanical properties of Al2O3/YAG eutectic ceramic grown by horizontal directional solidification method. J. Alloys Compd. 2016, 657, 184–191. [Google Scholar] [CrossRef]
- Nie, Y.; Han, J.; Liu, Y.; Zhang, M.; Zhang, J. Isotropy in large-size Al2O3/Y3Al5O12 eutectic ceramic grown by Horizontal Directional Solidification method. Mater. Sci. Eng. A 2017, 704, 207–211. [Google Scholar] [CrossRef]
- Bagdasarov, K.S.; Goryainov, L.A. Development of horizontally oriented crystallization of high-melting dielectric single crystals. J. Eng. Phys. Thermoph. 1998, 71, 248–253. [Google Scholar] [CrossRef]
- Akulenok, E.M.; Bagdasarov, K.S.; Danileiko, Y.K.; Lebedeva, T.P.; Manenkov, A.A. Diffusion of intrinsic defects in dielectric and semiconductor crystals with impurities. Cryst. Chem. 2002, 47, 918–924. [Google Scholar] [CrossRef]
- Bagdasarov, K.S. Fundamentals of high-temperature crystallization. Crystallogr. Rep. 2002, 47 (Suppl. S1), S27–S34. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, H.; Han, J.; Zhang, H.; Xu, C. Distribution of Neodymium and properties of Nd:YAG crystal by horizontal directional solidification. J. Cryst. Growth 2012, 340, 130–134. [Google Scholar] [CrossRef]
- Chaika, M.; Ubizskii, S.; Kajan, J.; Gregor, T.; Gamazyan, G.; Marciniak, L. On the nature of CT luminescence in Yb3+:YAG single crystal under low photon energy. Opt. Mater. 2022, 130, 112548. [Google Scholar] [CrossRef]
- Palkech, J.; Kajan, J.; Malyukov, S.; Mikita, M.; Medvecky, S. Numerical simulation of heat transfer in a furnace heating unit for Horizontal Direct Crystallization of sapphire single-crystal. Am. J. Energy Eng. 2017, 4, 78–83. [Google Scholar]
- Medvecky, S.; Mikita, M.; Hoc, M.; Kajan, J. TRIZ and HDC sapphire growth process. J. Appl. Eng. Sci. 2017, 15, 9–14. [Google Scholar] [CrossRef]
- Yuferev, V.S.; Vasil’ev, M.G. Heat transfer in shaped thin-walled semi-transparent crystals pulled from the melt. J. Cryst. Growth 1987, 82, 31–38. [Google Scholar] [CrossRef]
- Mizutani, Y.; Yasuda, H.; Ohnaka, I.; Maeda, N.; Waku, Y. Coupled growth of unidirectionally solidified Al2O3–YAG eutectic ceramics. J. Cryst. Growth. 2002, 244, 384–392. [Google Scholar] [CrossRef]
- Mizutani, Y.; Yasuda, H.; Ohnaka, I.; Waku, Y. Phase selection of the Al2O3-Y2O3 system controlled by nucleation. Mater. Trans. 2001, 42, 238–244. [Google Scholar] [CrossRef]
- Yasuda, H.; Ohnaka, I.; Mizutani, Y.; Waku, Y. Selection of eutectic systems in Al2O3–Y2O3 ceramics. Sci. Tech. Adv. Mater. 2001, 2, 67–71. [Google Scholar] [CrossRef]
- Beausir, B.; Fundenberger, J.J. Analysis Tools for Electron and X-ray Diffraction, ATEX-Software, Université de Lorraine—Metz. 2017. Available online: http://www.atex-software.eu/ (accessed on 8 September 2024).
- Lukanina, M.A.; Hodosevitch, K.V.; Kalaev, V.V.; Semenov, V.B.; Sytin, V.N.; Raevsky, V.L. 3D numerical simulation of heat transfer during horizontal direct crystallization of corundum single crystals. J. Cryst. Growth 2006, 287, 330–334. [Google Scholar] [CrossRef]
- Burden, M.H.; Hunt, J.D. Cellular and dendritic growth. J. Cryst. Growth 1974, 22, 99–108. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Zhong, Y.; Jiang, B.; Lou, L.; Zhang, J.; Wang, J. Microstructure evolution and crystallography of directionally solidified Al2O3/Y3Al5O12 eutectic ceramics prepared by the modified Bridgman method. J. Mater. Sci. Technol. 2019, 35, 1982–1988. [Google Scholar] [CrossRef]
- Simpson, J.E.; Garimella, S.V.; De Groh, H.C.; Abbaschian, R. Bridgman Crystal growth of an alloy with thermosolutal convection under microgravity conditions. Trans. ASME 2001, 123, 990–998. [Google Scholar] [CrossRef][Green Version]
- Ashbrook, R.L. Directionally solidified Ceramic Eutectics. J. Am. Ceram. 1977, 60, 428–435. [Google Scholar] [CrossRef]
- Mullins, W.W.; Sekerka, R.F. Stability of a planar interface during solidification of a dilute binary alloy. J. Am. Ceram. Soc. 1964, 35, 444–451. [Google Scholar] [CrossRef]
- Viechnicki, D.; Schmid, F. Investigation of the eutectic point in the system Al2O3/Y3Al5O12. Mat. Res. Bull. 1969, 4, 129–135. [Google Scholar] [CrossRef]
- Liu, Y.; Su, H.; Shen, Z.; Li, X.; Jiang, H.; Di Zhao, N.; Guo, Y.; Zhang, Z.; Guo, M. Insight into the complex coupled growth behavior of Al2O3/YAG eutectic ceramic based on the evolutions of microstructure and crystallographic texture. J. Eur. Ceram. Soc. 2023, 43, 4482–4497. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Sun, L.; Zhang, H.; Wang, L.; Lou, L.; Zhang, J. Microstructure evolution of Al2O3/Y3Al5O12 eutectic crystal during directional solidification. Scr. Mater. 2015, 108, 31–34. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajan, J.; Damazyan, G.; Tinkova, V.; Prnová, A.; Michálková, M.; Švančárek, P.; Gregor, T.; Akusevich, A.; Hruška, B.; Galusek, D. A Feature of the Horizontal Directional Solidification (HDS) Method Affects the Microstructure of Al2O3/YAG Eutectic Ceramics. Crystals 2024, 14, 858. https://doi.org/10.3390/cryst14100858
Kajan J, Damazyan G, Tinkova V, Prnová A, Michálková M, Švančárek P, Gregor T, Akusevich A, Hruška B, Galusek D. A Feature of the Horizontal Directional Solidification (HDS) Method Affects the Microstructure of Al2O3/YAG Eutectic Ceramics. Crystals. 2024; 14(10):858. https://doi.org/10.3390/cryst14100858
Chicago/Turabian StyleKajan, Juraj, Grigori Damazyan, Vira Tinkova, Anna Prnová, Monika Michálková, Peter Švančárek, Tomáš Gregor, Alena Akusevich, Branislav Hruška, and Dušan Galusek. 2024. "A Feature of the Horizontal Directional Solidification (HDS) Method Affects the Microstructure of Al2O3/YAG Eutectic Ceramics" Crystals 14, no. 10: 858. https://doi.org/10.3390/cryst14100858
APA StyleKajan, J., Damazyan, G., Tinkova, V., Prnová, A., Michálková, M., Švančárek, P., Gregor, T., Akusevich, A., Hruška, B., & Galusek, D. (2024). A Feature of the Horizontal Directional Solidification (HDS) Method Affects the Microstructure of Al2O3/YAG Eutectic Ceramics. Crystals, 14(10), 858. https://doi.org/10.3390/cryst14100858





