Green Synthesized Composite AB-Polybenzimidazole/TiO2 Membranes with Photocatalytic and Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bio-Mediated TiO2 Particles
2.3. Preparation of AB-PBI/Bio-TiO2 Membranes
2.4. Physicochemical Characterization of Prepared AB-PBI/Bio-TiO2 Membranes of Bio-Synthesized TiO2 Particles
2.5. Physicochemical Characterization of Prepared AB-PBI/Bio-TiO2 Membranes
2.6. Photocatalytic Activity Tests
2.7. Antibacterial Properties
- Media dia and test microorganisms
- Photocatalytic Antibacterial Activity of Bare and PBI-Stabilized TiO2 Nanoparticles
3. Results
3.1. TiO2 Particles
3.2. Preparation of AB-PBI/Bio-TiO2 Membranes
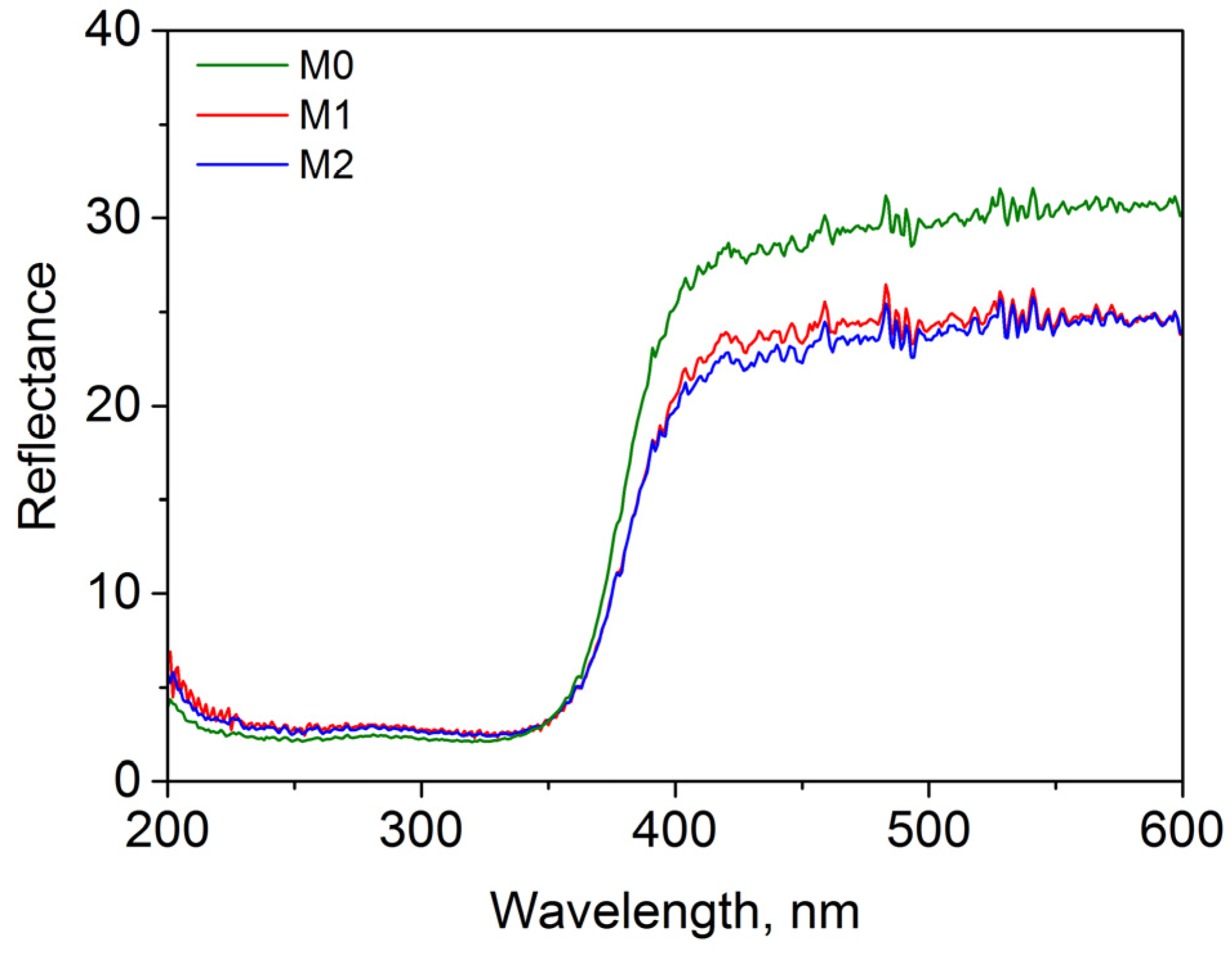
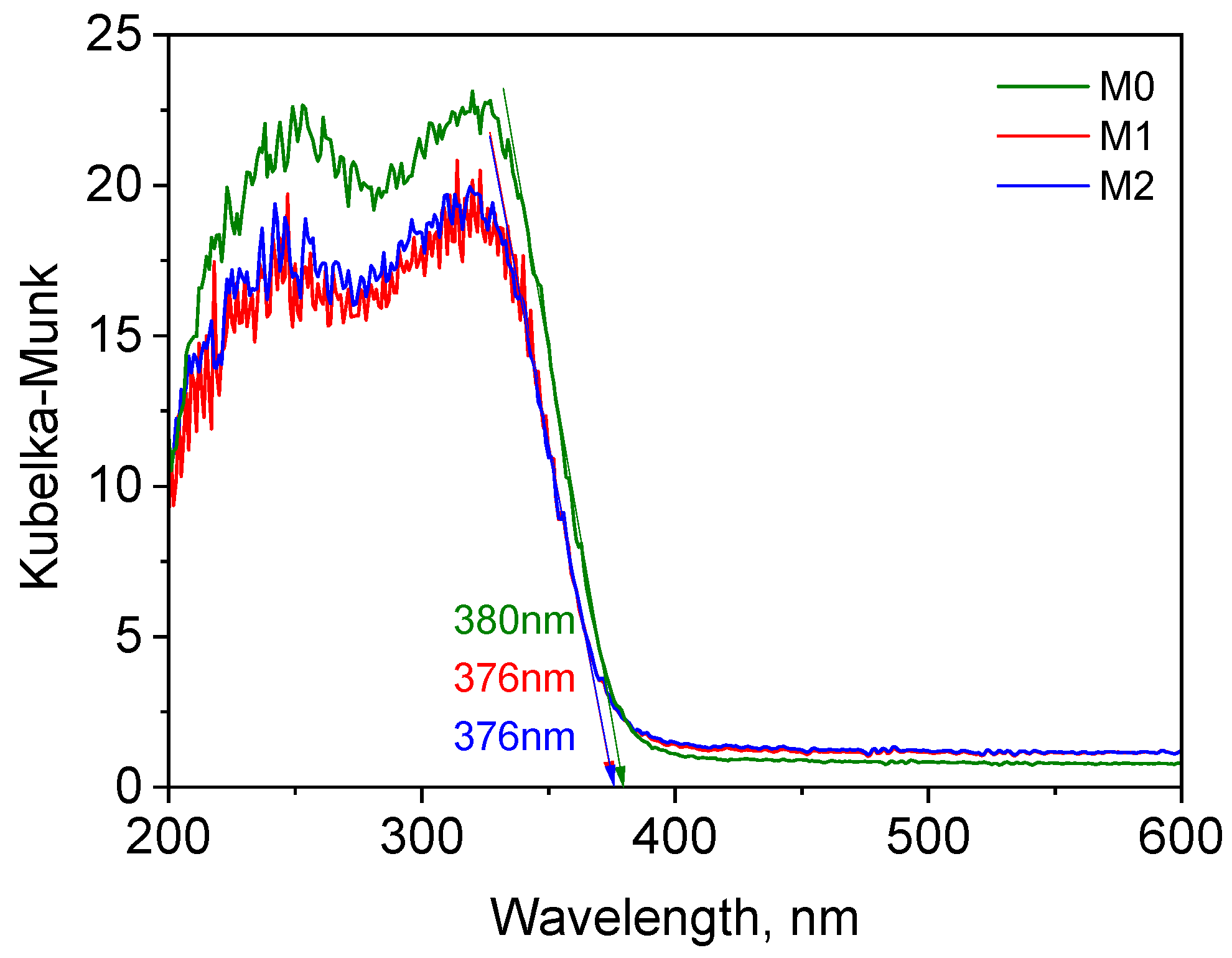
3.3. Characterization of Prepared AB-PBI/Bio-TiO2 Membranes
3.3.1. XRD Study
3.3.2. XPS Study
3.3.3. EPR Spectroscopy
3.3.4. SEM and EDS Mapping/Spectra Analysis of the AB-PBI/Bio-TiO2 Membranes
3.3.5. FTIR Investigations
3.3.6. Thermogravimetric Analysis (TG) of the Prepared AB-PBI/Bio-TiO2 Membranes
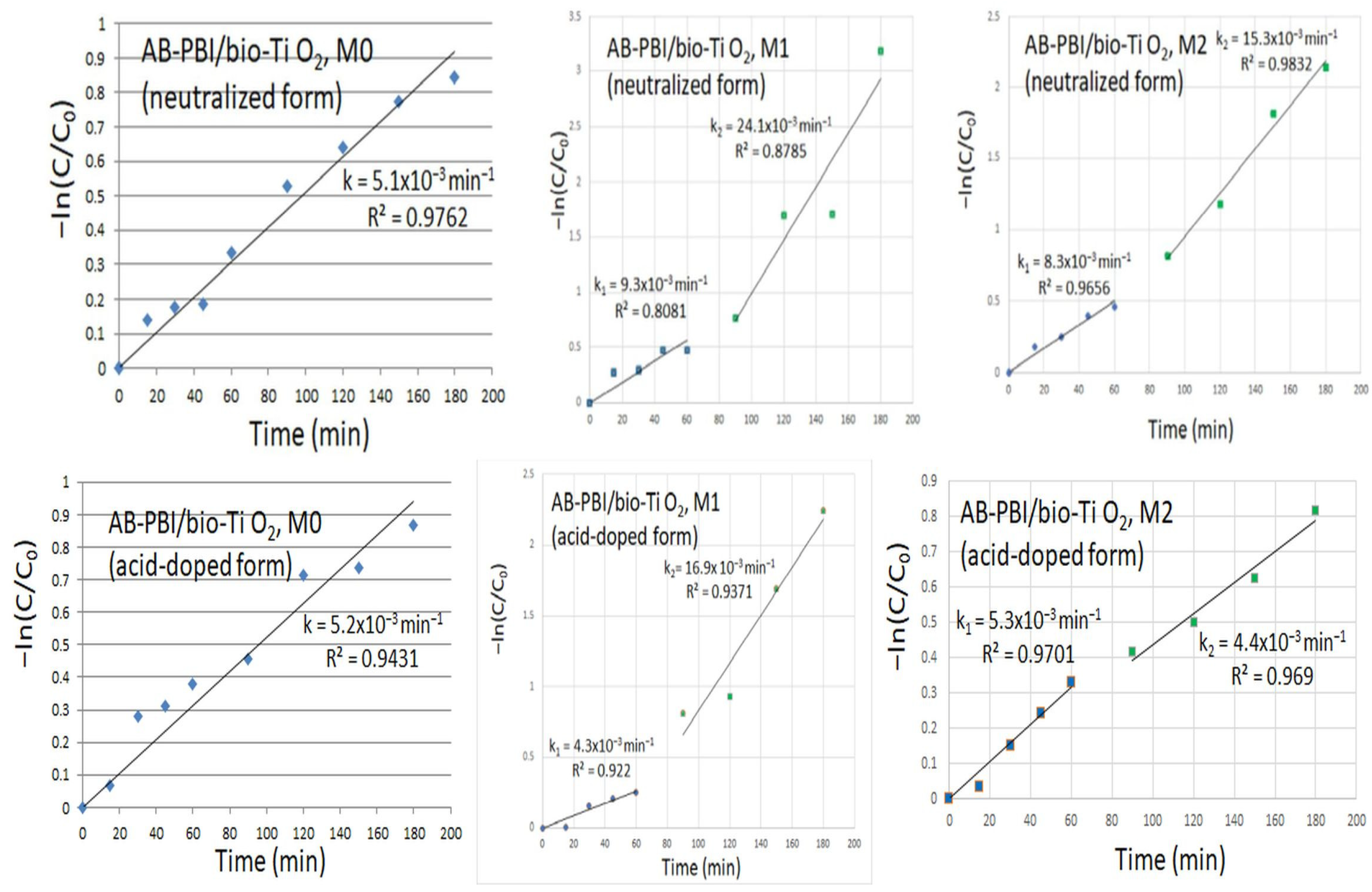
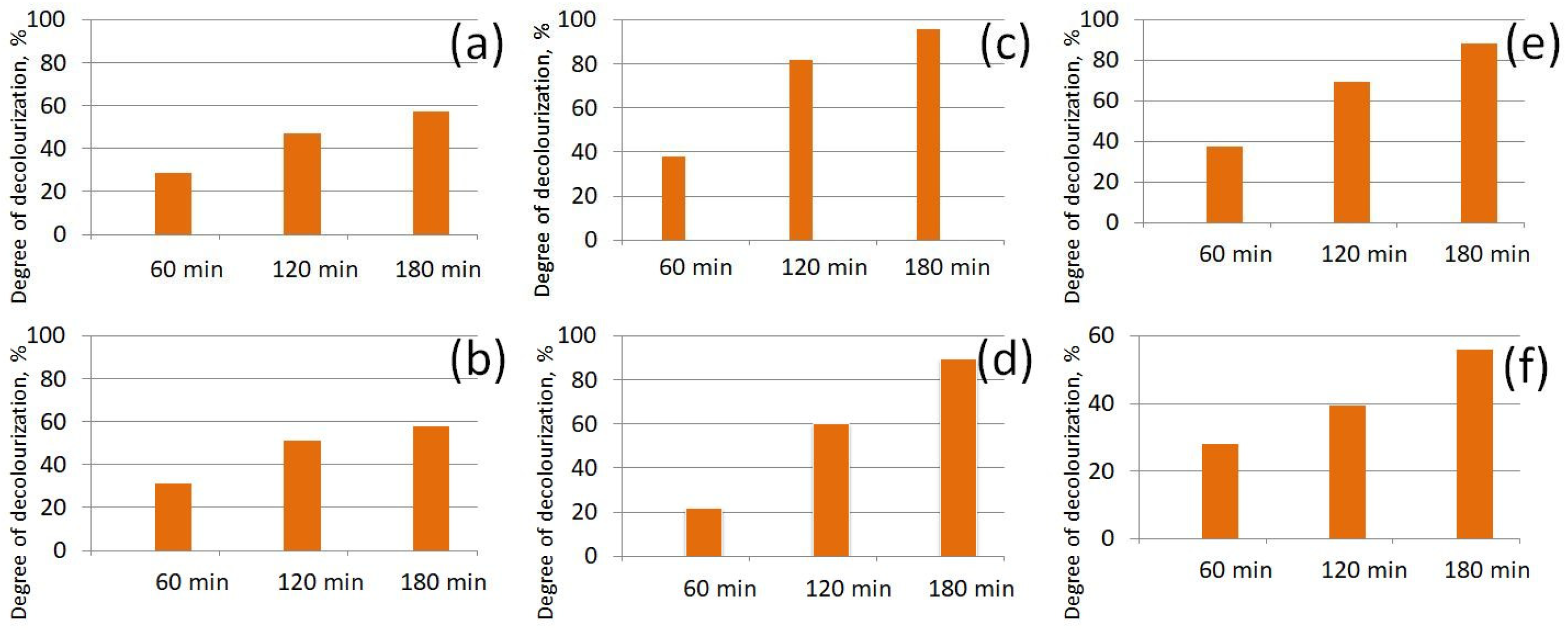
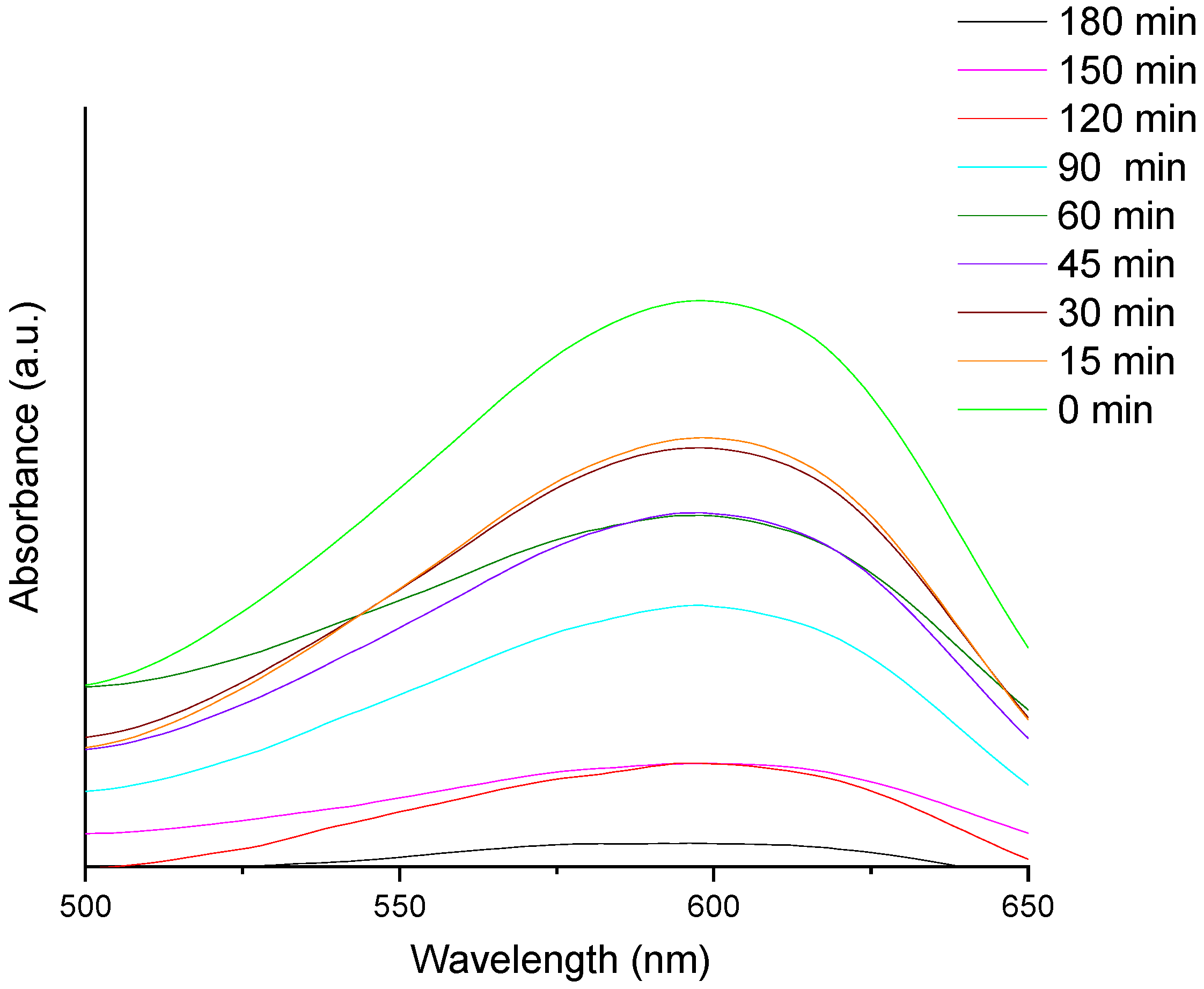
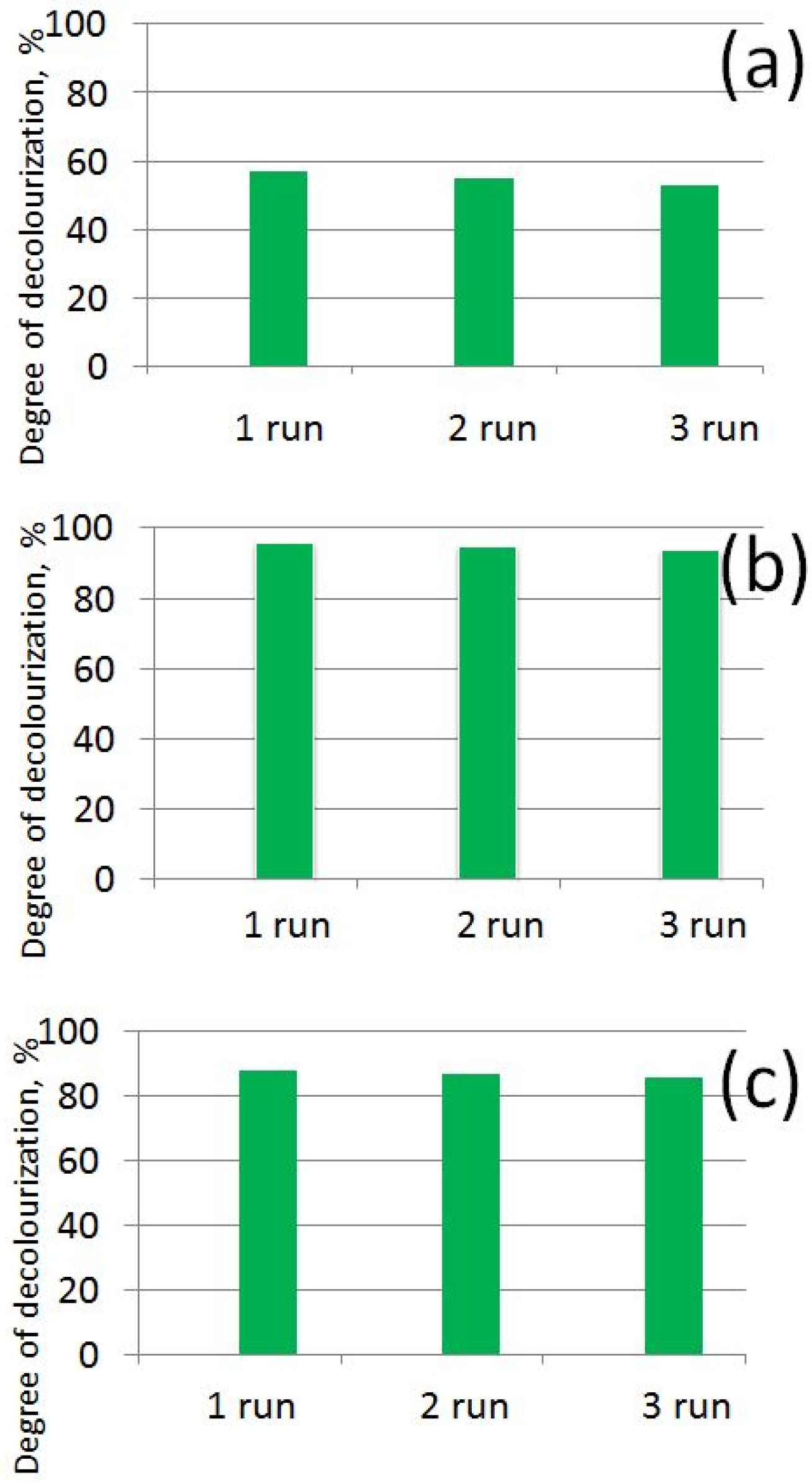
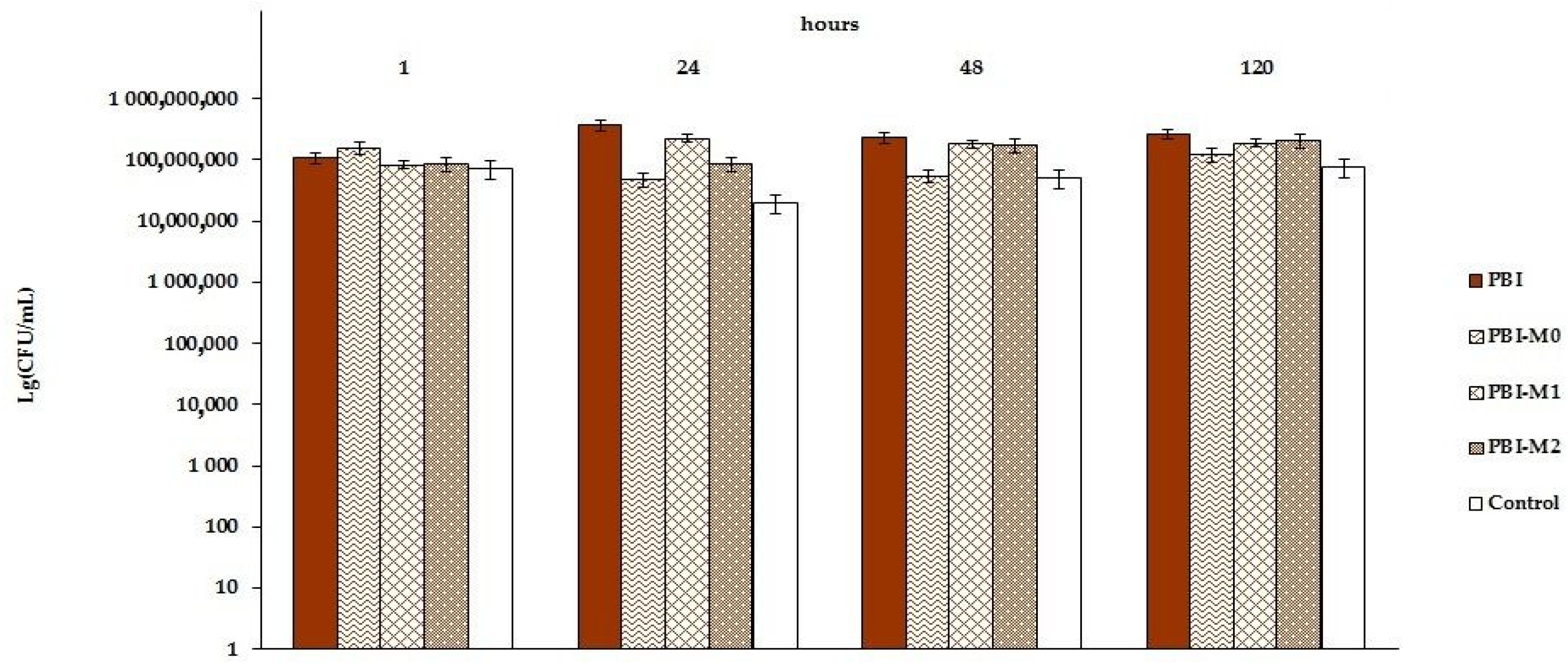
3.4. Photocatalytic Study of Prepared AB-PBI/Bio-TiO2 Membranes
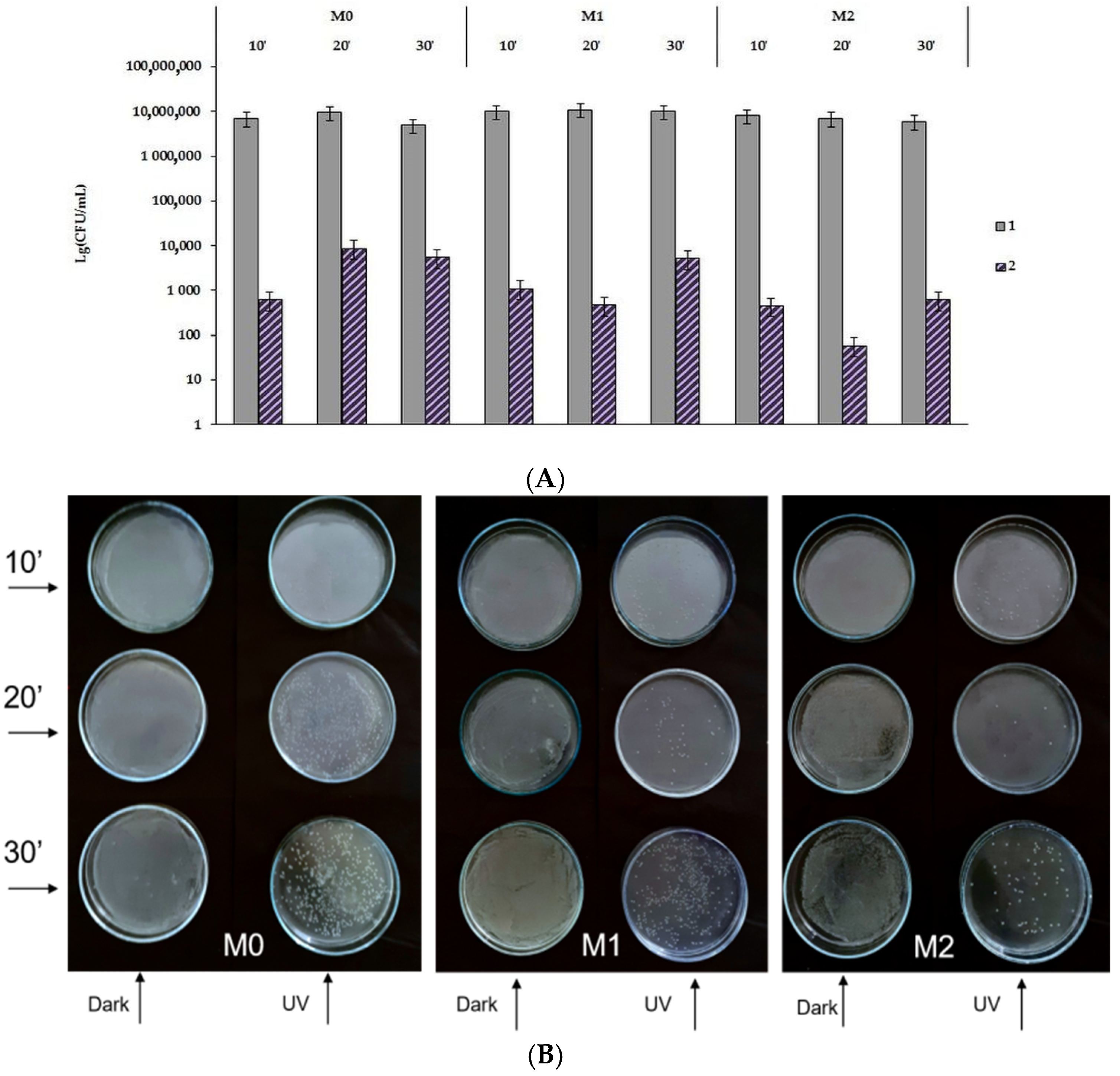
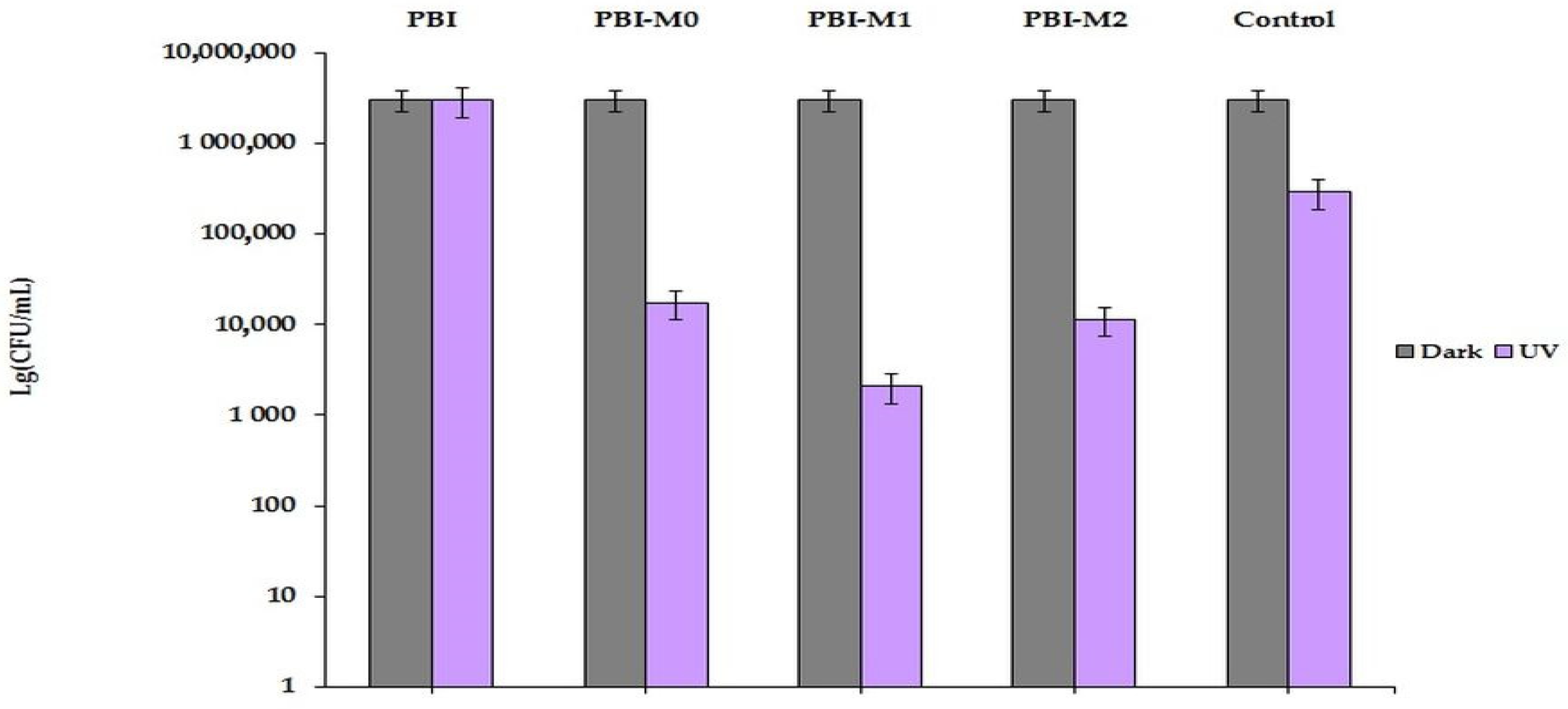
3.5. Antibacterial Activity of Prepared AB-PBI/Bio-TiO2 Membranes
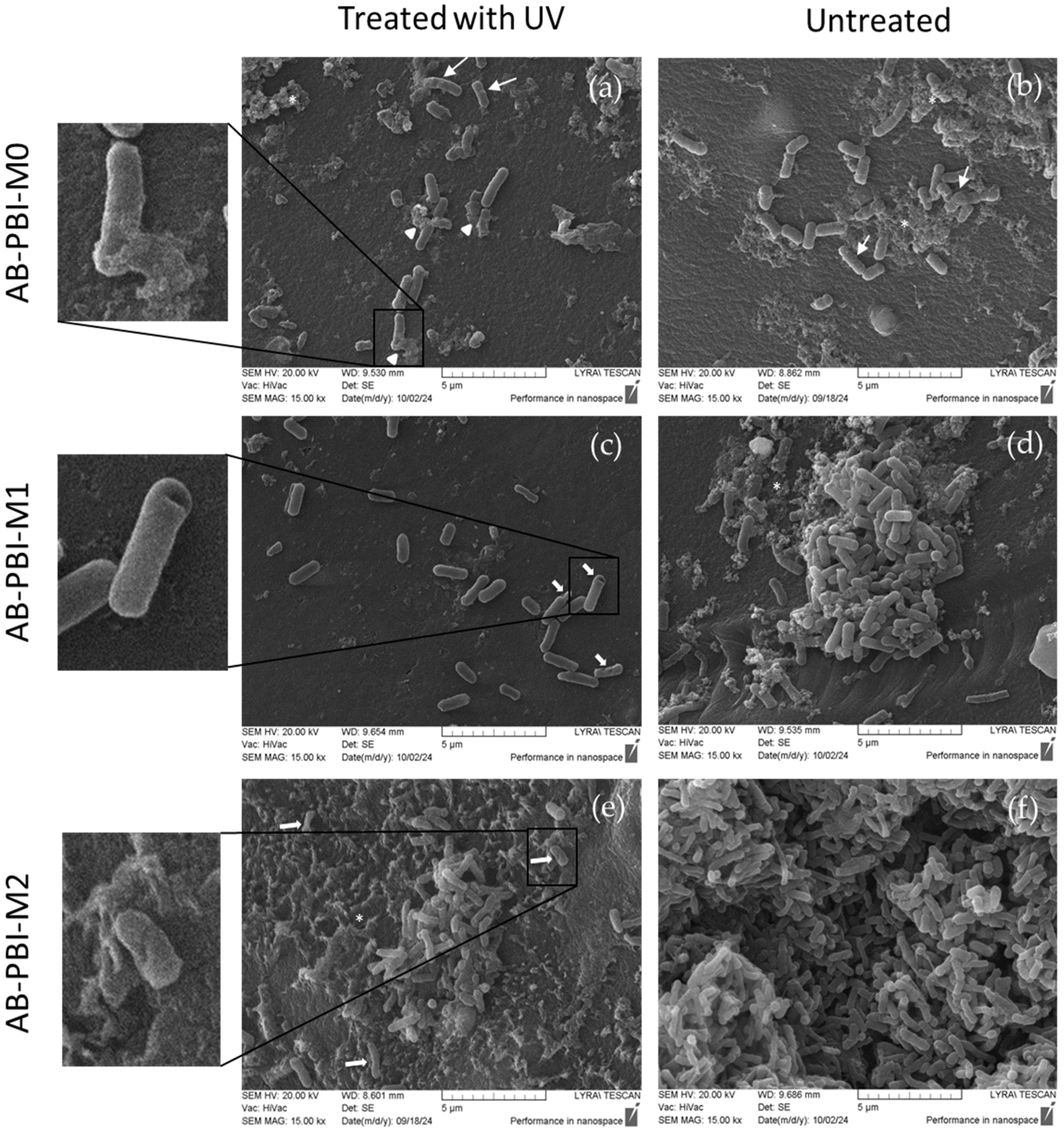
- Bacterial morphology assessment by SEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honma, I.; Nomura, S.; Nakajima, H. Protonic conducting organic/inorganic nanocomposites for polymerelectrolyte membrane. J. Membr. Sci. 2001, 185, 83–94. [Google Scholar] [CrossRef]
- Sengodu, P.; Deshmukh, A. Conducting polymers and its Inorganic composites for Advanced Li ion Batteries: A review. RSC Advances 2012, 1–3, 1–23. [Google Scholar] [CrossRef]
- Morales-Acevedo, A. (Ed.) Solar Cells-Research and Application Perspectives; InTech: Rijeka, Croatia, 2013; 386p, ISBN 978-953-51-1003-3. [Google Scholar]
- Laberty-Robert, C.; Vallé, K.; Pereira, F.; Sanchez, C. Design and properties of functional hybrid organic-inorganic membranes for fuel cells. Chem. Soc. Rev. 2011, 40, 961–1005. [Google Scholar] [CrossRef] [PubMed]
- Liras, M.; Barawi, M.; de la Peña O’Shea, V.A. Hybrid materials based on conjugated polymers and inorganicsemiconductors as photocatalysts: From environmental to energy applications. Chem. Soc. Rev. 2019, 48, 5454–5487. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Thakur, M.S.; Byrappa, K. Biocompatibility assessment and photocatalytic activity of bio-hydrothermal synthesis of ZnO nanoparticles by Thymus vulgaris leaf extract. Mater. Res. Bull. 2019, 109, 49–59. [Google Scholar] [CrossRef]
- Alamdari, S.; Ghamsari, M.S.; Lee, C.; Han, W.; Park, H.-H.; Tafreshi, M.J.; Afarideh, H.; Majles Ara, M.H. Preparation and Characterization of Zinc Oxide Nanoparticles Using Leaf Extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green Approach for Fabrication and Applications of Zinc Oxide Nanoparticles. Bioinorg. Chem. Appl. 2014, 2014, 523869. [Google Scholar] [CrossRef]
