Computational Fluid Dynamic Analysis of a High-Pressure Spatial Chemical Vapor Deposition (HPS-CVD) Reactor for Flow Stability
Abstract
:1. Introduction
2. Scope of Work
3. Governing Equations
4. Numerical Method
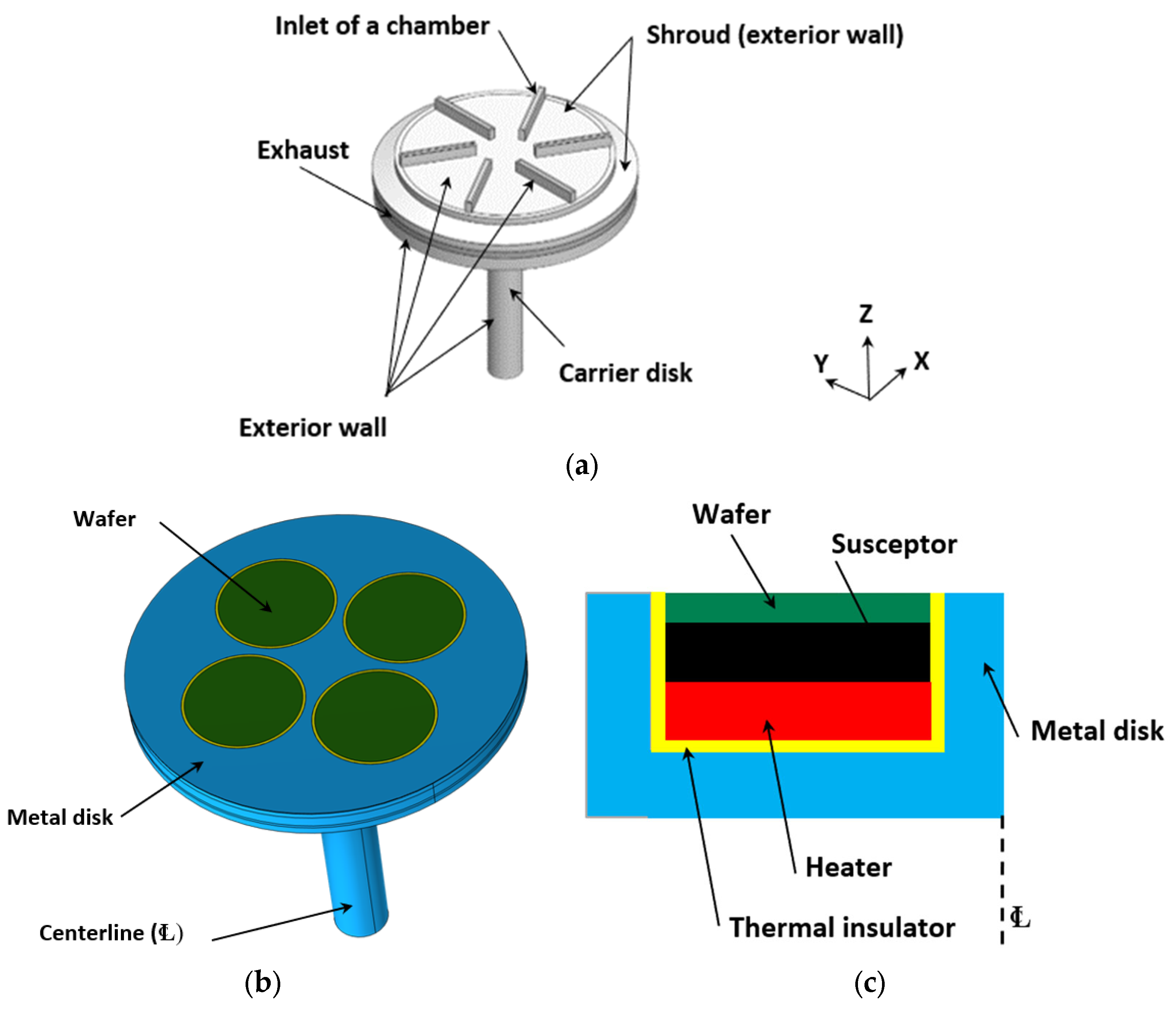
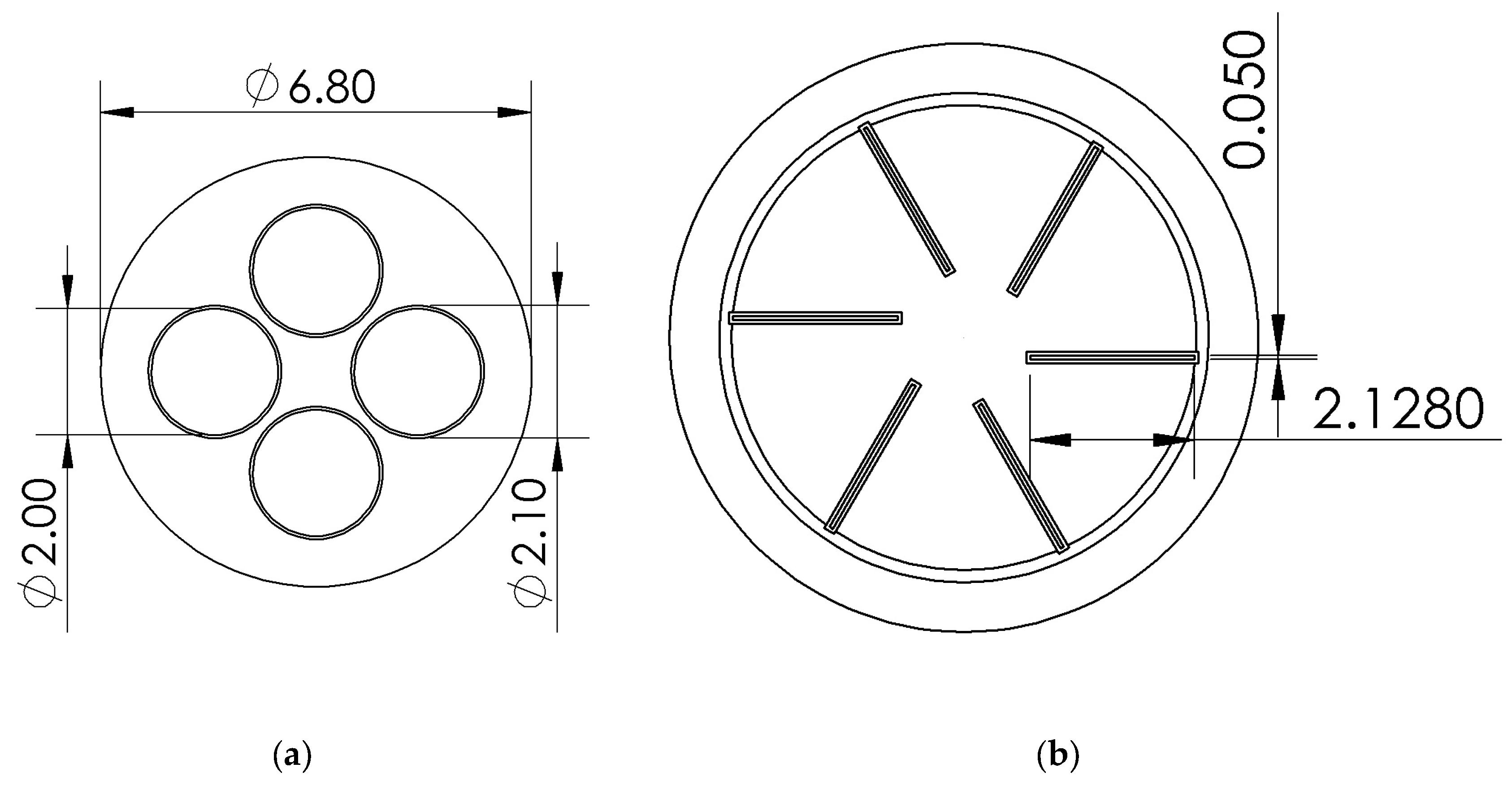
4.1. Geometry and Corresponding Numerical Domain
4.2. Physics
4.3. Boundary and Initial Conditions
4.4. Numerical Set Up
4.5. Mesh Convergence Study
5. Results and Discussion
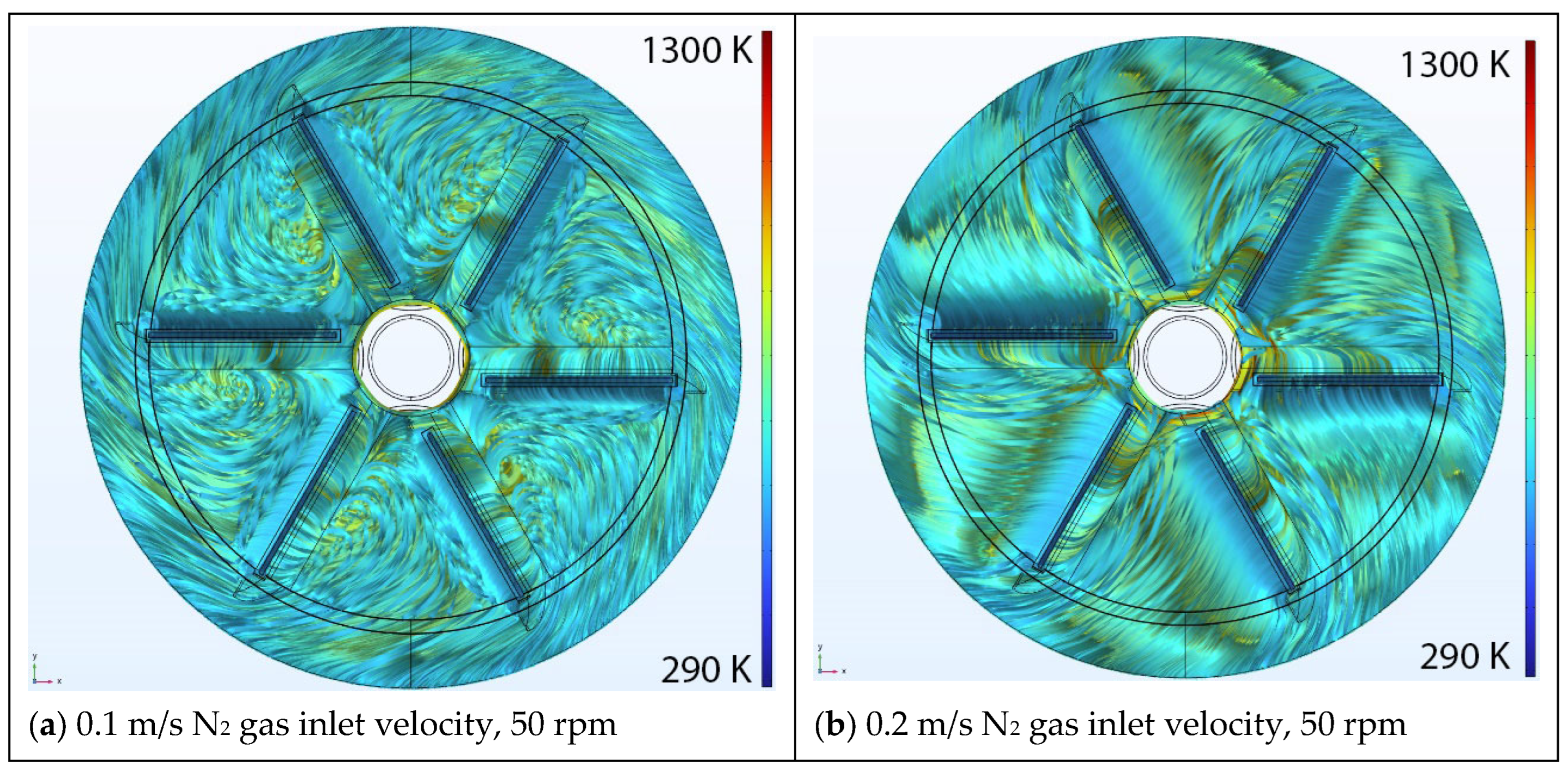
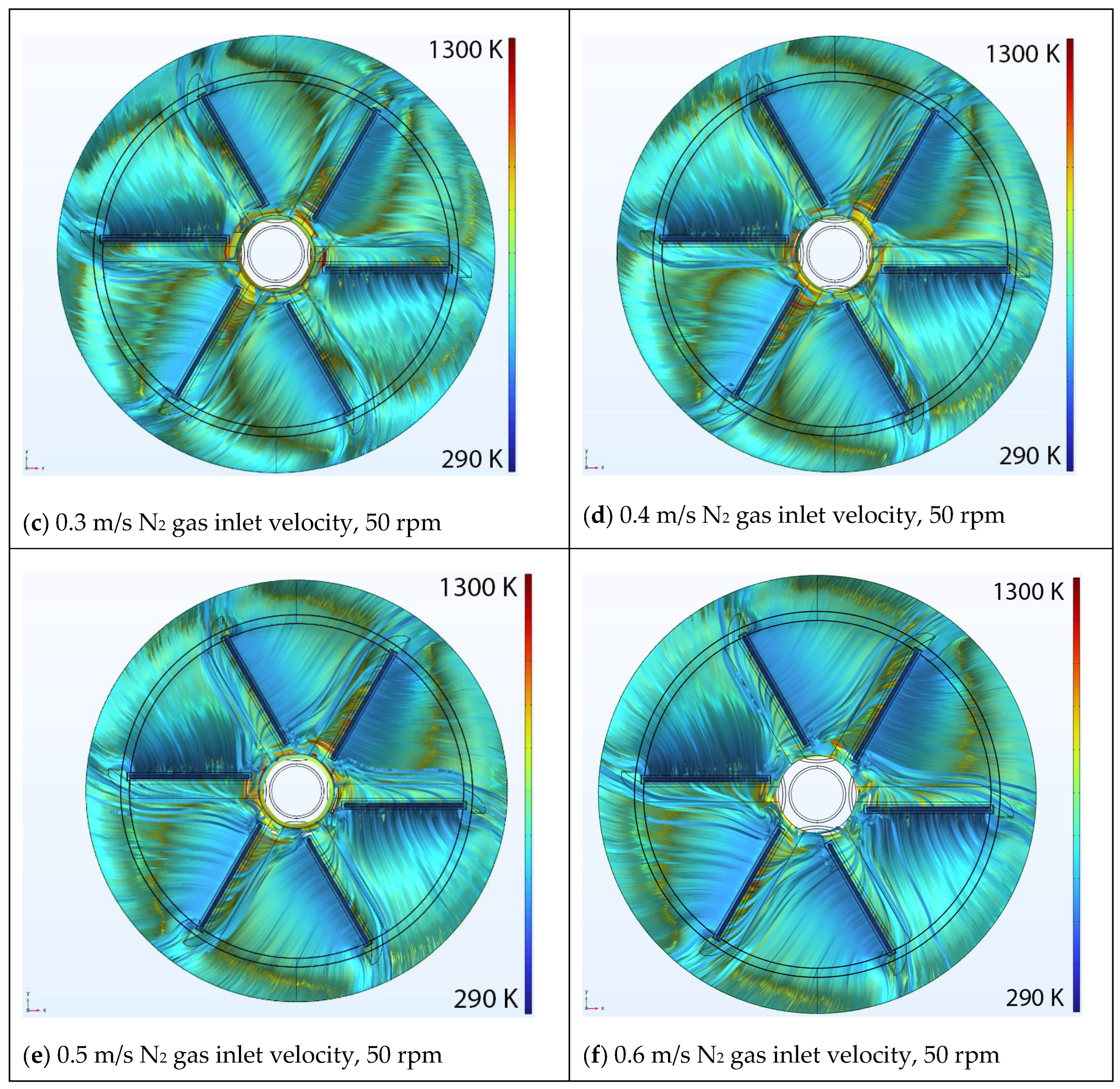
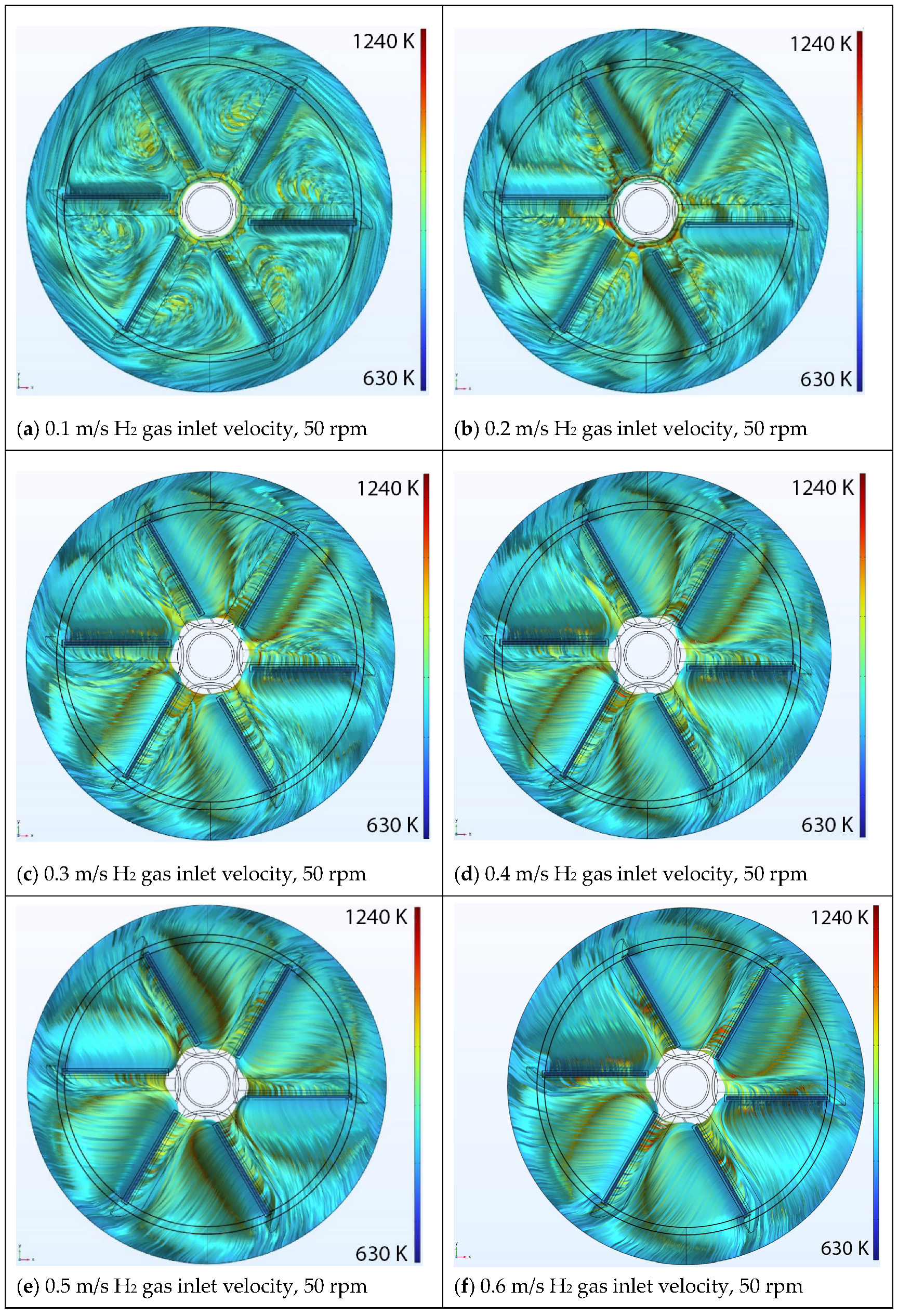
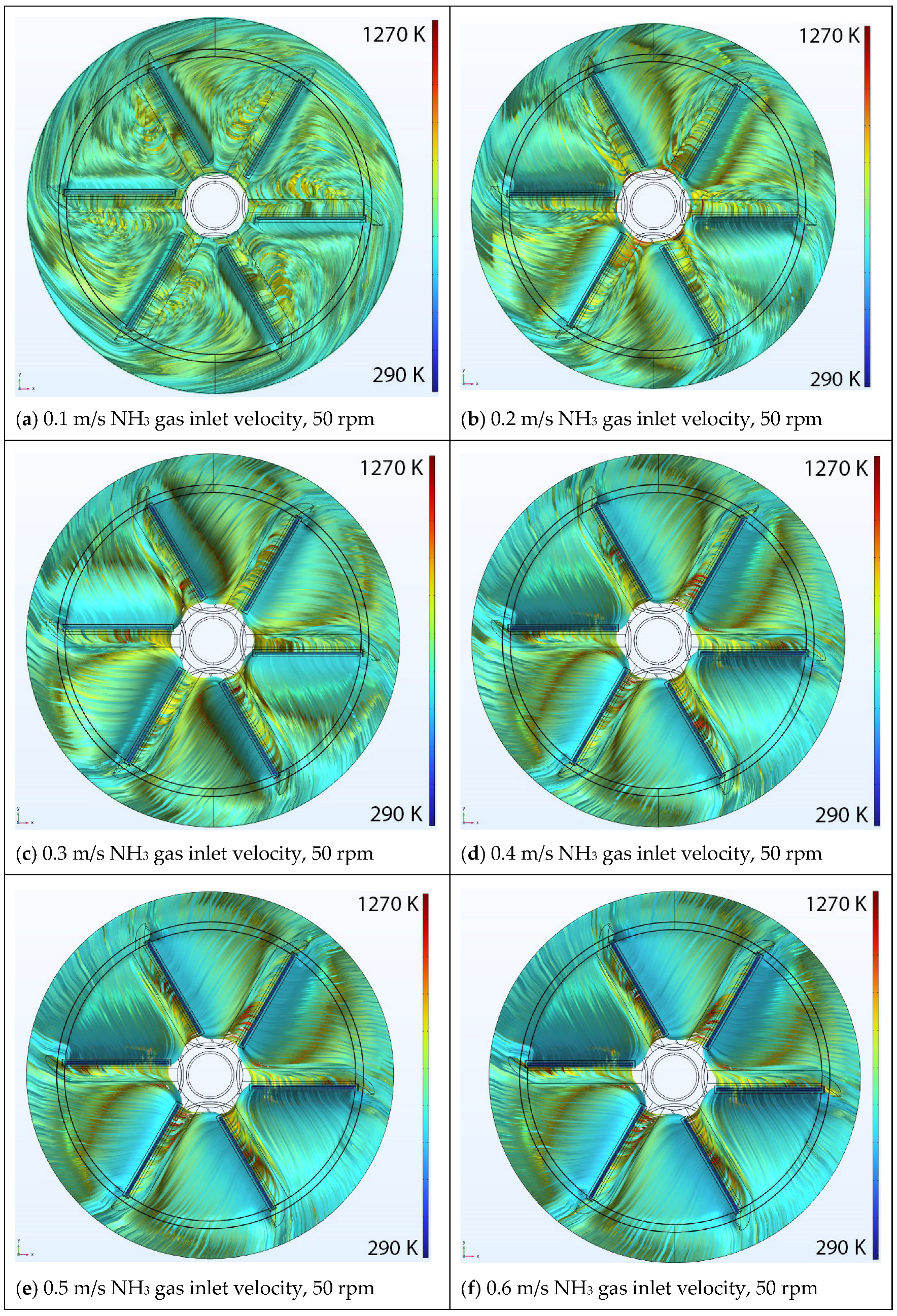
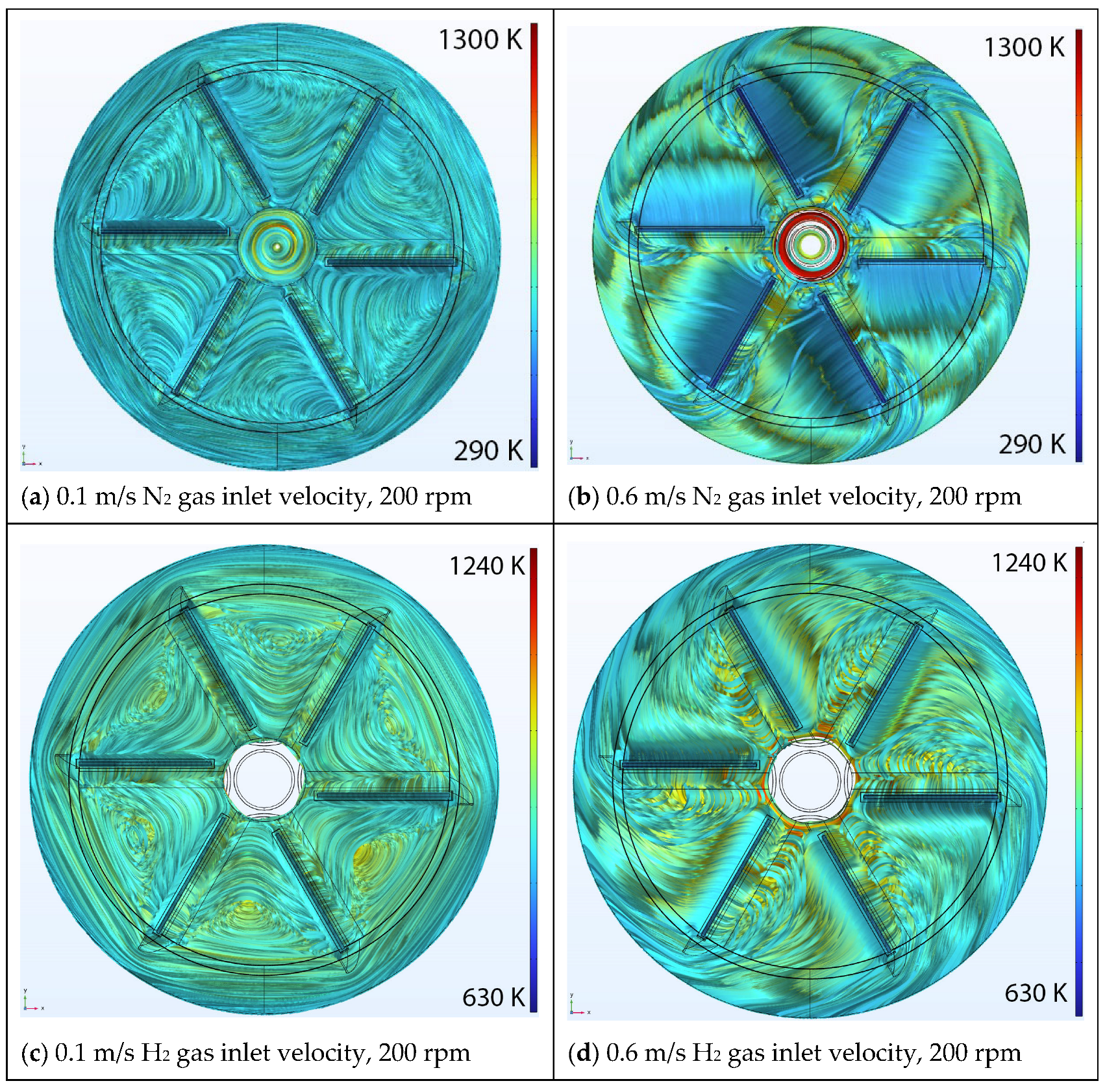
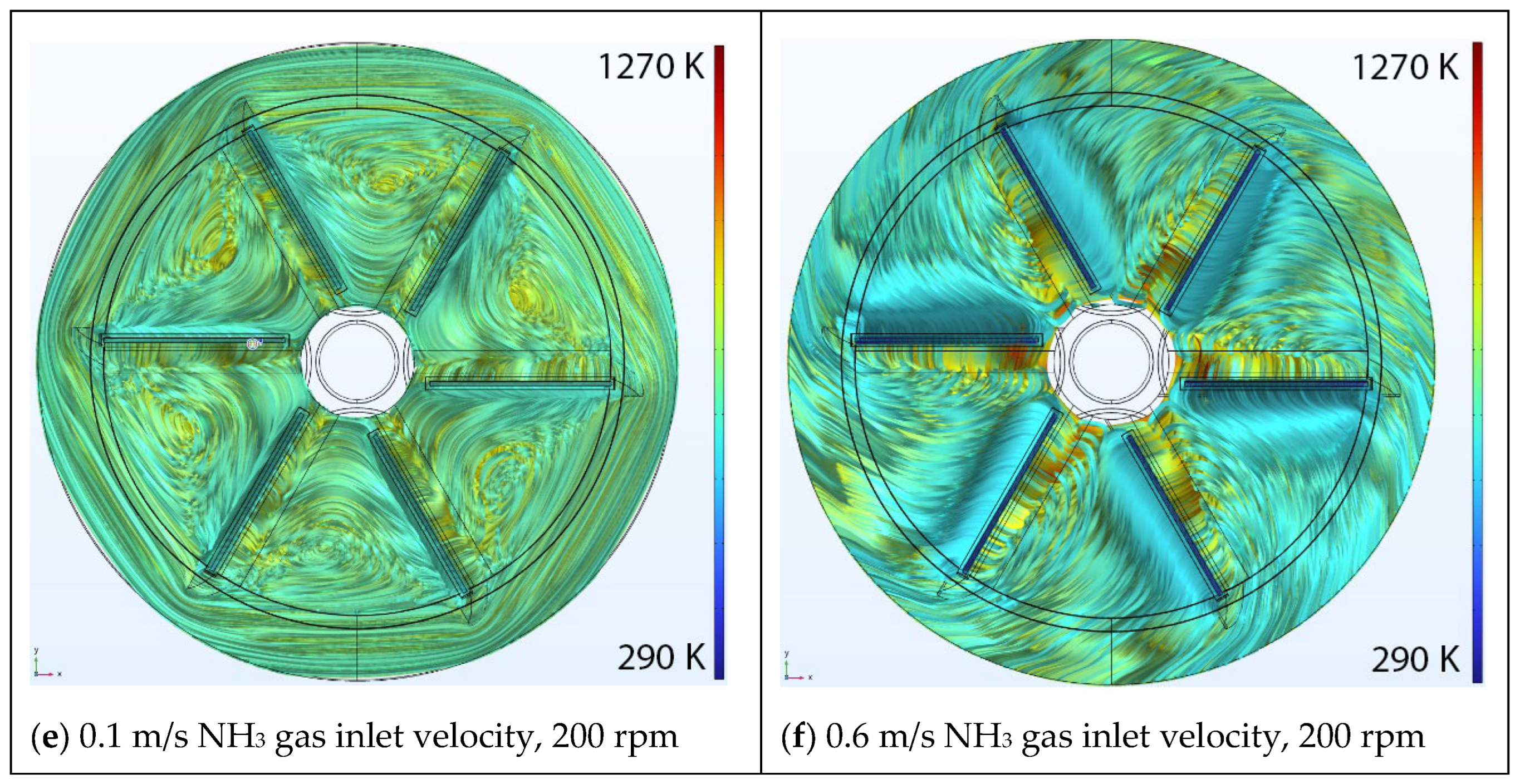
5.1. Effect of Inlet Velocity
Velocity Streamlines
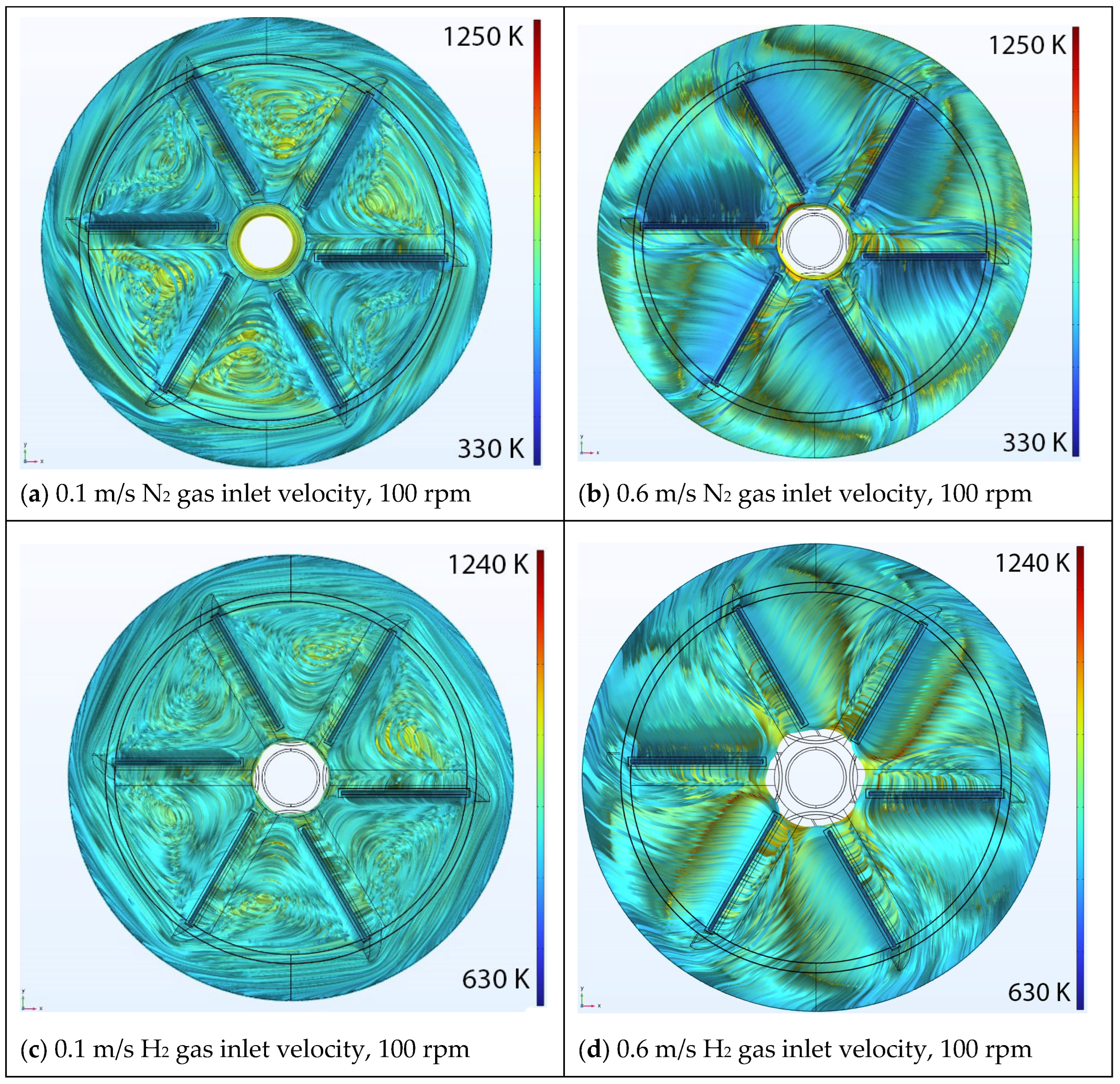
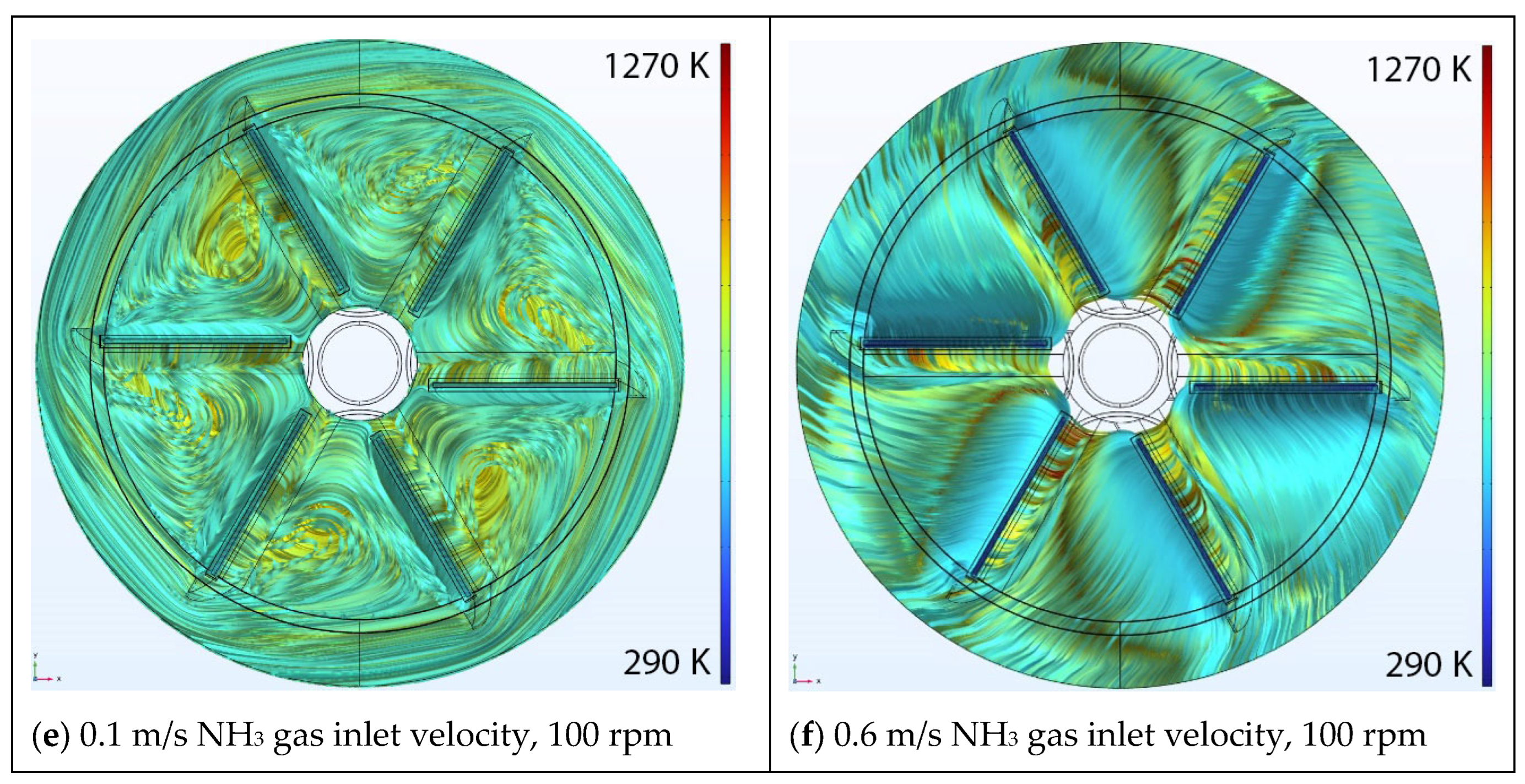
5.2. Effect of Rotational Speed
5.3. Effect of Inlet Area
6. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scholz, F. MOVPE of Group-III Heterostructures for Optoelectronic Applications. Cryst. Res. Technol. 2020, 55, 1900027. [Google Scholar] [CrossRef]
- Wang, C.A. Perspectives on the Development of Metalorganic Vapor Phase Epitaxy for III-V Optoelectronic Devices. IEEE J. Quantum Electron. 2022, 58, 5000111. [Google Scholar] [CrossRef]
- Wang, C.A. Early history of MOVPE reactor development. J. Cryst. Growth 2019, 506, 190–200. [Google Scholar] [CrossRef]
- Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Prospects for LED lighting. Nat. Photonics 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Amano, H.; Collazo, R.; De Santi, C.; Einfeldt, S.; Funato, M.; Glaab, J.; Hagedorn, S.; Hirano, A.; Hirayama, H.; Ishii, R.; et al. The 2020 UV emitter roadmap. J. Phys. D Appl. Phys. 2020, 53, 503001. [Google Scholar] [CrossRef]
