Microwave-Assisted Fabrication and Characterization of Carbon Fiber-Sodium Bismuth Titanate Composites
Abstract
1. Introduction
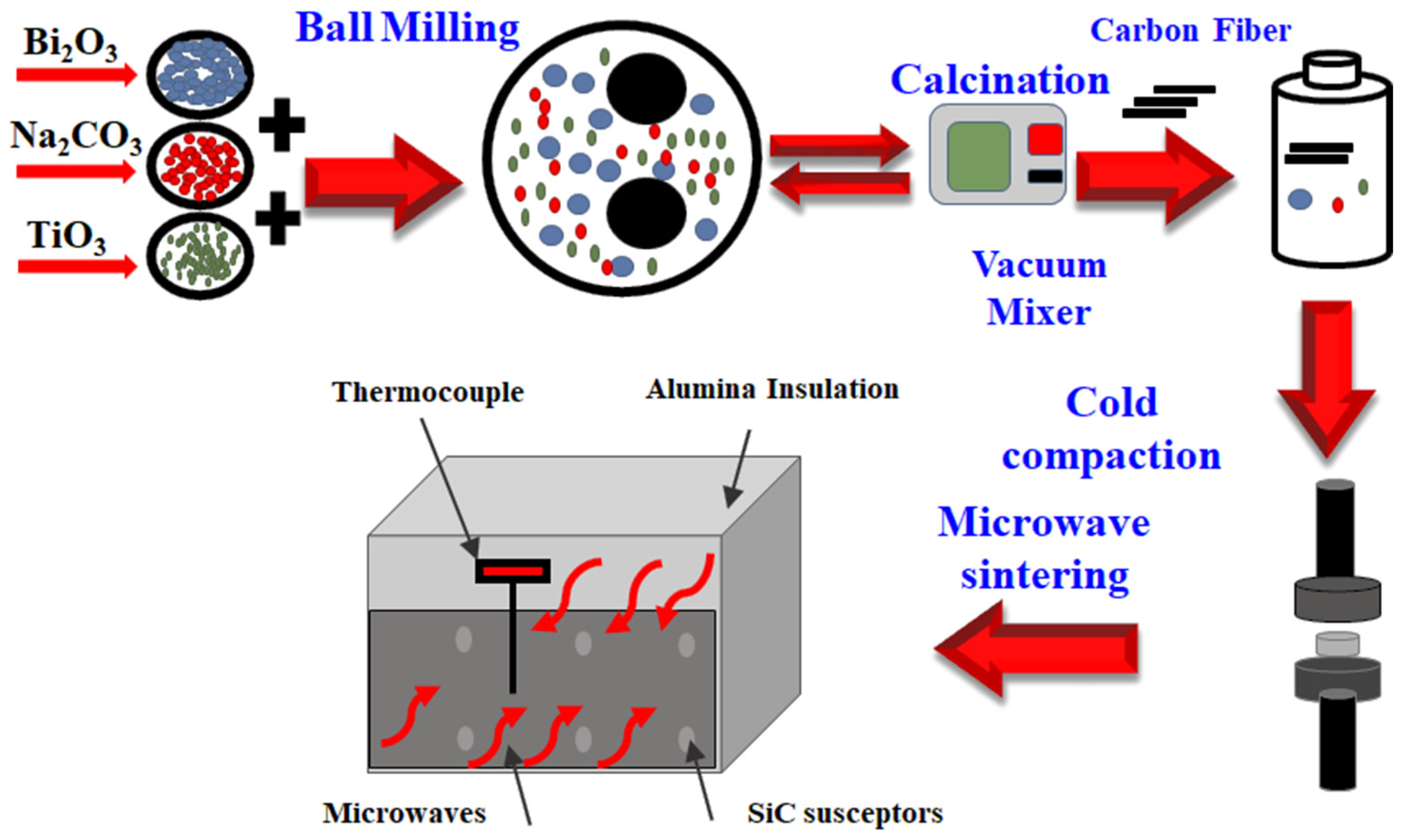
2. Materials and Method
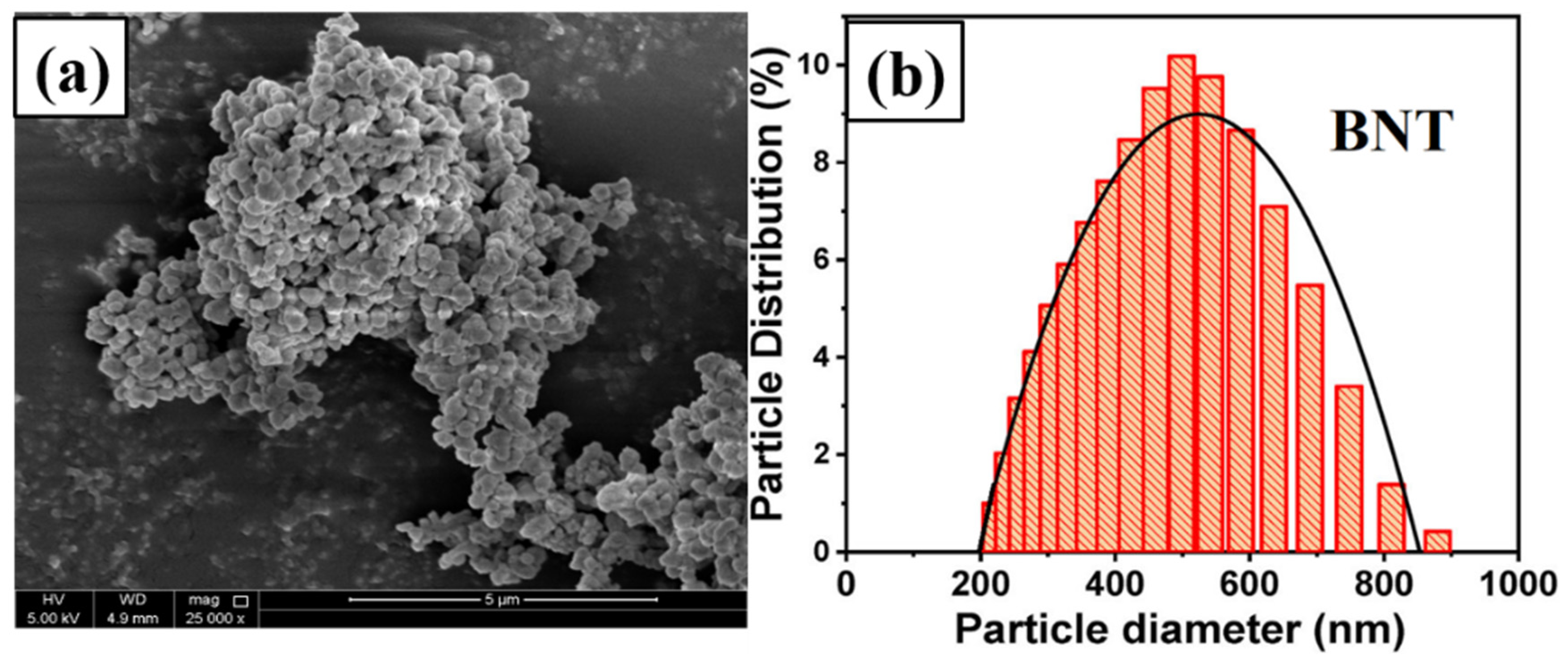
2.1. BNT Powder Preparation
2.2. BNT-Carbon Fiber Composite
2.3. Characterization
3. Results and Discussions
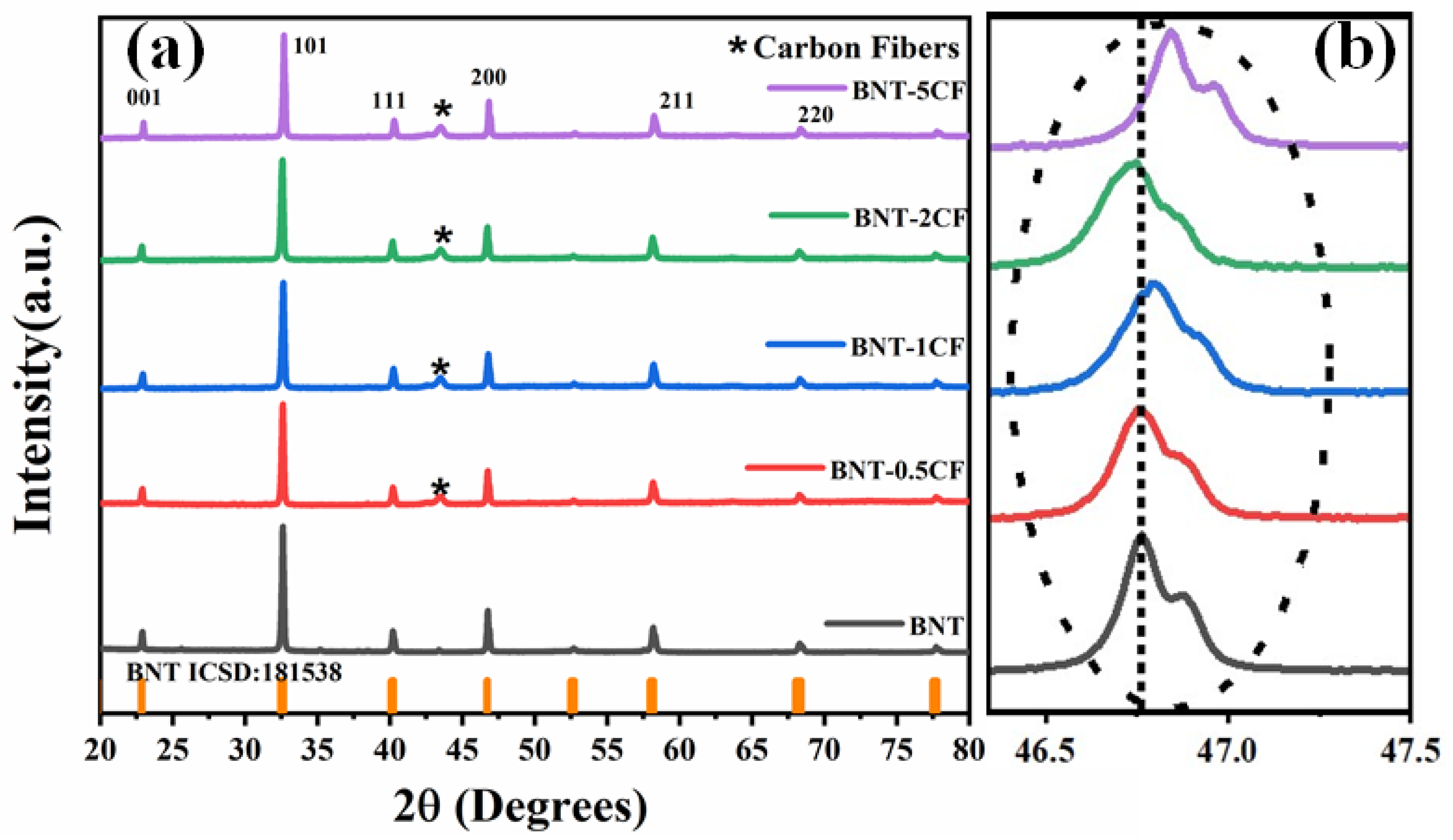
3.1. X-ray Diffraction
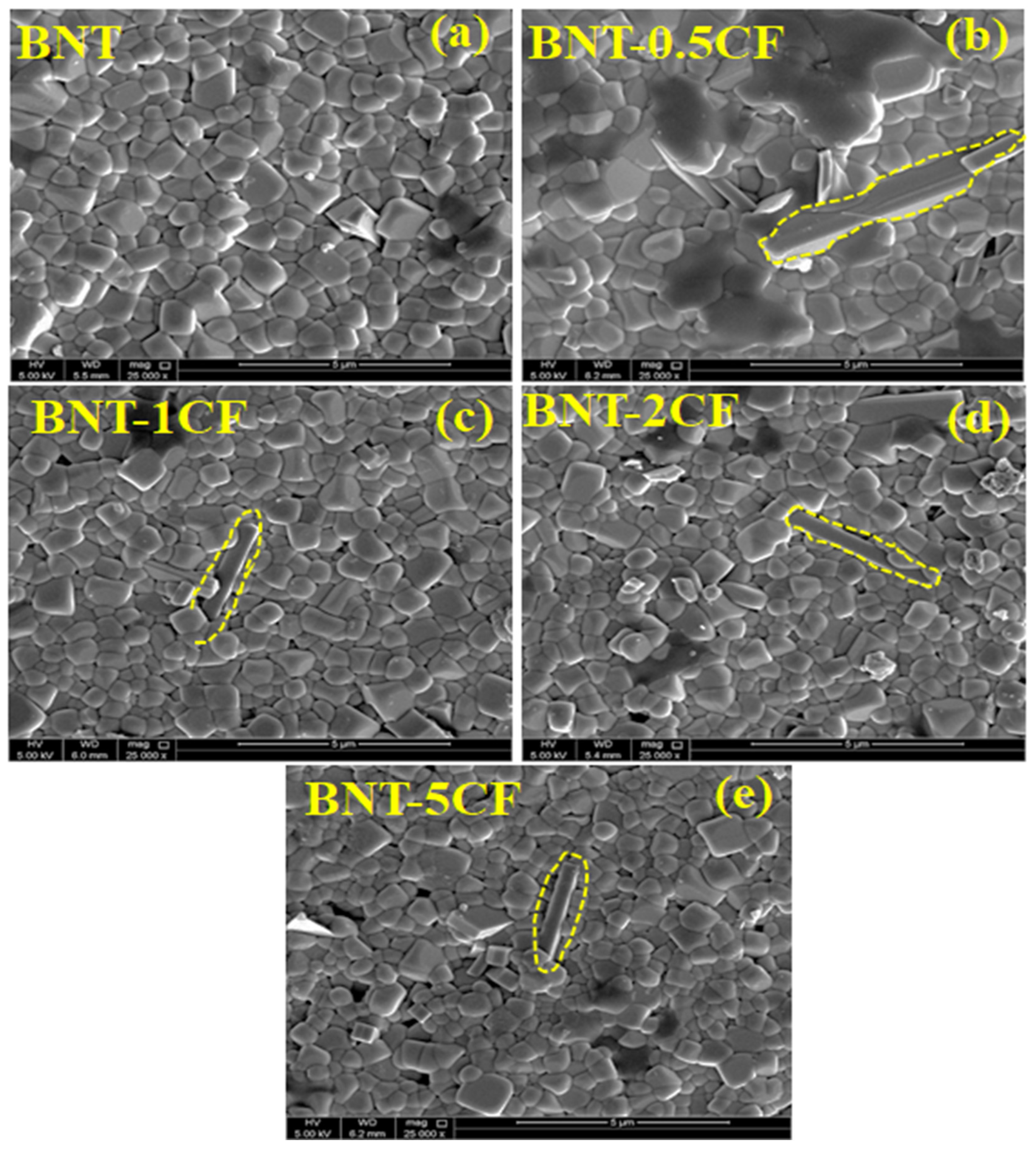
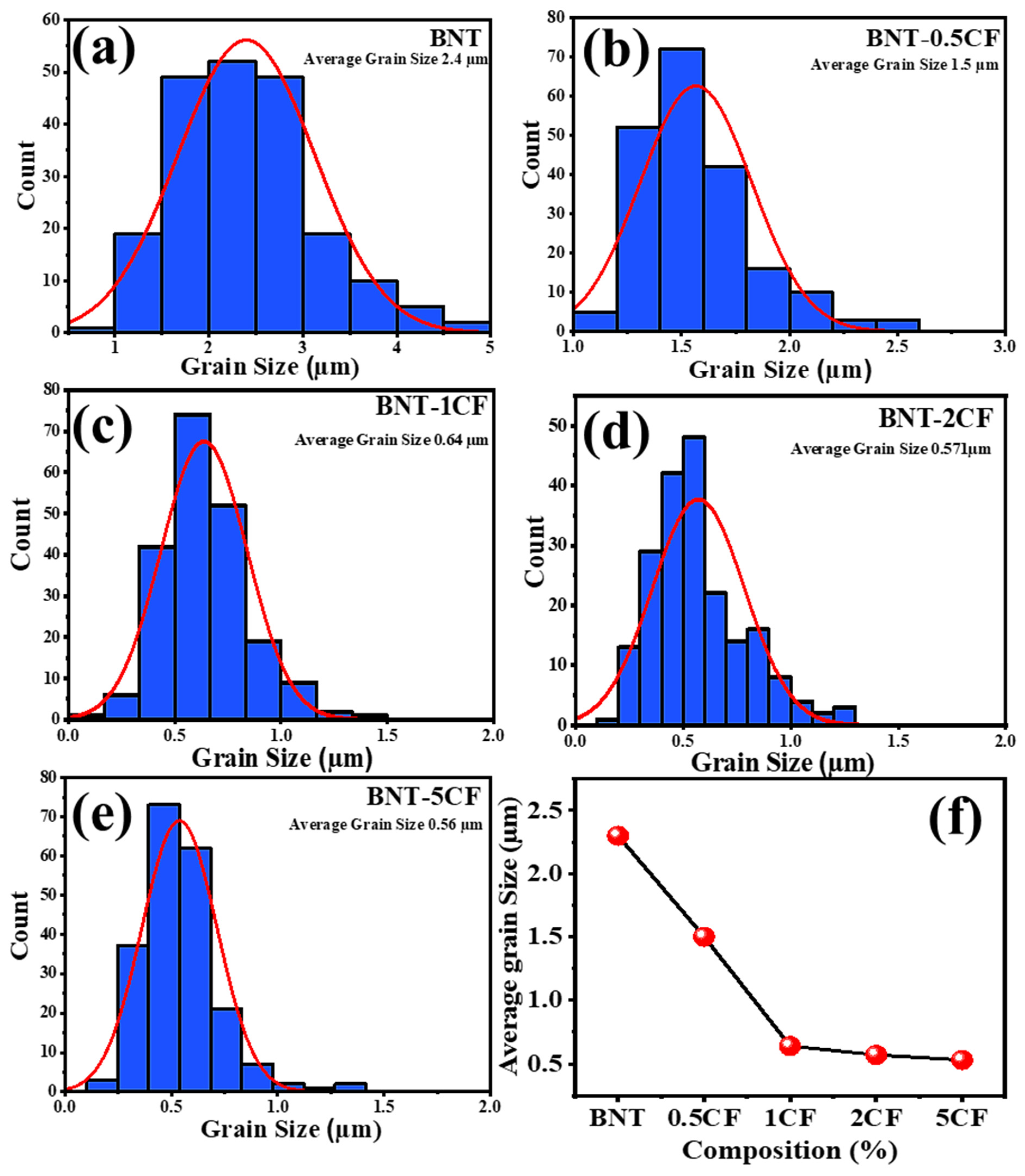
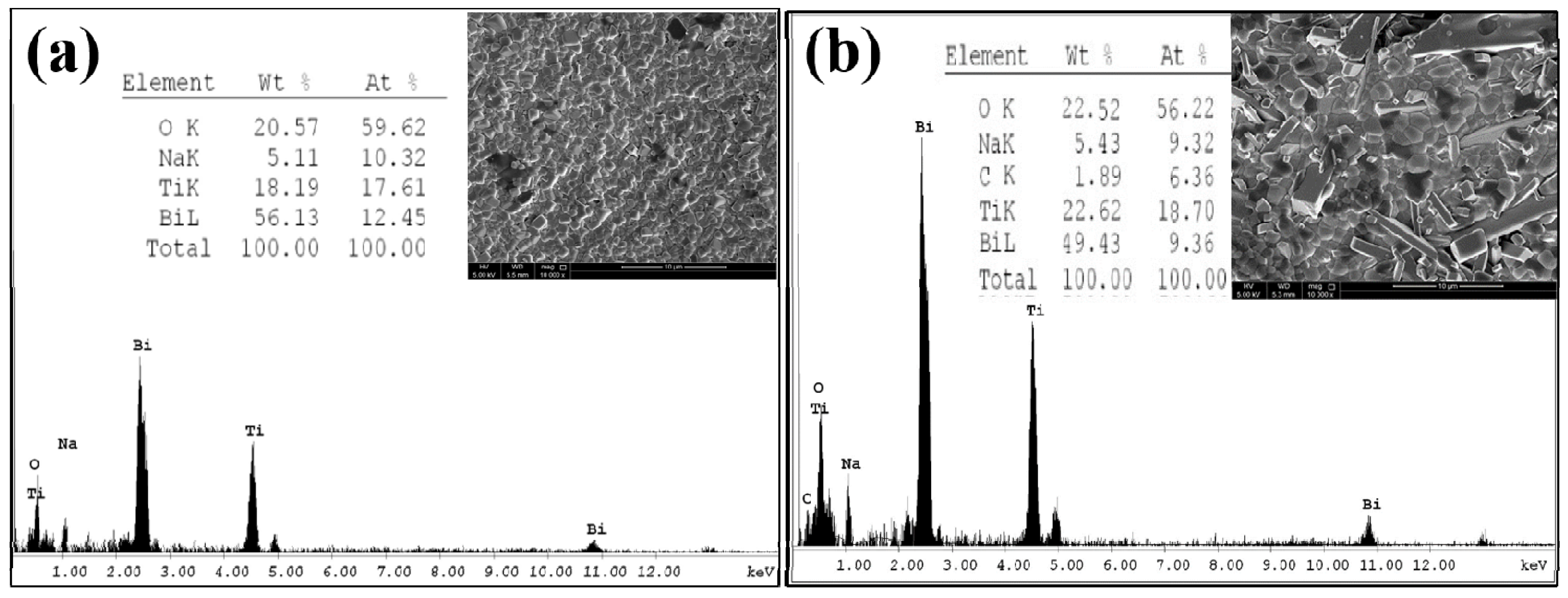
3.2. Scanning Electron Microscopy
3.3. Raman Spectroscopy
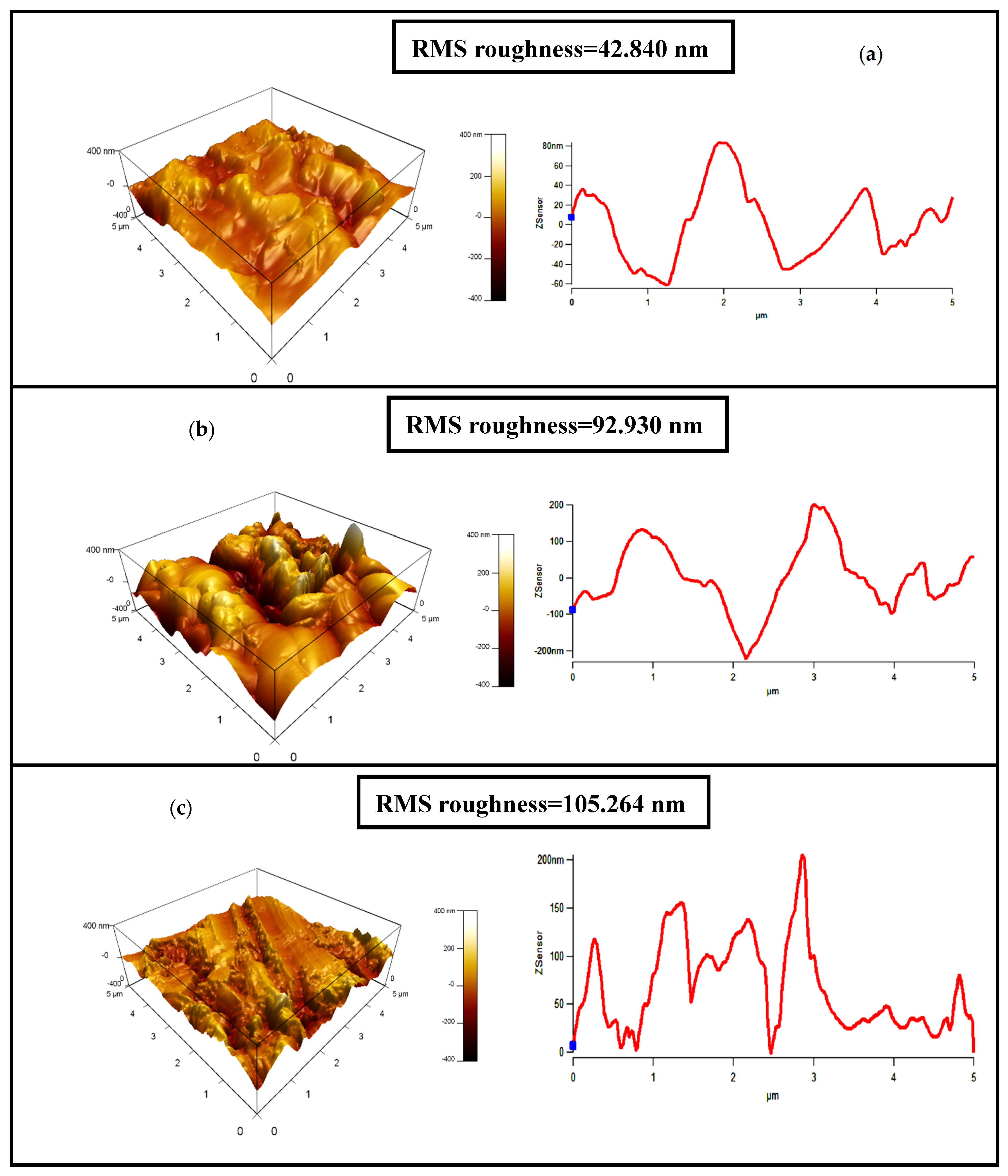
3.4. Atomic Force Microscopy
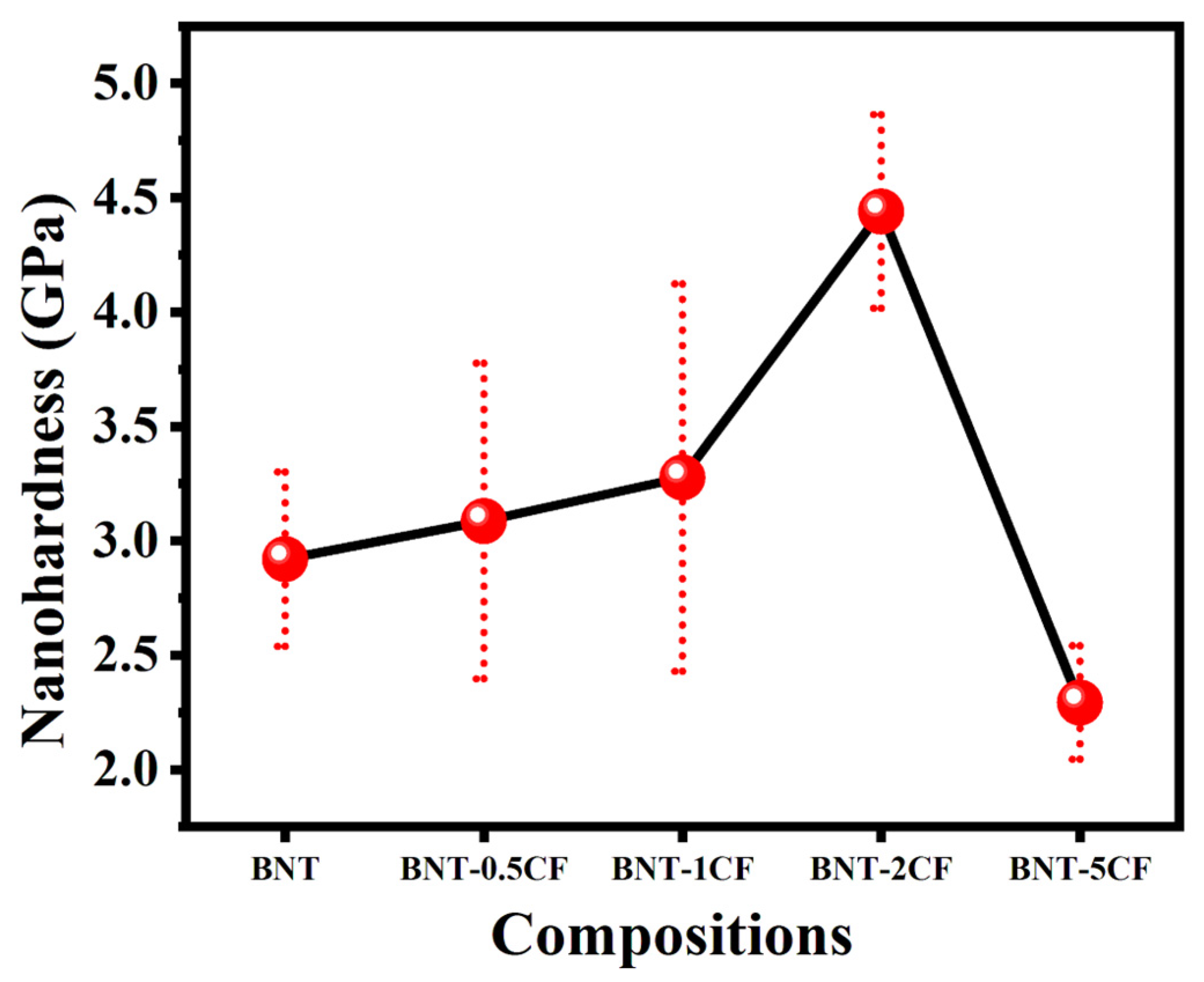
3.5. Nanohardness
3.6. Conductivity Study
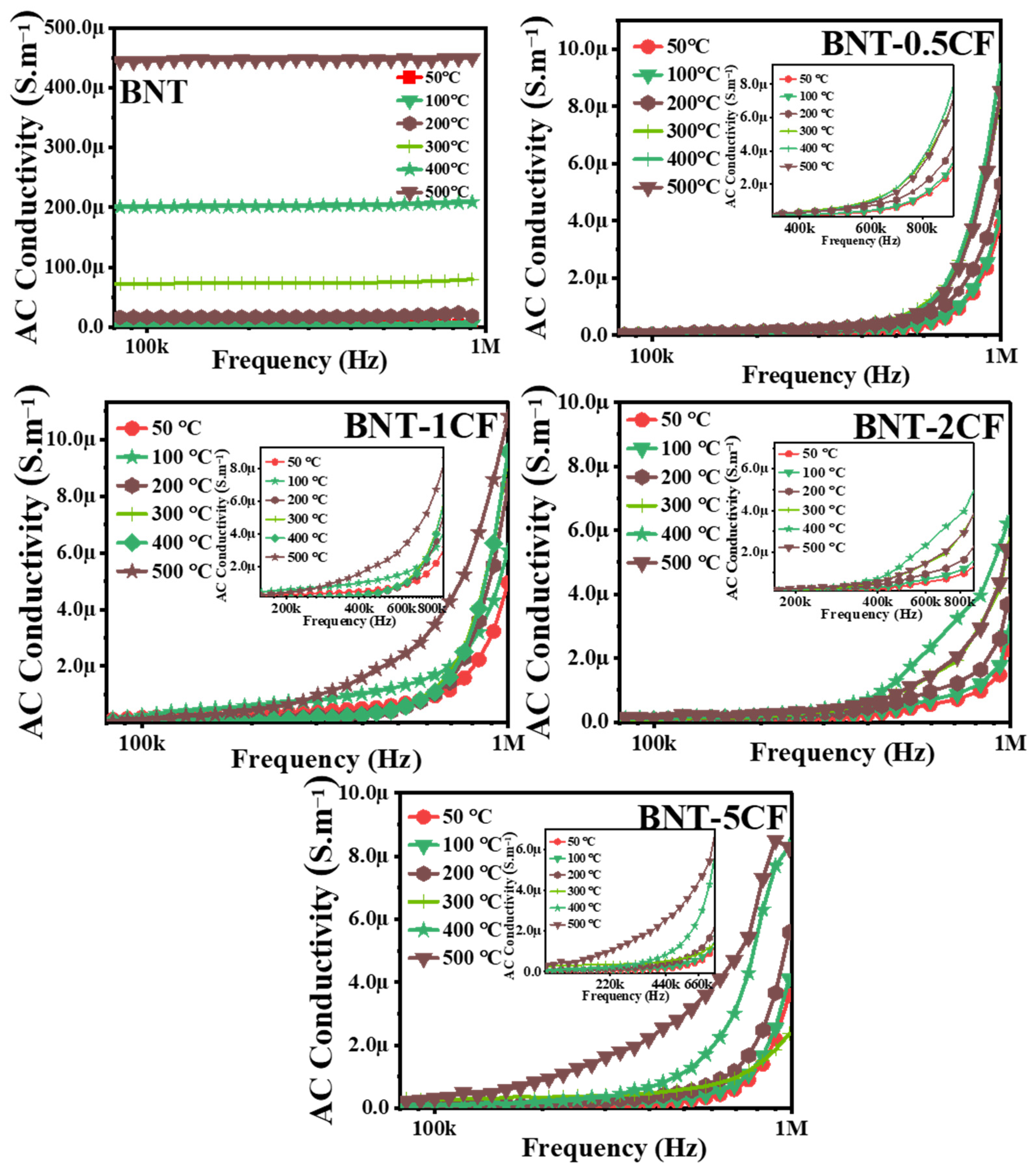
3.6.1. Effect of Frequency on Conductivity
3.6.2. Activation Energy
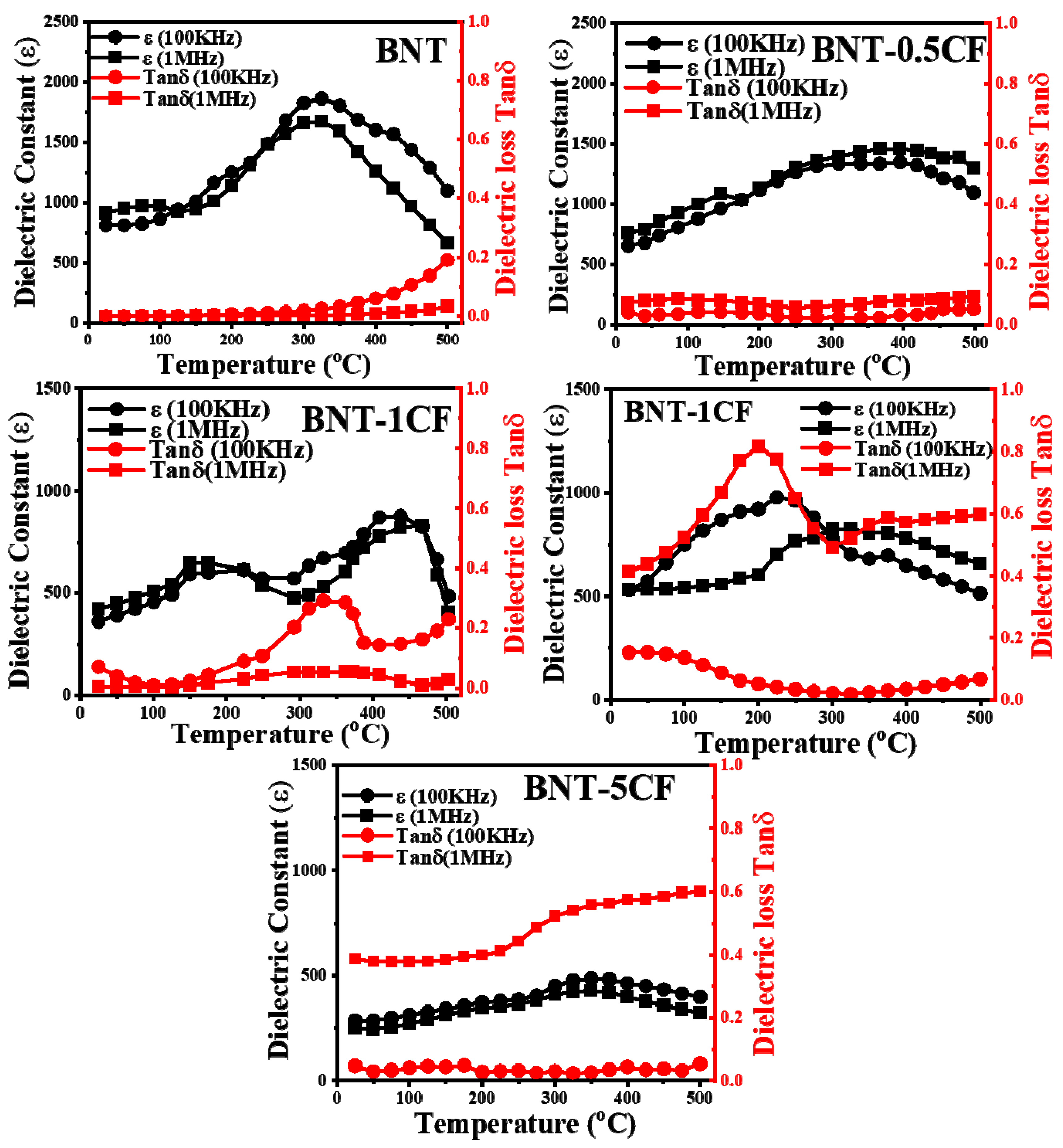
3.6.3. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milewski, J.; Dybiński, O.; Szczęśniak, A.; Martsinchyk, A.; Ćwieka, K.; Xing, W.; Szabłowski, Ł. Identification of Oxygen Ion Conductivity of Ba Doped Bi0.5Na0.5TiO3 (Ba-BNT) Based Matrix Impregnated by Lithium/Potassium Electrolyte for Molten Carbonate Fuel Cells. Int. J. Hydrog. Energy 2024, 51, 412–423. [Google Scholar] [CrossRef]
- Tan, J.; Huang, R.; Dai, Y.; Liang, Z.; Feng, J.; Lin, H.-T. Improvement of Electrostrain Properties of BNT-Based Piezoelectric Ceramics by Co-Doping of Sr2+Ta5+ in A and B Sites. Ceram. Int. 2023, 49, 35399–35405. [Google Scholar] [CrossRef]
- Manotham, S.; Jaita, P.; Butnoi, P.; Lertcumfu, N.; Rujijanagul, G. Improvements of Depolarization Temperature, Piezoelectric and Energy Harvesting Properties of BNT-Based Ceramics by Doping an Interstitial Dopant. J. Alloys Compd. 2022, 897, 163021. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Dong, R.; Tian, W.; Liu, X. Polarization Enhancement in Fe Doped BNT Based Relaxors Using Bi Compensation. J. Alloys Compd. 2021, 889, 161720. [Google Scholar] [CrossRef]