- Punnoose, A.; Dodge, K.; Rassmussen, J.; Chess, J.; Wingett, D.; Ander, C. Cytotoxicity of ZnO nanoparticles can be tailored by modifying their surface structure: A green chemistry approach for safer nanomaterials. ACS Sustain. Chem. Eng. 2014, 2, 1666–1673. [Google Scholar] [CrossRef]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Photocatalysis and Antimicrobial Applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef]
- Muniandy, S.S.; NH Mohd, K.; Jiang, Z.T.; Altarawneh, M.; Lee, H.L. Green synthesis of mesoporous anataseTiO2 nanoparticles and their photocatalytic activities. RSC Adv. 2017, 7, 48083–48094. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Bama, K.; Bhavani, M.; Jegatheeswaran, S.; Sumathi, R. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J. Photochem. Photobiol. B Biol. 2017, 171, 117–124. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Ganesh, V.; Che Abdullah, C.A.; Yahia, I.S.; Zyoud, A.H.; Abdelkader, A.F.I.; Daher, M.G.; Nasor, M.; Shahwan, M.; Zahran, H.Y.; et al. Facile Synthesis of Ni-Doped ZnO Nanostructures via Laser-Assisted Chemical Bath Synthesis with High and Durable Photocatalytic Activity. Crystals 2023, 13, 1087. [Google Scholar] [CrossRef]
- Li, S.; Dong, K.; Cai, M.; Li, X.; Chen, X. A plasmonic S-scheme Au/MIL-101(Fe)/BiOBr photocatalyst for efficient synchronous decontamination of Cr(VI) and norfloxacin antibiotic. eScience 2024, 4, 100208. [Google Scholar] [CrossRef]
- Rajesh, D.; Ahuja, T.; Kumar, D. Recent progress in the development of nano-structured conducting polymers/nanocomposites for sensor applications. Sens. Actuators B 2009, 136, 275–286. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Kleine, J.; Peinemann, K.V.; Schuster, C.; Warnecke, H.J. Multifunctional system for treatment of wastewaters from adhesive-producing industries: Separation of solids and oxidation of dissolved pollutants using doted microfiltration membranes. Chem. Eng. Sci. 2002, 57, 1661–1664. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, anti fouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, S.-Y.; Sohn, B.-H.; Park, T.H. Design of TiO2 nanoparticle self assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem. J. Membr. Sci. 2003, 211, 157–165. [Google Scholar] [CrossRef]
- Alaoui, O.T.; Nguyen, Q.T.; Mbareck, C.; Rhlalou, T. Elaboration and study of poly (vinylidene fluoride)–anatase TiO2 composite membranes in photocatalytic degradation of dyes. Appl. Catal. A Gen. 2009, 358, 13–20. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Taheri, A.H.; Mansourpanah, Y. Coupling TiO2 nanoparticles with UV irradiation for modification of polyethersulfone ultra filtration membranes. J. Membr. Sci. 2008, 313, 158–169. [Google Scholar] [CrossRef]
- Liu, P.; Liu, H.; Liu, G.; Yao, K.; Lv, W. Preparation of TiO2 nanotubes coated on polyurethane and study of their photocatalytic activity. Appl. Surf. Sci. 2012, 258, 9593–9598. [Google Scholar] [CrossRef]
- Liu, L.; Chen, F.; Yang, F. Stable photocatalytic activity of immobilized Fe0/TiO2/ACF on composite membrane in degradation of 2,4-dichlorophenol. Sep. Purif. Technol. 2009, 70, 173–178. [Google Scholar] [CrossRef]