- Flack, T.J.; Pushpakaran, B.N.; Bayne, S.B. GaN Technology for Power Electronic Applications: A Review. J. Electron. Mater. 2016, 45, 2673–2682. [Google Scholar] [CrossRef]
- Nakamura, S. Nobel Lecture: Background story of the invention of efficient blue InGaN light emitting diodes. Rev. Mod. Phys. 2015, 87, 1139–1151. [Google Scholar] [CrossRef]
- Nakamura, S.; Harada, Y.; Seno, M. Novel metalorganic chemical vapor deposition system for GaN growth. Appl. Phys. Lett. 1991, 58, 2021–2023. [Google Scholar] [CrossRef]
- MacChesney, J.B.; Bridenbaugh, P.M.; O’Connor, P.B. Thermal stability of indium nitride at elevated temperatures and nitrogen pressures. Mater. Res. Bull. 1970, 5, 783–791. [Google Scholar] [CrossRef]
- Lymperakis, L.; Schulz, T.; Freysoldt, C.; Anikeeva, M.; Chen, Z.; Zheng, X.; Shen, B.; Chèze, C.; Siekacz, M.; Wang, X.Q.; et al. Elastically frustrated rehybridization: Origin of chemical order and compositional limits in InGaN quantum wells. Phys. Rev. Mater. 2018, 2, 11601. [Google Scholar] [CrossRef]
- Ivanov, S.V.; Shubina, T.V.; Komissarova, T.A.; Jmerik, V.N. Metastable nature of InN and In-rich InGaN alloys. J. Cryst. Growth 2014, 403, 83–89. [Google Scholar] [CrossRef]
- Borovac, D.; Sun, W.; Peart, M.R.; Song, R.; Wierer, J.J.; Tansu, N. Low background doping in AlInN grown on GaN via metalorganic vapor phase epitaxy. J. Cryst. Growth 2020, 548, 125847. [Google Scholar] [CrossRef]
- Miyoshi, M.; Yamanaka, M.; Egawa, T.; Takeuchi, T. Microstructure variation in thick AlInN films grown on c-plane GaN on sapphire by metalorganic chemical vapor deposition. J. Cryst. Growth 2019, 506, 40–44. [Google Scholar] [CrossRef]