- Chen, B.; Tian, Y.; Lu, J.; Wu, D.; Qiao, X.; Liang, P.; Du, H.; Peng, Z.; Ren, X.; Chao, X.; et al. Ultrahigh Storage Density Achieved with (1-x)KNN-xBZN Ceramics. J. Eur. Ceram. Soc. 2020, 40, 2936–2944. [Google Scholar] [CrossRef]
- Sebastián, E.-G.; Raigoza, C.F.V.; Sonia, G.J. Structural and Magnetic Characterization of (1-x) KNN-xBLFO Ceramic Powders Obtained by the Combustion Reaction Method. J. Magn. Magn. Mater. 2020, 498, 166197. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Li, H.; Zhai, J. Effects of CuO on KNN-Based Ceramics. Inorg. Chem. Commun. 2020, 122, 108299. [Google Scholar] [CrossRef]
- Amu-Darko, J.N.O.; Zhang, C.; Hussain, S.; Issaka, E.; Otoo, S.L.; Agyemang, M.F.; Okoroafor, E.C. Dielectric Properties and Relaxor Behavior of (1-x)Ba(Zr0.15Ti0.85)O3—xBa(Mg(1⁄3)Nb(2⁄3))O3 Ceramics for Capacitor Applications. Mater. Chem. Phys. 2022, 287, 126328. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Xu, K.; Wang, B.; Wang, F.; Li, C.; Diao, C.; Huang, H.; Zheng, H. Significantly Improved Energy Storage Performance of Flexible PVDF-Based Nanocomposite by Loading Surface-Hydroxylated BaZr0.2Ti0.8O3 Nanofibers. Ceram. Int. 2022, 48, 16114–16122. [Google Scholar] [CrossRef]
- Qi, X.; Ren, P.; Tong, X. Effect of Heterovalent-Ion Doping and Oxygen-Vacancy Regulation on Piezoelectric Properties of KNN Based Lead-Free Ceramics. Ceram. Int. 2023, 49, 34795–34804. [Google Scholar] [CrossRef]
- Takenaka, T.; Kei-ichi Maruyama, K.M.; Koichiro Sakata, K.S. (Bi1/2N 1/2)TiO3-BaTiO3 System for Lead-Free Piezoelectric Ceramics. Jpn. J. Appl. Phys. 1991, 30, 2236. [Google Scholar] [CrossRef]
- Xu, Q.; Song, Z.; Tang, W.; Hao, H.; Zhang, L.; Appiah, M.; Cao, M.; Yao, Z.; He, Z.; Liu, H. Ultra-Wide Temperature Stable Dielectrics Based on Bi 0.5 Na 0.5 TiO3–NaNbO3 System. J. Am. Ceram. Soc. 2015, 98, 3119–3126. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, G.; Luo, H.; Bowen, C.R.; Zhang, D. Phase Structure and Properties of Sodium Bismuth Titanate Lead-Free Piezoelectric Ceramics. Prog. Mater. Sci. 2021, 122, 100836. [Google Scholar] [CrossRef]