- Rota, F.; Cavassi, M.; Niego, D.; Gorlani, R.; Vianelli, L.; Tatti, L.; Bruzzi, P.; Moroni, A.; Bellobono, I.R.; Bianchi, M.; et al. Mathematical modelling of photomi neralization of phenols in aqueous solution, by photocatalytic membranes immobilizing titanium dioxide. Chemosphere 1996, 33, 2159–2173. [Google Scholar] [CrossRef]
- You, S.-J.; Semblante, G.U.; Lu, S.-C.; Damodar, R.A.; Wei, T.-C. Evaluation of the antifouling and photocatalytic properties of poly(vinylidene fluoride) plasma grafted poly(acrylic acid) membrane with self-assembled TiO2. J. Hazard. Mater. 2012, 237–238, 10–19. [Google Scholar] [CrossRef]
- Morris, R.E.; Krikanova, E.; Shadman, F. Photocatalytic membrane for removal of organic contaminants during ultra-purification of water. Clean Technol. Environ. Policy 2004, 6, 96–104. [Google Scholar] [CrossRef]
- Wang, K.Y.; Weber, M.; Chung, T.-S. Polybenzimidazoles (PBIs) and state-of-the-art PBI hollow fiber membranes for water, organic solvent and gas separations: A review. J. Mater. Chem. A 2022, 10, 8687–8718. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Úbeda, D.; Javier Pinar, F. A novel titanium PBI-based composite membrane for high temperature PEMFCs. J. Membr. Sci. 2011, 369, 105–111. [Google Scholar] [CrossRef]
- Javier Pinar, F.; Cañizares, P.; Rodrigo, M.A.; Ubeda, D.; Lobato, J. Titanium composite PBI-based membranes for high temperature polymer electrolyte membrane fuel cells. Effect on titanium dioxide amount. RSC Adv. 2012, 2, 1547–1556. [Google Scholar] [CrossRef]
- Díaz-Abad, S.; Rodrigo, M.A.; Sáez, C.; Lobato, J. Enhancement of the Green H2 Production by Using TiO2 Composite Polybenzimidazole Membranes. Nanomaterials 2022, 12, 2920. [Google Scholar] [CrossRef]
- Li, Q.; Aili, D.; Hjuler, H.A.; Jensen, J.O. Editors, High Temperature Polymer Electrolyte Membrane Fuel Cells Approaches, Status, and Perspectives; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Murdock, L.A.; Benicewicz, B.C. Preparation of Dense Polybenzimidazole Films without Organic Solvents. Macromolecules 2023, 56, 2729–2735. [Google Scholar] [CrossRef]
- Penchev, H.; Zaharieva, K.; Milenova, K.; Ublekov, F.; Dimova, S.; Budurova, D.; Staneva, M.; Stambolova, I.; Sinigersky, V.; Blaskov, V. Novel meta- and AB-Polybenzimidazole/Zinc oxide polymer hybrid nanomaterials for photocatalytic degradation of organic dyes. Mater. Lett. 2018, 230, 187–190. [Google Scholar] [CrossRef]
- Borisova, D.; Haladjova, E.; Kyulavska, M.; Petrov, P.; Pispas, S.; Stoitsova, S.; Paunova-Krasteva, T. Application of cationic polymer micelles for the dispersal of bacterial biofilms. Eng. Life Sci. 2018, 18, 943–948. [Google Scholar] [CrossRef]
- Paunova-Krasteva, T.; Haladjova, E.; Petrov, P.; Forys, A.; Trzebicka, B.; Topouzova-Hristova, T.; Stoitsova, S.R. Destruction of Pseudomonas aeruginosa pre-formed biofilms by cationic polymer micelles bearing silver nanoparticles. Biofouling 2020, 36, 679–695. [Google Scholar] [CrossRef]
- Paunova-Krasteva, T.; Hemdan, B.A.; Dimitrova, P.D.; Damyanova, T.; El-Feky, A.M.; Elbatanony, M.M.; Stoitsova, S.; Azab El-Liethy, M.; El-Taweel, G.E.; El Nahrawy, A.M. Hybrid Chitosan/CaO-Based Nanocomposites Doped with Plant Extracts from Azadirachta indica and Melia azedarach: Evaluation of Antibacterial and Antibiofilm Activities. BioNanoSci 2023, 13, 88–102. [Google Scholar] [CrossRef]