- Sangiovanni, D.G.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Ab initio molecular dynamics of atomic-scale surface reactions: Insights into metal organic chemical vapor deposition of AlN on graphene. Phys. Chem. Chem. Phys. 2018, 20, 17751–17761. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, D.G.; Faccio, R.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Discovering atomistic pathways for supply of metal atoms from methyl-based precursors to graphene surface. Phys. Chem. Chem. Phys. 2022, 25, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.R.Q.; Gueorguiev, G.K.; De Brito Mota, F.; De Castilho, C.M.C.; Stafström, S.; Kakanakova-Georgieva, A. Reactivity of adducts relevant to the deposition of hexagonal BN from first-principles calculations. Chem. Phys. Lett. 2013, 583, 119–124. [Google Scholar] [CrossRef]
- Stringfellow, G.B. Fundamental aspects of organometallic vapor phase epitaxy. Mater. Sci. Eng. 2001, 87, 97–116. [Google Scholar] [CrossRef]
- Kuzmík, J.; Adikimenakis, A.; Ťapajna, M.; Gregušová, D.; Haščík, Š.; Dobročka, E.; Tsagaraki, K.; Stoklas, R.; Georgakilas, A. InN: Breaking the limits of solid-state electronics. AIP Adv. 2021, 11, 125325. [Google Scholar] [CrossRef]
- Dietz, N. Real-time optical characterization of thin film growth. Mater. Sci. Eng. B 2001, 87, 1–22. [Google Scholar] [CrossRef]
- Dietz, N.; Alevli, M.; Kang, H.; Strassburg, M.; Woods, V.; Ferguson, I.T.; Moore, C.E.; Cardelino, B.H. The growth of InN and related alloys by high-pressure CVD. In Operational Characteristics and Crystal Growth of Nonlinear Optical Materials II; SPIE: Bellingham, WA, USA, 2005; p. 5912. [Google Scholar]