- Mahboob, S.; Prasad, G.; Kumar, G.S. Impedance Spectroscopy and Conductivity Studies on B Site Modified (Na0.5Bi0.5)(NdxTi1−2xNbx)O3 Ceramics. J. Mater. Sci. 2007, 42, 10275–10283. [Google Scholar] [CrossRef]
- Lian, H.; Liang, X.; Shi, M.; Liu, L.; Chen, X. Improved Dielectric Temperature Stability and Energy Storage Properties of BNT-BKT-Based Lead-Free Ceramics. Ceram. Int. 2024, 50, 5021–5031. [Google Scholar] [CrossRef]
- Bai, W.; Li, L.; Li, W.; Shen, B.; Zhai, J.; Chen, H. Effect of SrTiO3 Template on Electric Properties of Textured BNT–BKT Ceramics Prepared by Templated Grain Growth Process. J. Alloys Compd. 2014, 603, 149–157. [Google Scholar] [CrossRef]
- Devi, C.S.; Kumar, G.S.; Prasad, G. Control of Ferroelectric Phase Transition in Nano Particulate NBT–BT Based Ceramics. Mater. Sci. Eng. B 2013, 178, 283–292. [Google Scholar] [CrossRef]
- Machado, R.; Ochoa, D.A.; Dos Santos, V.B.; Cerdeiras, E.; Mestres, L.; García, J.E. High Stability of Properties in Morphotropic Phase Boundary Bi0.5Na0.5TiO3–BaTiO3 Piezoceramics. Mater. Lett. 2016, 183, 73–76. [Google Scholar] [CrossRef]
- Nagata, H.; Shinya, T.; Hiruma, Y.; Takenaka, T.; Sakaguchi, I.; Haneda, H. Piezoelectric Properties of Bismuth Sodium Titanate Ceramics. In Ceramic Transactions Series; Nair, K.M., Guo, R., Bhalla, A.S., Hirano, S., Suvorov, D., Eds.; Wiley: Hoboken, NJ, USA, 2006; Volume 167, pp. 213–221. ISBN 978-1-57498-188-9. [Google Scholar]
- Kainz, T.; Naderer, M.; Schütz, D.; Fruhwirth, O.; Mautner, F.A.; Reichmann, K. Solid State Synthesis and Sintering of Solid Solutions of BNT–xBKT. J. Eur. Ceram. Soc. 2014, 34, 3685–3697. [Google Scholar] [CrossRef]
- Julphunthong, P.; Bongkarn, T.; Maensiri, S. The Effect of Firing Temperatures on Phase Formation, Microstructure and Dielectric Properties of Bi0.5(Na0.74K0.16Li0.10)0.5TiO3 Ceramics Synthesized via the Combustion Route. Ceram. Int. 2015, 41, S143–S151. [Google Scholar] [CrossRef]
- Badapanda, T.; Venkatesan, S.; Panigrahi, S.; Kumar, P. Structure and Dielectric Properties of Bismuth Sodium Titanate Ceramic Prepared by Auto-Combustion Technique. Process. Appl. Ceram. 2013, 7, 135–141. [Google Scholar] [CrossRef][Green Version]