- Stancheva, R.; Paunova-Krasteva, T.; Topouzova-Hristova, T.; Stoitsova, S.; Petrov, P.; Haladjova, E. Ciprofloxacin-Loaded Mixed Polymeric Micelles as Antibiofilm Agents. Pharmaceutics 2023, 15, 1147. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Environmental Application of Photocatalysis. MSF 2012, 734, 273–294. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Sukirtha, T.H.; Balakrishna, K.M.; Varghese, T. Microbicidal activity of TiO2 nanoparticles synthesised by sol-gel method. IET Nanobiotechnol. 2016, 10, 81–86. [Google Scholar] [CrossRef]
- Kőrösi, L.; Pertics, B.; Schneider, G.; Bognár, B.; Kovács, J.; Meynen, V.; Scarpellini, A.; Pasquale, L.; Prato, M. Photocatalytic Inactivation of Plant Pathogenic Bacteria Using TiO2 Nanoparticles Prepared Hydrothermally. Nanomaterials 2020, 10, 1730. [Google Scholar] [CrossRef]
- Anbumanip, D.; Vizhi Dhandapani, K.; Manoharan, J.; Babujanarthanam, R.; Bashir, A.K.H.; Muthusamy, K.; Alfarhan, A.; Kanimozhi, K. Green synthesis and antimicrobial efficacy of titanium dioxide nanoparticles using Luffa acutangula leaf extract. J. King Saud Univ. Sci. 2022, 34, 101896. [Google Scholar] [CrossRef]
- Serov, D.A.; Gritsaeva, A.V.; Yanbaev, F.M.; Simakin, A.V.; Gudkov, S.V. Review of Antimicrobial Properties of Titanium Dioxide Nanoparticles. Int. J. Mol. Sci. 2024, 25, 10519. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media-A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. Powder Cell for Windows; Federal Institute for Materials Research and Testing: Berlin, Germany, 2000. [Google Scholar]
- Williams, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and tungsten. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Penchev, H.; Zaharieva, K.; Dimova, S.; Grancharov, G.; Petrov, P.D.; Shipochka, M.; Dimitrov, O.; Lazarkevich, I.; Engibarov, S.; Eneva, R. Hybrid Cellulosic Substrates Impregnated with Meta-PBI-Stabilized Carbon Nanotubes/Plant Extract-Synthesized Zinc Oxide—Antibacterial and Photocatalytic Dye Degradation Study. Nanomaterials 2024, 14, 1346. [Google Scholar] [CrossRef]
- Micic, O.I.; Zhang, Y.; Cromack, K.R.; Trifunac, A.D.; Thurnauer, M.C. Trapped holes on TiO2 colloids studied by Electron Paramagnetic Resonance. J. Phys. Chem. 1993, 97, 7277–7283. [Google Scholar] [CrossRef]
- Nakaoka, Y.; Nosaka, Y. ESR investigation into the effects of heat treatment and crystal structure on radicals produced over irradiated TiO2 powder. J. Photochem. Photobiol. A Chem. 1997, 110, 299–305. [Google Scholar] [CrossRef]
- Kumar, C.P.; Gopal, N.O.; Wang, T.C.; Wong, M.-S.; Ke, S.C. EPR investigation of TiO2 nanoparticles with temperature-dependent properties. J. Phys. Chem. B 2006, 110, 5223–5229. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Surface engineering of g-C3N4 by stacked Bi-OBr sheets rich in oxygen vacancies for boosting photoctalytic performance. Angew. Chem. Int. Ed. Engl. 2020, 59, 4519–4524. [Google Scholar] [CrossRef] [PubMed]
- Asensio, J.A.; Gomez-Romero, P. Recent developments on proton conducting poly(2,5- ben-zimidazole) ABPBI membranes for high temperature polymer electrolyte membrane fuel cells. Fuel Cell. 2005, 5, 336–343. [Google Scholar] [CrossRef]