- Dietz, N. High Pressure Chemical Vapor Deposition Apparatuses, Methods, and Compositions Produced Therewith; EP2464760A4; ACM Press: New York, NY, USA, 2013. [Google Scholar]
- Alevli, M.; Durkaya, G.; Weerasekara, A.; Perera, A.G.U.; Dietz, N.; Fenwick, W.; Woods, V.; Ferguson, I. Characterization of InN layers grown by high-pressure chemical vapor deposition. Appl. Phys. Lett. 2006, 89, 1023–1026. [Google Scholar] [CrossRef]
- Senevirathna, M.K.I.; Gamage, S.; Atalay, R.; Acharya, A.R.; Perera, A.G.U.; Dietz, N.; Buegler, M.; Hoffmann, A.; Su, L.; Meltonc, A.; et al. Effect of reactor pressure on the electrical and structural properties of InN epilayers grown by high-pressure chemical vapor deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 2012, 30, 31511. [Google Scholar] [CrossRef]
- Yousefian, P.; Pimputkar, S. Computational fluid dynamics modeling of a new high-pressure chemical vapor deposition reactor design. J. Cryst. Growth 2021, 566, 126155. [Google Scholar] [CrossRef]
- Papavasileiou, P.; Koronaki, E.D.; Pozzetti, G.; Kathrein, M.; Czettl, C.; Boudouvis, A.G.; Mountziaris, T.J.; Bordas, S.P.A. An efficient chemistry-enhanced CFD model for the investigation of the rate-limiting mechanisms in industrial Chemical Vapor Deposition reactors. Chem. Eng. Res. Design 2022, 186, 314–325. [Google Scholar] [CrossRef]
- Theodoropoulos, C.; Mountziaris, T.J.; Moffat, H.K.; Han, J. Design of gas inlets for the growth of gallium nitride by metalorganic vapor phase epitaxy. J. Cryst. Growth 2000, 217, 65–81. [Google Scholar] [CrossRef]
- Barua, H.; Povitsky, A. Numerical model of carbon chemical vapor deposition at internal surfaces. Vacuum 2020, 175, 109234. [Google Scholar] [CrossRef]