- Pu, Y.; Zhang, L.; Cui, Y.; Chen, M. High Energy Storage Density and Optical Transparency of Microwave Sintered Homogeneous (Na0.5Bi0.5)(1–x)BaxTi(1–y)SnyO3 Ceramics. ACS Sustain. Chem. Eng. 2018, 6, 6102–6109. [Google Scholar] [CrossRef]
- Ramana, M.V.; Kiran, S.R.; Ramamanohar Reddy, N.; Siva Kumar, K.V.; Murthy, V.R.K.; Murty, B.S. Synthesis of Lead Free Sodium Bismuth Titanate (Nbt) Ceramic by Conventional and Microwave Sintering Methods. J. Adv. Dielectr. 2011, 01, 71–77. [Google Scholar] [CrossRef]
- Tian, L.; Nan, J.; Wang, H.; Shen, C. Effect of Microwave Sintering on the Properties of 0.95(Ca0.88Sr0.12) TiO3–0.05(Bi0.5Na0.5) TiO3 Ceramics. Materials 2019, 12, 803. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.K. Microwave Processing of Ceramics. Curr. Opin. Solid State Mater. Sci. 1998, 3, 480–485. [Google Scholar] [CrossRef]
- Fukushima, H.; Mori, H.; Hatanaka, T.; Matsui, M. Properties and Microstructure of PZT Ceramics Sintered by Microwave. J. Ceram. Soc. Jpn. 1995, 103, 1011–1016. [Google Scholar] [CrossRef][Green Version]
- Bandyopadhyay, P.; Ravi, D.; Ravichandran, R.; Mukhopadhyay, A.K. Carbon Fiber Reinforced Ceramics: A Flexible Material for Sophisticated Applications. In Advanced Flexible Ceramics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 551–566. ISBN 978-0-323-98824-7. [Google Scholar]
- Liu, T.; Li, R.; Pei, J.; Xing, X.; Guo, Q. Effect of Different Fibers Reinforcement on the Properties of PZT/PVA Composites. Mater. Chem. Phys. 2020, 239, 122063. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, H.; Yan, Z.; Xue, G.; Luo, H.; Zhang, D. Large Energy Density with Excellent Stability in Fine-Grained (Bi0.5Na0.5)TiO3-Based Lead-Free Ceramics. J. Eur. Ceram. Soc. 2019, 39, 4053–4059. [Google Scholar] [CrossRef]
- Tunkasiri, T.; Rujijanagul, G. Dielectric Strength of Fine Grained Barium Titanate Ceramics. J. Mater. Sci. Lett. 1996, 15, 1767–1769. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, J.; Nie, X.; Wei, D.; Zhang, N.; Peng, S.; Dong, X.; Li, Y.; Fang, P. One-Step Firing of Carbon Fiber and Ceramic Precursors for High Performance Electro-Thermal Composite: Influence of Graphene Coating. Mater. Des. 2020, 191, 108633. [Google Scholar] [CrossRef]