- Arlt, T.; Lüke, W.; Kardjilov, N.; Banhart, J.; Lehnert, W. Monitoring the hydrogen distribution in poly(2,5- benzimidazole) based membranes in operating high-temperature polymer electrolyte fuel cells by using H-D contrast neutron imaging. J. Power Sources 2015, 299, 125–129. [Google Scholar] [CrossRef]
- Vogel, H.; Marvel, C.S. Polybenzimidazoles, new thermally stable polymers. J. Polym. Sci. 1961, 50, 511–539. [Google Scholar] [CrossRef]
- Perry, K.A.; Eisman, G.A.; Benicewicz, B.C. Electrochemical hydrogen pumping using a high-temperature polybenzimidazole (PBI) membrane. J. Power Sources 2008, 177, 478–484. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, H.; Scanlon, E.; Ramanathan, L.S.; Choe, E.-W.; Rogers, D.; Apple, T.; Benicewicz, B.C. High-Temperature Polybenzimidazole Fuel Cell Membranes via a Sol-Gel Process. Chem. Mater. 2005, 17, 5328–5333. [Google Scholar] [CrossRef]
- Zhou, D.; Ji, Z.; Jiang, X.; Dunphy, D.R.; Brinker, J.; Kel-ler, A.A. Influence of material properties on TiO2 nanoparticle agglomeration. PLoS ONE 2013, 8, e81239. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, S.; Wu, L. De-Agglomeration and Dispersion Behaviour of TiO2 Nanoparticles in Organic Media Using 3-Methacryloxypropyltrimethoxysilane as a Surface Modifier. J. Dispers. Sci. Technol. 2012, 33, 497–505. [Google Scholar] [CrossRef]
- Lohokare, H.R.; Chaudhari, H.D.; Kharul, U.K. Solvent and pH-stable poly(2,5-benzimidazole) (ABPBI) based UF membranes: Preparation and characterizations. J. Membr. Sci. 2018, 563, 743–751. [Google Scholar] [CrossRef]
- Brunel, R.; Marestin, C.; Martin, V.; Mercier, R. Water-Borne Polyimides via Microwave-Assisted Polymerization. High Perform. Polym. 2010, 22, 82–94. [Google Scholar] [CrossRef]
- Yang, J.; He, R.; Che, Q.; Gao, X.; Shi, L. A Copolymer of Poly[2,20-(m-Phenylene)-5,50- Bibenzimidaz-ole] and Poly(2,5-Benzimidazole) for High-Temperature Proton-Conducting Membranes. Polym. Int. 2010, 59, 1695–1700. [Google Scholar] [CrossRef]
- Olvera-Mancilla, J.; Palacios-Alquisira, J.; Escobar-Barrios, V.A.; Alexandrova, L. Some aspects of polybenzimidazoles’ synthesis in Eaton reagent under different temperatures and microwave irradiation. J. Macromol. Sci. Part A Pure Appl. Chem. 2019, 56, 609–617. [Google Scholar] [CrossRef]
- Chaudhari, H.D.; Illathvalappil, R.; Kurungot, S.; Kharul, U.K. Preparation and investigations of ABPBI membrane for HT-PEMFC by immersion precipitation method. J. Membr. Sci. 2018, 564, 211–217. [Google Scholar] [CrossRef]
- Bhatti, M.A.; Tahira, A.; Chandio, A.d.; Almani, K.F.; Bhatti, A.L.; Waryani, B.; Nafady, A.; Ibupoto, Z.H. Enzymes and phytochemicals from neem extract robustly tuned the photocatalytic activity of ZnO for the degradation of malachite green (MG) in aqueous media. Res. Chem. Intermed. 2021, 47, 1581–1599. [Google Scholar] [CrossRef]
- Song, C.; Jiao, Y.; Tian, Z.; Qin, Q.; Qin, S.; Zhang, J.; Li, J.; Cui, Z. Fabrication of hollow-fiber nanofiltration membrane with negative-positive dual-charged separation layer to remove low concentration CuSO4. Sep. Purif. Technol. 2022, 296, 121352. [Google Scholar] [CrossRef]