- Psarellis, G.M.; Aviziotis, I.G.; Duguet, T.; Vahlas, C.; Koronaki, E.D.; Boudouvis, A.G. Investigation of reaction mechanisms in the chemical vapor deposition of al from DMEAA. Chem. Eng. Sci. 2018, 177, 464–470. [Google Scholar] [CrossRef]
- Zou, S.; Xiao, J.; Wu, V.; Chen, X.D. Analyzing industrial CVD reactors using a porous media approach. Chem. Eng. J. 2021, 415, 129038. [Google Scholar] [CrossRef]
- Cheimarios, N.; Kavousanakis, M.; Kokkoris, G.; Boudouvis, A.G. Beware of symmetry breaking and periodic flow regimes in axisymmetric CVD reactor setups. Comput. Chem. Eng. 2019, 124, 124–132. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, R.; Zhou, X.; Wang, H.; Hu, Y.; Nie, D.; Bao, D. Numerical Modelling on the Effect of Temperature on MOCVD Growth of ZnO Using Diethylzinc and Tertiarybutanol. Coatings 2022, 12, 1991. [Google Scholar] [CrossRef]
- Huang, Z.; Qie, S.; Quan, X.; Guo, K.; Liu, C. Numerical simulation of multiple polysilicon CVD reactors connected in series using CFD method. Can. J. Chem. Eng. 2015, 93, 1721–1729. [Google Scholar] [CrossRef]
- Ni, H.; Lu, S.; Chen, C. Modeling and simulation of silicon epitaxial growth in Siemens CVD reactor. J. Cryst. Growth 2014, 404, 89–99. [Google Scholar] [CrossRef]
- Gakis, G.P.; Koronaki, E.D.; Boudouvis, A.G. Numerical investigation of multiple stationary and time-periodic flow regimes in vertical rotating disc CVD reactors. J. Cryst. Growth 2015, 432, 152–159. [Google Scholar] [CrossRef]
- Raj, E.; Lisik, Z.; Niedzielski, P.; Ruta, L.; Turczynski, M.; Wang, X.; Waag, A. Modelling of MOCVD reactor: New 3D approach. J. Phys. Conf. Ser. 2014, 494, 12019. [Google Scholar] [CrossRef]