- Ahmad, I.; Yazdani, B.; Zhu, Y. Recent Advances on Carbon Nanotubes and Graphene Reinforced Ceramics Nanocomposites. Nanomaterials 2015, 5, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Randall, C.A. An Overview of Oxygen Vacancy Dynamics in (1 − x)(Bi1/2Na1/2)TiO3 − xBaTiO3 Solid Solution. J. Mater. Chem. C 2021, 9, 10303–10308. [Google Scholar] [CrossRef]
- Rafiq, M.A.; Tkach, A.; Costa, M.E.; Vilarinho, P.M. Defects and Charge Transport in Mn-Doped K0.5Na0.5NbO3 Ceramics. Phys. Chem. Chem. Phys. 2015, 17, 24403–24411. [Google Scholar] [CrossRef]
- Jayakrishnan, A.R.; Alex, K.V.; Kamakshi, K.; Silva, J.P.B.; Sekhar, K.C.; Gomes, M.J.M. Enhancing the Dielectric Relaxor Behavior and Energy Storage Properties of 0.6Ba(Zr0.2Ti0.8)O3–0.4(Ba0.7Ca0.3)TiO3 Ceramics through the Incorporation of Paraelectric SrTiO3. J. Mater. Sci. Mater. Electron. 2019, 30, 19374–19382. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Xue, J. Phase Transition, Ferroelectric Behaviors and Domain Structures of (Na1/2Bi1/2)1−xTiPbxO3 Thin Films. Acta Mater. 2006, 54, 1691–1698. [Google Scholar] [CrossRef]
- Shan, D.; Qu, Y.; Song, J. Ionic Doping Effects on Crystal Structure and Relaxation Character in Bi0.5Na0.5TiO3 Ferroelectric Ceramics. J. Mater. Res. 2007, 22, 730–734. [Google Scholar] [CrossRef]
- Yoon, M.-S.; Khansur, N.H.; Ur, S.-C. The Effect of Pre-Milling/Pre-Synthesis Process and Excess Ba on the Microstructure and Dielectric/Piezoelectric Properties of Nano-Sized 0.94[(Bi0.5Na0.5)TiO3]–0.06[Ba(1+x)TiO3]. Ceram. Int. 2010, 36, 1265–1275. [Google Scholar] [CrossRef]
- Bai, W.; Li, P.; Li, L.; Zhang, J.; Shen, B.; Zhai, J. Structure Evolution and Large Strain Response in BNT–BT Lead-Free Piezoceramics Modified with Bi(Ni0.5Ti0.5)O3. J. Alloys Compd. 2015, 649, 772–781. [Google Scholar] [CrossRef]
- Kandemir, S.; Bohlen, J.; Dieringa, H. Influence of Recycled Carbon Fiber Addition on the Microstructure and Creep Response of Extruded AZ91 Magnesium Alloy. J. Magnes. Alloys 2023, 11, 2518–2529. [Google Scholar] [CrossRef]