- Coppola, R.E.; Lozano, H.E.; Contin, M.; Canneva, A.; Mo-linari, F.N.; Abuinaan, G.C.; D’Accorso, N.B. Polybenzimidazole membrane for efficient copper removal from aqueous solutions. Polym. Int. 2022, 71, 1143–1151. [Google Scholar] [CrossRef]
- Andreeva, R.; Stoyanova, E.; Tsanev, A.; Stoychev, D. Influence of the Pre-Treatment and Post-Treatment Operations on the Surface Chemistry and Corrosion Behavior of Cerium-Based Conversion Coatings on Aluminum. Curr. Adv. Chem. Bio-Chem. 2021, 7, 1–28. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Cappelletti, G.; Ardizzone, S.; Gialanella, S.; Naldoni, A.; Oliva, C.; Pirola, C. N-doped TiO2 from TiCl3 for photodegradation of air pollutants. Catal. Today 2009, 144, 31–36. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; Zhang, M.; Yang, J.; Zhang, Z. Enhanced visible light photocatalytic activity of N-doped TiO2 in relation to single-electron-trapped oxygen vacancy and doped-nitrogen. Appl. Catal. B Environ. 2010, 100, 84–90. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Seristatidou, E.; Deligiannakis, Y.; Konstantinou, I. Photocatalytic activity of N-doped and N-F co-doped TiO2 and reduction of chromium (VI) in aqueous solution: An EPR study. Appl. Catal. B Environ. 2013, 132–133, 460–468. [Google Scholar] [CrossRef]
- Khade, G.V.; Suwarnkar, M.B.; Gavade, N.L.; Garadkar, K.M. Green synthesis of TiO2 and its photocatalytic activity. J. Mater. Sci. Mater. Electron. 2015, 26, 3309–3315. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Ashok, M.; Kashinath, L.; Sanjeeviraja, C.; Rajendran, A. Phytosynthesis and Characterization of TiO2 Nanoparticles using Diospyros ebenum Leaf Extract and their Antibacterial and Photocatalytic Degradation of Crystal Violet. Smart Sci. 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.; An, L.; Zhao, G.; Yan, X. Physicochemical properties of alkaline doped polybenzimidazole membranes for anion exchange membrane fuel cells. J. Membr. Sci. 2015, 493, 340–348. [Google Scholar] [CrossRef]
- Kumbharkar, S.C.; Islam, M.N.; Potrekar, R.A.; Kharul, U.K. Variation in acid moiety of polybenzimidazoles: Investigation of physico-chemical properties towards their applicability as proton exchange and gas separation membrane materials. Polymer 2009, 50, 1403–1413. [Google Scholar] [CrossRef]
- Kumbharkar, S.C.; Kharul, U.K. New N-substituted ABPBI: Synthesis and evaluation of gas permeation properties. J. Membr. Sci. 2010, 360, 418–425. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, S.; Wu, Y.; Yan, F.; Qin, S.; Yang, J.; Li, J.; Cui, Z. Fabrication of polymer@TiO2 NPs hybrid membrane based on covalent bonding and coordination and its mechanism of enhancing photocatalytic performance. J. Alloys Compd. 2022, 910, 164887. [Google Scholar] [CrossRef]
- Lou, L.; Kendall, R.J.; Ramkumar, S. Comparison of hydrophilic PVA/TiO2 and hydrophobic PVDF/TiO2 microfiber webs on the dye pollutant photo-catalyzation. J. Environ. Chem. Eng. 2020, 8, 103914. [Google Scholar] [CrossRef]
- Jumat, N.A.; Wai, P.S.; Ching, J.J.; Basirun, W.J. Synthesis of Polyaniline-TiO2 nanocomposites and their Application in Photocatalytic degradation. Polym. Polym. Compos. 2017, 25, 507–513. [Google Scholar] [CrossRef]