- Lin, C.H.; Cheng, W.T.; Lee, J.H. Effect of embedding a porous medium on the deposition rate in a vertical rotating MOCVD reactor based on CFD modeling. Int. Commun. Heat. Mass. Transfer. 2009, 36, 680–685. [Google Scholar] [CrossRef]
- Sytniewski, Ł.J.; Lapkin, A.A.; Stepanov, S.; Wang, W.N. CFD optimisation of up-flow vertical HVPE reactor for GaN growth. J. Cryst. Growth 2008, 310, 3358–3365. [Google Scholar] [CrossRef]
- Tabatabaei, S. Evaluation of Fluid Dynamic Effect on Thin Film Growth in a Horizontal Type Meso-Scale Chemical Vapor Deposition Reactor Using Computational Fluid Dynamics; University of Alabama: Tuscaloosa, AL, USA, 2013. [Google Scholar]
- Kempisty, P.; Łucznik, B.; Pastuszka, B.; Grzegory, I.; Boćkowski, M.; Krukowski, S.; Porowski, S. CFD and reaction computational analysis of the growth of GaN by HVPE method. J. Cryst. Growth 2006, 296, 31–42. [Google Scholar] [CrossRef]
- Bao, Q.; Zhu, T.; Zhou, N.; Guo, S.; Luo, J.; Zhao, C. Effect of hydrogen carrier gas on AlN and AlGaN growth in AMEC Prismo D-Blue® MOCVD platform. J. Cryst. Growth 2015, 419, 52–56. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Luo, T.; Pei, Y. Analysis and optimization of MOCVD flow ratio based on machine learning and PSO algorithm. J. Cryst. Growth 2022, 590. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Zhang, J.; Li, J.; Jiang, H.; Fu, X.; Han, Y.; Xia, Y.; Huang, Y.; Yin, J.; et al. A susceptor with partial-torus groove in vertical MOCVD reactor by induction heating. Int. J. Heat Mass Transf. 2014, 75, 410–413. [Google Scholar] [CrossRef]
- Shinde, V.M.; Deivendran, B.; Kumar, H.; Eswara Prasad, N. Investigation of transport processes in a commercial hot wall CVD reactor with multi-substrates for high-quality pyrocarbon deposition. Surf. Coat. Technol. 2021, 425. [Google Scholar] [CrossRef]