- Hao, X. A Review on the Dielectric Materials for High Energy-Storage Application. J. Adv. Dielectr. 2013, 03, 1330001. [Google Scholar] [CrossRef]
- Prado, A.; Ramajo, L.; Camargo, J.; Del Campo, A.; Öchsner, P.; Rubio-Marcos, F.; Castro, M. Stabilization of the Morphotropic Phase Boundary in (1 − x)Bi0.5Na0.5TiO3–xBaTiO3 Ceramics through Two Alternative Synthesis Pathways. J. Mater. Sci. Mater. Electron. 2019, 30, 18405–18412. [Google Scholar] [CrossRef]
- Sahoo, S.; Hajra, S.; De, M.; Choudhary, R.N.P. Resistive, Capacitive and Conducting Properties of Bi0.5Na0.5TiO3-BaTiO3 Solid Solution. Ceram. Int. 2018, 44, 4719–4726. [Google Scholar] [CrossRef]
- Prado, A.; Rubio-Marcos, F.; Ramajo, L.; Castro, M.S. Dielectric and Ferroelectric Properties Evolution of (1−x)(Bi0.5Na0.5TiO3)–xK0.5Na0.5NbO3 Piezoceramics. Bull. Mater. Sci. 2020, 43, 282. [Google Scholar] [CrossRef]
- Lidjici, H.; Lagoun, B.; Berrahal, M.; Rguitti, M.; Hentatti, M.A.; Khemakhem, H. XRD, Raman and Electrical Studies on the (1−x)(Na0.5Bi0.5)TiO3−xBaTiO3 Lead Free Ceramics. J. Alloys Compd. 2015, 618, 643–648. [Google Scholar] [CrossRef]
- Nesterović, A.; Vukmirović, J.; Stijepović, I.; Milanović, M.; Bajac, B.; Tóth, E.; Cvejić, Ž.; Srdić, V.V. Structure and Dielectric Properties of (1-x)Bi0.5Na0.5TiO3–x BaTiO3 Piezoceramics Prepared Using Hydrothermally Synthesized Powders. R. Soc. Open Sci. 2021, 8, 202365. [Google Scholar] [CrossRef]
- Alaoui, I.H.; Lemée, N.; Le Marrec, F.; Mebarki, M.; Cantaluppi, A.; Favry, D.; Lahmar, A. Effect of Oxygen Annealing Atmosphere on Structural, Electrical and Energy Storage Properties of Bi0.5Na0.5TiO3 Polycrystalline Thin Film. Quantum Beam Sci. 2023, 7, 29. [Google Scholar] [CrossRef]
- Anthoniappen, J.; Tu, C.S.; Chen, P.-Y.; Chen, C.-S.; Idzerda, Y.U.; Chiu, S.-J. Raman Spectra and Structural Stability in B-Site Manganese Doped (Bi0.5Na0.5)0.925Ba0.075TiO3 Relaxor Ferroelectric Ceramics. J. Eur. Ceram. Soc. 2015, 35, 3495–3506. [Google Scholar] [CrossRef]