- Sangareswari, M.; Sundaram, M.M. Development of efficiency improved polymer-modified TiO2 for the photocatalytic degradation of an organic dye from wastewater environment. Appl. Water Sci. 2017, 7, 1781–1790. [Google Scholar] [CrossRef]
- Shah, L.A.; Malik, T.; Siddiq, M.; Haleem, A.; Sayed, M.; Naeem, A. TiO2 nanotubes doped poly(vinylidene fluoride) polymer membranes (PVDF/TNT) for efficient photocatalytic degradation of brilliant green dye. J. Environ. Chem. Eng. 2019, 7, 103291. [Google Scholar] [CrossRef]
- Song, L.; Qiu, R.; Mo, Y.; Zhang, D.; Wei, H.; Xiong, Y. Photodegradation of phenol in a polymer-modified TiO2 semiconductor particulate system under the irradiation of visible light. Catal. Commun. 2007, 8, 429–433. [Google Scholar] [CrossRef]
- Xiong, L.; Ouyang, M.; Yan, L.; Li, J.; Qiu, M.; Yu, Y. Visible-light energy storage by Ti3+ in TiO2/Cu2O bilayer film. Chem. Lett. 2009, 38, 1154–1155. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, J.; Chen, F.; Tian, B. An economic method to prepare vacuum activated photocatalysts with high photo-activities and photosensitivities. Chem. Commun. 2011, 47, 4947–4949. [Google Scholar] [CrossRef]
- Chakansin, C.; Yostaworakul, J.; Warin, C.; Kulthong, K.; Boonrungsiman, S. Resazurin rapid screening for antibacterial activities of organic and inorganic nanoparticles: Potential, limitations and precautions. Anal. Biochem. 2022, 637, 114449. [Google Scholar] [CrossRef]
- Akhtar, S.; Ali, I.; Tauseef, S. Synthesis, characterization and antibacterial activity of titanium dioxide (TiO2) nanoparticles. FUUAST J. Biol. 2016, 6, 141–147. [Google Scholar]
- Anandgaonker, P.; Kulkarni, G.; Gaikwad, S.; Rajbhoj, A. Synthesis of TiO2 nanoparticles by electrochemical method and their antibacterial application. Arab. J. Chem. 2019, 12, 1815–1822. [Google Scholar] [CrossRef]
- Rathi, V.; Rejo Jeice, A. Green fabrication of titanium dioxide nanoparticles and their applications in photocatalytic dye degradation and microbial activities. Chem. Phys. Impact 2023, 6, 100197. [Google Scholar] [CrossRef]
- Arora, B.; Murar, M.; Dhumale, V. Antimicrobial potential of TiO2 nanoparticles against MDR Pseudomonas aeruginosa. J. Exp. Nanosci. 2014, 10, 819–827. [Google Scholar] [CrossRef]
- Kassalia, M.-E.; Chorianopoulos, N.; Nychas, G.-J.; Pavlatou, E.A. Investigation of the Photoinduced Antimicrobial Properties of N-Doped TiO2 Nanoparticles under Visible-Light Irradiation on Salmonella Typhimurium Biofilm. Appl. Sci. 2023, 13, 4498. [Google Scholar] [CrossRef]
- Damyanova, T.; Dimitrova, P.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An overview of biofilm-associated infections and the role of phytochemicals and nanomaterials in their control and prevention. Pharmaceutics 2024, 16, 162. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J. Photochem. Photobiol. B 1992, 14, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, L.; Si, Y.; Shu, K. Size-Dependent Cytotoxicity of Silver Nanoparticles to Azotobacter Vinelandii: Growth Inhibition, Cell Injury, Oxidative Stress and Internalization. PLoS ONE 2018, 13, e0209020. [Google Scholar] [CrossRef] [PubMed]
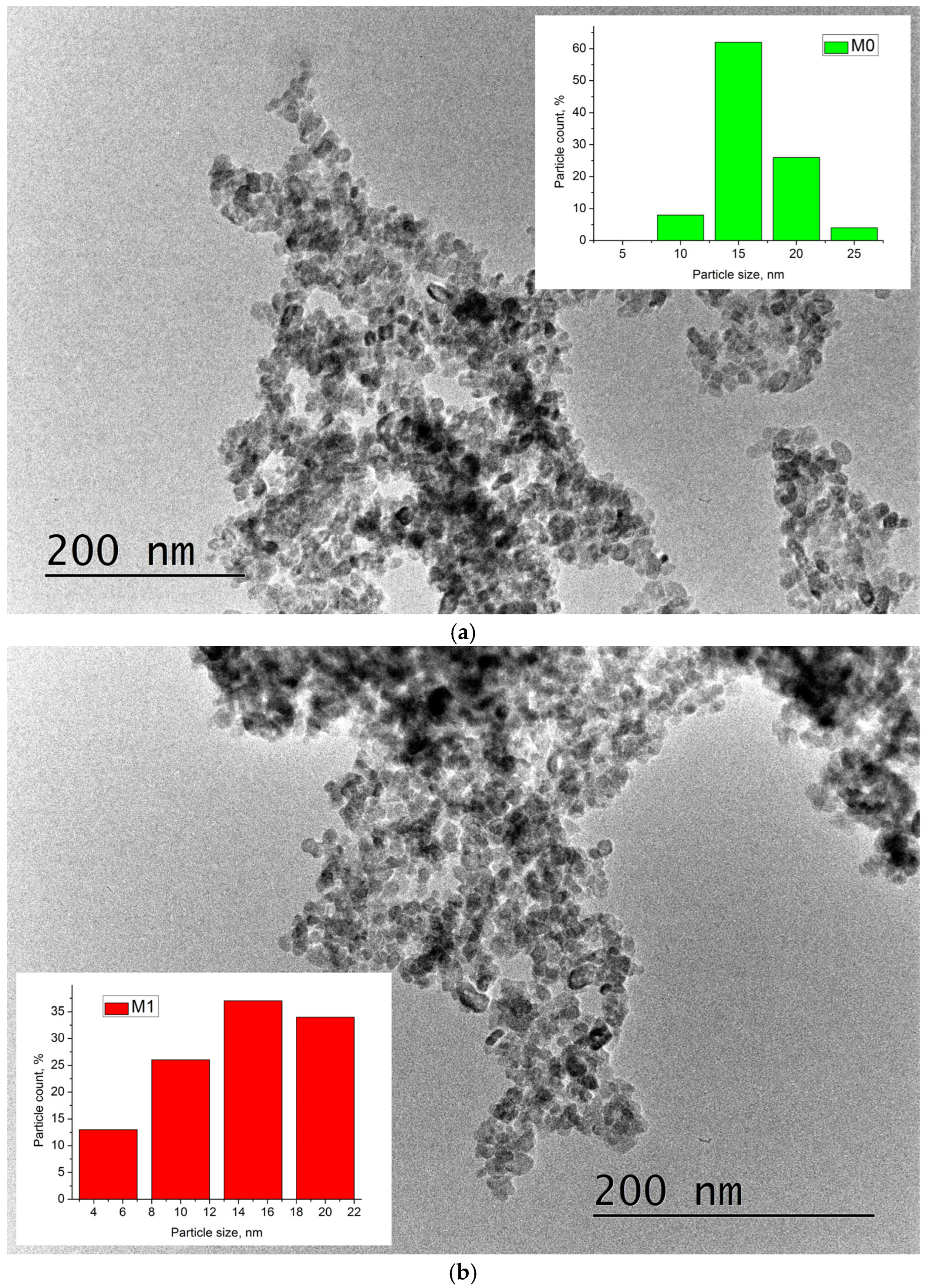
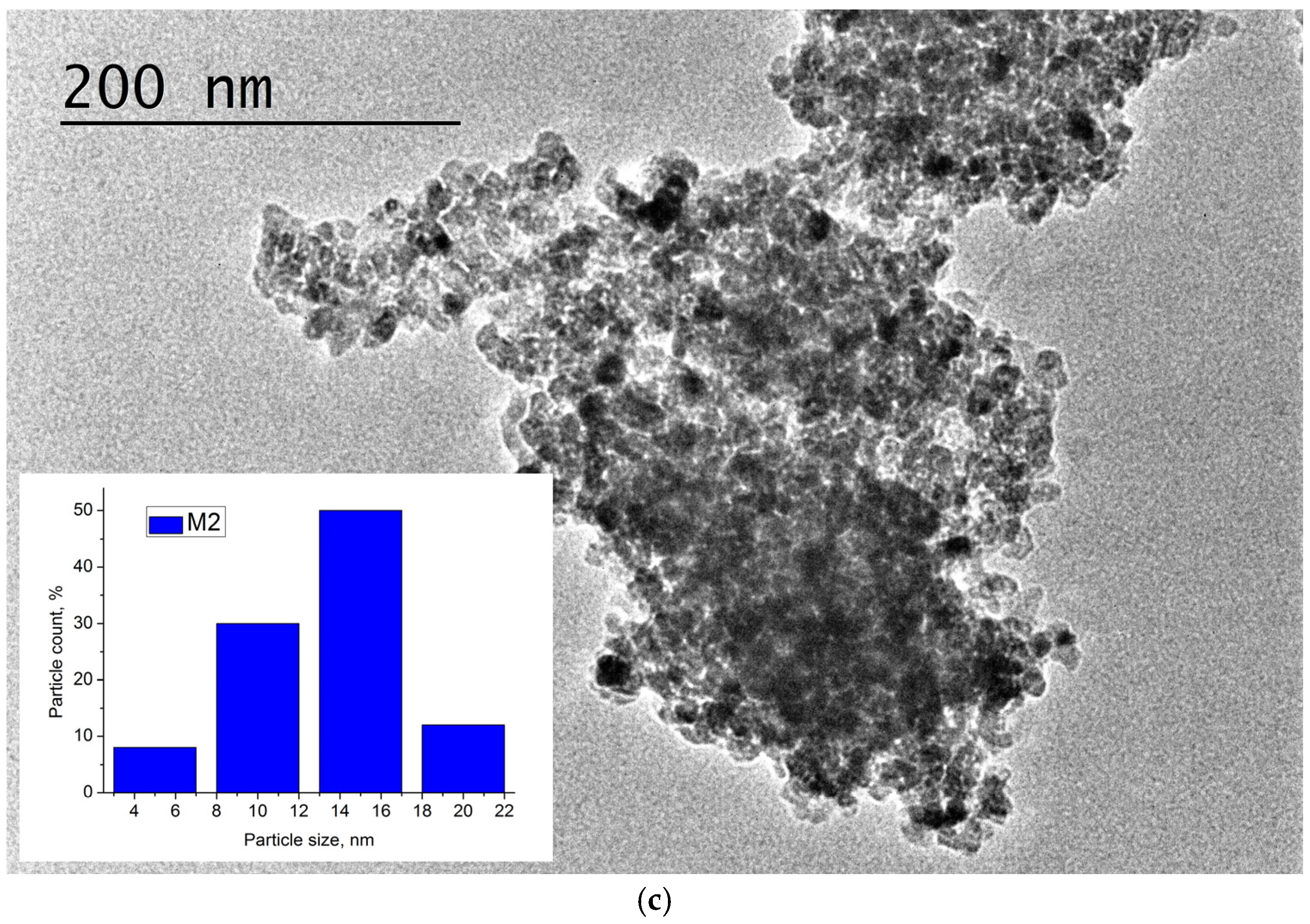
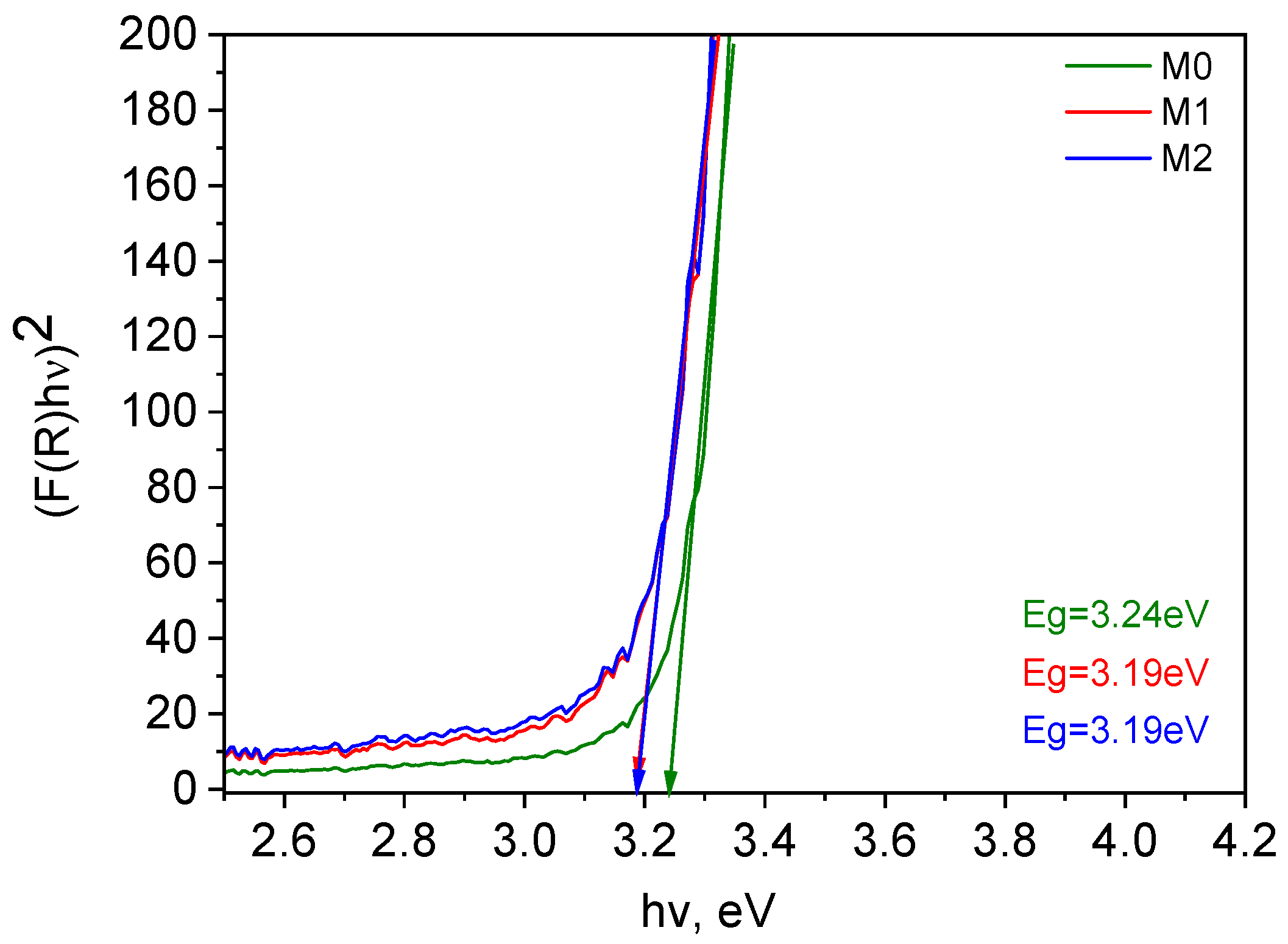






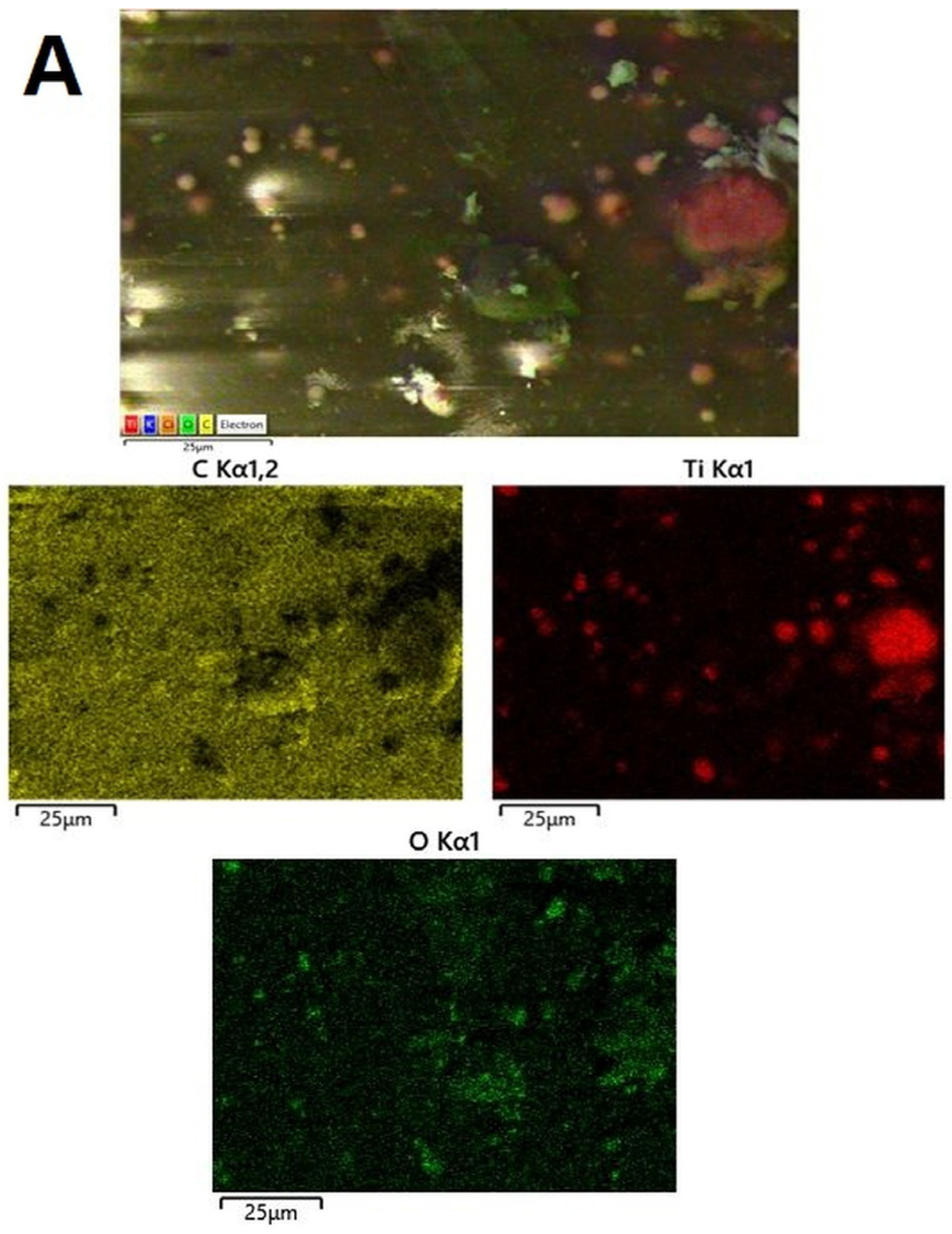