- Gkinis, P.A.; Aviziotis, I.G.; Koronaki, E.D.; Gakis, G.P.; Boudouvis, A.G. The effects of flow multiplicity on GaN deposition in a rotating disk CVD reactor. J. Cryst. Growth 2017, 458, 140–148. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Chen, C.T. Mathematical modeling and optimal design of an MOCVD reactor for GaAs film growth. J. Taiwan Inst. Chem. Eng. 2014, 45, 254–267. [Google Scholar] [CrossRef]
- Comsol. The CFD Module User’s Guide. 2022. Available online: www.comsol.com (accessed on 1 December 2023).
- Comsol. The Heat Transfer Module User’s Guide. 2022. Available online: www.comsol.com (accessed on 1 December 2023).
- Monogenidou, S.A.; Assael, M.J.; Huber, M.L. Reference Correlation for the Viscosity of Ammonia from the Triple Point to 725 K and up to 50 MPa. J. Phys. Chem. Ref. Data 2018, 47, 23102. [Google Scholar] [CrossRef] [PubMed]


| Solid Domain | Material | Thermal Conductivity (W/m·K) | Density (kg/m3) | Heat Capacity at Constant Pressure (J/kg·K) | Emissivity |
|---|---|---|---|---|---|
| Thermal insulator | Boron nitride | 19 | 1907 | 770 | 0.9 |
| Susceptor | Graphite | 350 | 2265 | 707 | 0.85 |
| Heater | Graphite | 350 | 2265 | 707 | - |
| Disk | Stainless Steel | 16.2 | 8000 | 500 | 0.5 |
| Wafer | Sapphire | 46.06 | 3908 | 1171 | - |
| Side walls | Molybdenum | 138 | 10,200 | 250 | 0.1 |
| Parameter | Value (s) |
|---|---|
| Rotational speed (rpm) | 50, 100, 200 |
| Operating pressure (atm) | 1 |
| Heat transfer coefficient (W/m2·K) | 50 |
| Inlet velocity/chamber (m/s) | 0.1–0.6 |
| Inlet gas temperature (K) | 298.15 |
| Input power/wafer (watt) | Varied |
| Surrounding temperature (K) | 298.15 |
| Area per inlet (in2) | 0.1064 |
| Molybdenum emissivity | 0.1 |