- Rout, D.; Moon, K.-S.; Kang, S.-J.L.; Kim, I.W. Dielectric and Raman Scattering Studies of Phase Transitions in the (100−x)Na0.5Bi0.5TiO3–xSrTiO3 System. J. Appl. Phys. 2010, 108, 084102. [Google Scholar] [CrossRef]
- Kreisel, J.; Glazer, A.M.; Bouvier, P.; Lucazeau, G. High-Pressure Raman Study of a Relaxor Ferroelectric: The Na0.5Bi0.5TiO3 Perovskite. Phys. Rev. B 2001, 63, 174106. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, J.; Shen, B. Local Phenomena in Bismuth Sodium Titanate Perovskite Studied by Raman Spectroscopy. J. Am. Ceram. Soc. 2018, 101, 5604–5614. [Google Scholar] [CrossRef]
- Trelcat, J.-F.; Courtois, C.; Rguiti, M.; Leriche, A.; Duvigneaud, P.-H.; Segato, T. Morphotropic Phase Boundary in the BNT–BT–BKT System. Ceram. Int. 2012, 38, 2823–2827. [Google Scholar] [CrossRef]
- Yan, K.; Ren, S.; Fang, M.; Ren, X. Crucial Role of Octahedral Untilting R3m/P4Mm Morphotropic Phase Boundary in Highly Piezoelectric Perovskite Oxide. Acta Mater. 2017, 134, 195–202. [Google Scholar] [CrossRef]
- Parmar, K.; Negi, N.S. Influence of Na/Bi Excess on Structural, Dielectric and Multiferroic Properties of Lead Free (Na0.5Bi0.5)0.99La0.01Ti0.988Fe0.012O3 Ceramic. J. Alloys Compd. 2015, 618, 781–787. [Google Scholar] [CrossRef]
- Ureña, A.; Rams, J.; Escalera, M.D.; Sánchez, M. Characterization of Interfacial Mechanical Properties in Carbon Fiber/Aluminium Matrix Composites by the Nanoindentation Technique. Compos. Sci. Technol. 2005, 65, 2025–2038. [Google Scholar] [CrossRef]
- Pattanayak, R.; Panigrahi, S.; Dash, T.; Muduli, R.; Behera, D. Electric Transport Properties Study of Bulk BaFe12O19 by Complex Impedance Spectroscopy. Phys. B Condens. Matter 2015, 474, 57–63. [Google Scholar] [CrossRef]
- Kanuru, S.R.; Baskar, K.; Dhanasekaran, R. Synthesis, Structural, Morphological and Electrical Properties of NBT–BT Ceramics for Piezoelectric Applications. Ceram. Int. 2016, 42, 6054–6064. [Google Scholar] [CrossRef]
- Nie, X.; Wang, J.; Peng, Z.; Chai, Q.; Zhou, Q.; Chao, X.; Yang, Z. High Transparency and Good Electric Properties in Low Symmetry BNT-Based Ceramics. Solid State Sci. 2022, 129, 106906. [Google Scholar] [CrossRef]