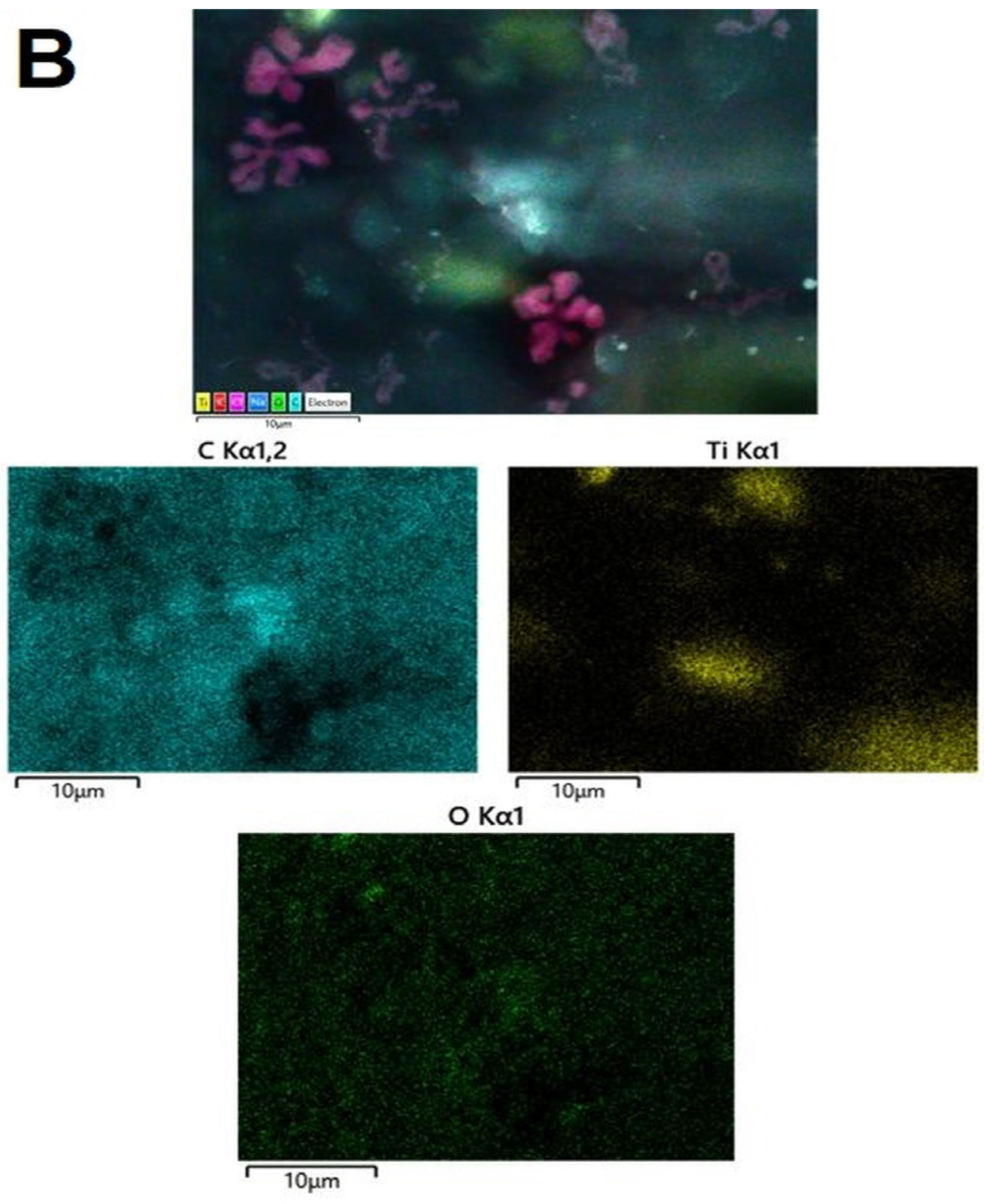
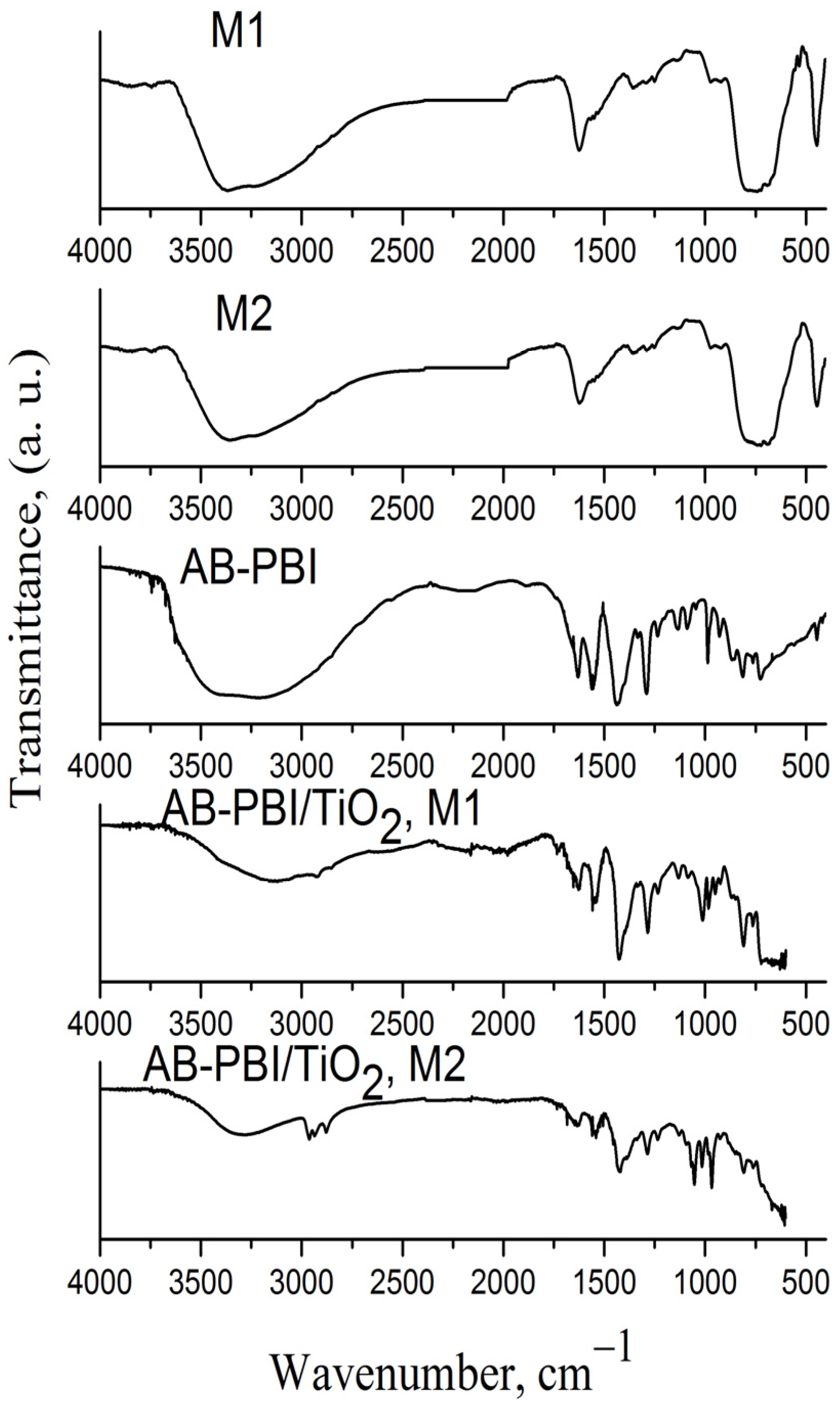


| C, at.% | O, at.% | Ti, at.% | OT/OL | |
|---|---|---|---|---|
| MO | 22.1 | 54.1 | 23.8 | 1.49 |
| M1 | 20.4 | 56.8 | 22.8 | 1.56 |
| M2 | 21.7 | 55.8 | 22.5 | 1.85 |
| Samples | C, at. % | O, at. % | Ti, at. % | N, at. % | P, at. % | Na, at. % |
|---|---|---|---|---|---|---|
| AB-PBI-TiO2, M1 acid-doped form | 57.0 | 26.8 | 0.3 | 9.6 | 5.3 | 1.0 |
| AB-PBI-TiO2, M1 neutralized form | 72.4 | 17.1 | 0.2 | 8.3 | 0.8 | 1.2 |
| Binding Energy, eV | Chemical Bonding | Concentration, % | ||
|---|---|---|---|---|
| AB-PBI-TiO2, M1 Acidic Form | AB-PBI-TiO2, M1 Neutral Form | |||
| 285.0 | C-C | 61.4 | 76.1 | |
| 286.5 | C-O/C=N | 26.0 | 14.6 | |
| 288.3 | C=O | 9.5 | 9.3 | |
| 289.4 | COOH | 3.1 | - | |
| 398.2 | Imine, -N=C | 28.7 | 71.3 | |
| 399.9 | Amine, -NH- | 27.9 | 72.1 | |
| Element | wt. % | Atomic % |
|---|---|---|
| C | 75.28 | 83.37 |
| O | 16.96 | 14.10 |
| Na | 0.86 | 0.50 |
| Cl | 1.30 | 0.49 |
| K | 0.80 | 0.27 |
| Ti | 4.04 | 1.12 |
| Zn | 0.76 | 0.15 |
| Total | 100.00 | 100.00 |
| Materials | Processes | Type of Pollutant Removed | Lamp Source | Removal Efficiency | References |
|---|---|---|---|---|---|
| PVDF@TiO2 | blending, dip coating, and grafting | Methylene blue dye | UV light | 95.4% | [81] |
| PVA /TiO2 | electrospinning | Rhodamine B dye | Vis light | 80% | [82] |
| PANI-TiO2 | template free method | Reactive Black 5 dye | Vis light | 96% | [83] |
| PPy-TiO2 | chemical oxidative polymerization method | Methylene blue dye | Solar light | 93% | [84] |
| PVDF/TiO2 | phase inversion Method | brilliant green dye | UV light | ~45% | [85] |
| TiO2/PFT | surface modification | phenol | Vis light | 74.3% | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penchev, H.; Zaharieva, K.; Dimova, S.; Tsacheva, I.; Eneva, R.; Engibarov, S.; Lazarkevich, I.; Paunova-Krasteva, T.; Shipochka, M.; Mladenova, R.; et al. Green Synthesized Composite AB-Polybenzimidazole/TiO2 Membranes with Photocatalytic and Antibacterial Activity. Crystals 2024, 14, 1081. https://doi.org/10.3390/cryst14121081
Penchev H, Zaharieva K, Dimova S, Tsacheva I, Eneva R, Engibarov S, Lazarkevich I, Paunova-Krasteva T, Shipochka M, Mladenova R, et al. Green Synthesized Composite AB-Polybenzimidazole/TiO2 Membranes with Photocatalytic and Antibacterial Activity. Crystals. 2024; 14(12):1081. https://doi.org/10.3390/cryst14121081
Chicago/Turabian StylePenchev, Hristo, Katerina Zaharieva, Silvia Dimova, Ivelina Tsacheva, Rumyana Eneva, Stephan Engibarov, Irina Lazarkevich, Tsvetelina Paunova-Krasteva, Maria Shipochka, Ralitsa Mladenova, and et al. 2024. "Green Synthesized Composite AB-Polybenzimidazole/TiO2 Membranes with Photocatalytic and Antibacterial Activity" Crystals 14, no. 12: 1081. https://doi.org/10.3390/cryst14121081
APA StylePenchev, H., Zaharieva, K., Dimova, S., Tsacheva, I., Eneva, R., Engibarov, S., Lazarkevich, I., Paunova-Krasteva, T., Shipochka, M., Mladenova, R., Dimitrov, O., Stoyanova, D., & Stambolova, I. (2024). Green Synthesized Composite AB-Polybenzimidazole/TiO2 Membranes with Photocatalytic and Antibacterial Activity. Crystals, 14(12), 1081. https://doi.org/10.3390/cryst14121081