| Metric | Min Value | Average |
|---|---|---|
| Skewness | 0.003 | 0.67 |
| Growth rate | 0.0 | 0.62 |
| Maximum angle | 0.05 | 0.77 |
| Volume versus length | 2.2 × 10−4 | 0.51 |
| Gas: | N2 | H2 | NH3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inlet Velocity (m/s) | Total Flow Rate (slm) | Disk Rotational Speed (rpm) | ||||||||
| 50 | 100 | 200 | 50 | 100 | 200 | 50 | 100 | 200 | ||
| 0.1 | 2.5 | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ |
| 0.2 | 5 | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ |
| 0.3 | 7.5 | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ |
| 0.4 | 10 | ✓ | ❌ | ❌ | ❌ | ❌ | ❌ | ✓ | ❌ | ❌ |
| 0.5 | 12 | ✓ | ✓ | ❌ | ✓ | ❌ | ❌ | ✓ | ❌ | ❌ |
| 0.6 | 15 | ✓ | ✓ | ❌ | ✓ | ❌ | ❌ | ✓ | ✓ | ❌ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enayati, H.; Pimputkar, S. Computational Fluid Dynamic Analysis of a High-Pressure Spatial Chemical Vapor Deposition (HPS-CVD) Reactor for Flow Stability. Crystals 2024, 14, 105. https://doi.org/10.3390/cryst14020105
Enayati H, Pimputkar S. Computational Fluid Dynamic Analysis of a High-Pressure Spatial Chemical Vapor Deposition (HPS-CVD) Reactor for Flow Stability. Crystals. 2024; 14(2):105. https://doi.org/10.3390/cryst14020105
Chicago/Turabian StyleEnayati, Hooman, and Siddha Pimputkar. 2024. "Computational Fluid Dynamic Analysis of a High-Pressure Spatial Chemical Vapor Deposition (HPS-CVD) Reactor for Flow Stability" Crystals 14, no. 2: 105. https://doi.org/10.3390/cryst14020105
APA StyleEnayati, H., & Pimputkar, S. (2024). Computational Fluid Dynamic Analysis of a High-Pressure Spatial Chemical Vapor Deposition (HPS-CVD) Reactor for Flow Stability. Crystals, 14(2), 105. https://doi.org/10.3390/cryst14020105