- Schulz, T.; Veerapandiyan, V.; Deluca, M.; Töpfer, J. Synthesis and Properties of Lead-Free BNT-BT-xCZ Ceramics as High-Temperature Dielectrics. Mater. Res. Bull. 2022, 145, 111560. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, Y.; Yang, Y. Growth, Properties and Applications of Bi0.5Na0.5TiO3 Ferroelectric Nanomaterials. Nanomaterials 2021, 11, 1724. [Google Scholar] [CrossRef]
- Arya, B.B.; Choudhary, R.N.P. Studies of Structural and Electrical Characteristics of Multi-Substituted Bi0.5Na0.5TiO3 Ferroelectric Ceramics. J. Mater. Sci. Mater. Electron. 2021, 32, 11547–11567. [Google Scholar] [CrossRef]

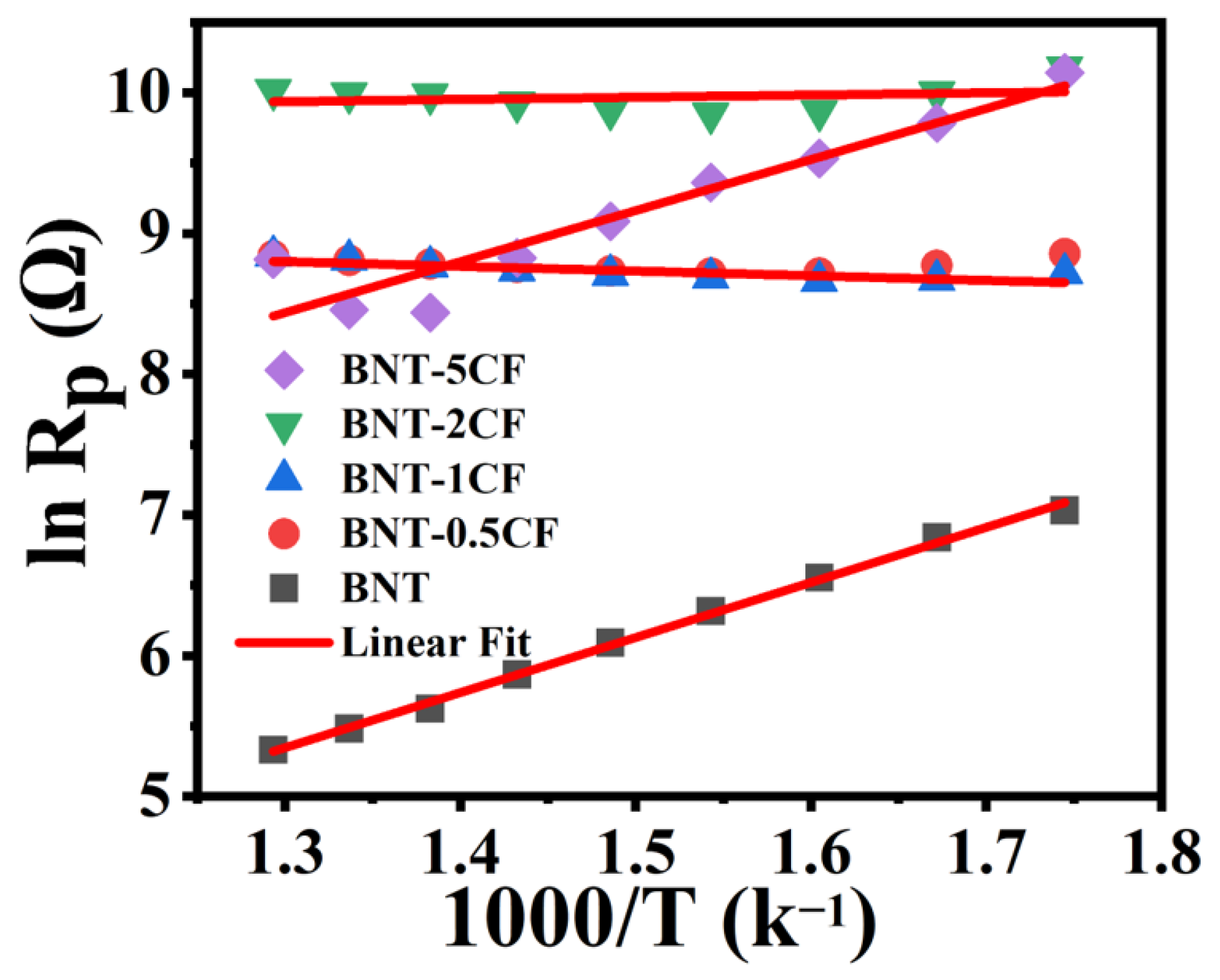
| SR. Number | Sample | Ea |
|---|---|---|
| 1. | BNT | 0.34 |
| 2. | BNT-0.5CF | 0.15 |
| 3. | BNT-1CF | 0.1 |
| 4. | BNT-2CF | 0.03 |
| 5. | BNT-5CF | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azam, F.; Rafiq, M.A.; Ahmed, F.; Moqbool, A.; Fayyaz, O.; Imran, Z.; Habib, M.S.; Shakoor, R.A. Microwave-Assisted Fabrication and Characterization of Carbon Fiber-Sodium Bismuth Titanate Composites. Crystals 2024, 14, 798. https://doi.org/10.3390/cryst14090798
Azam F, Rafiq MA, Ahmed F, Moqbool A, Fayyaz O, Imran Z, Habib MS, Shakoor RA. Microwave-Assisted Fabrication and Characterization of Carbon Fiber-Sodium Bismuth Titanate Composites. Crystals. 2024; 14(9):798. https://doi.org/10.3390/cryst14090798
Chicago/Turabian StyleAzam, Fareeha, Muhammad Asif Rafiq, Furqan Ahmed, Adnan Moqbool, Osama Fayyaz, Zerfishan Imran, Muhammad Salman Habib, and Rana Abdul Shakoor. 2024. "Microwave-Assisted Fabrication and Characterization of Carbon Fiber-Sodium Bismuth Titanate Composites" Crystals 14, no. 9: 798. https://doi.org/10.3390/cryst14090798
APA StyleAzam, F., Rafiq, M. A., Ahmed, F., Moqbool, A., Fayyaz, O., Imran, Z., Habib, M. S., & Shakoor, R. A. (2024). Microwave-Assisted Fabrication and Characterization of Carbon Fiber-Sodium Bismuth Titanate Composites. Crystals, 14(9), 798. https://doi.org/10.3390/cryst14090798









