Insights into the Spray Synthesis of UiO-66 and UiO-66-NH2 Metal–Organic Frameworks: Effect of Zirconium Precursors and Process Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Precursor Solution of UiO-66 Using ZrCl4 as a Zr Source
2.3. Precursor Solution of UiO-66 and UiO-66-NH2 Using Zr(OnPr)4 as a Zr Source
2.4. Spray Synthesis of UiO-66 and UiO-66-NH2
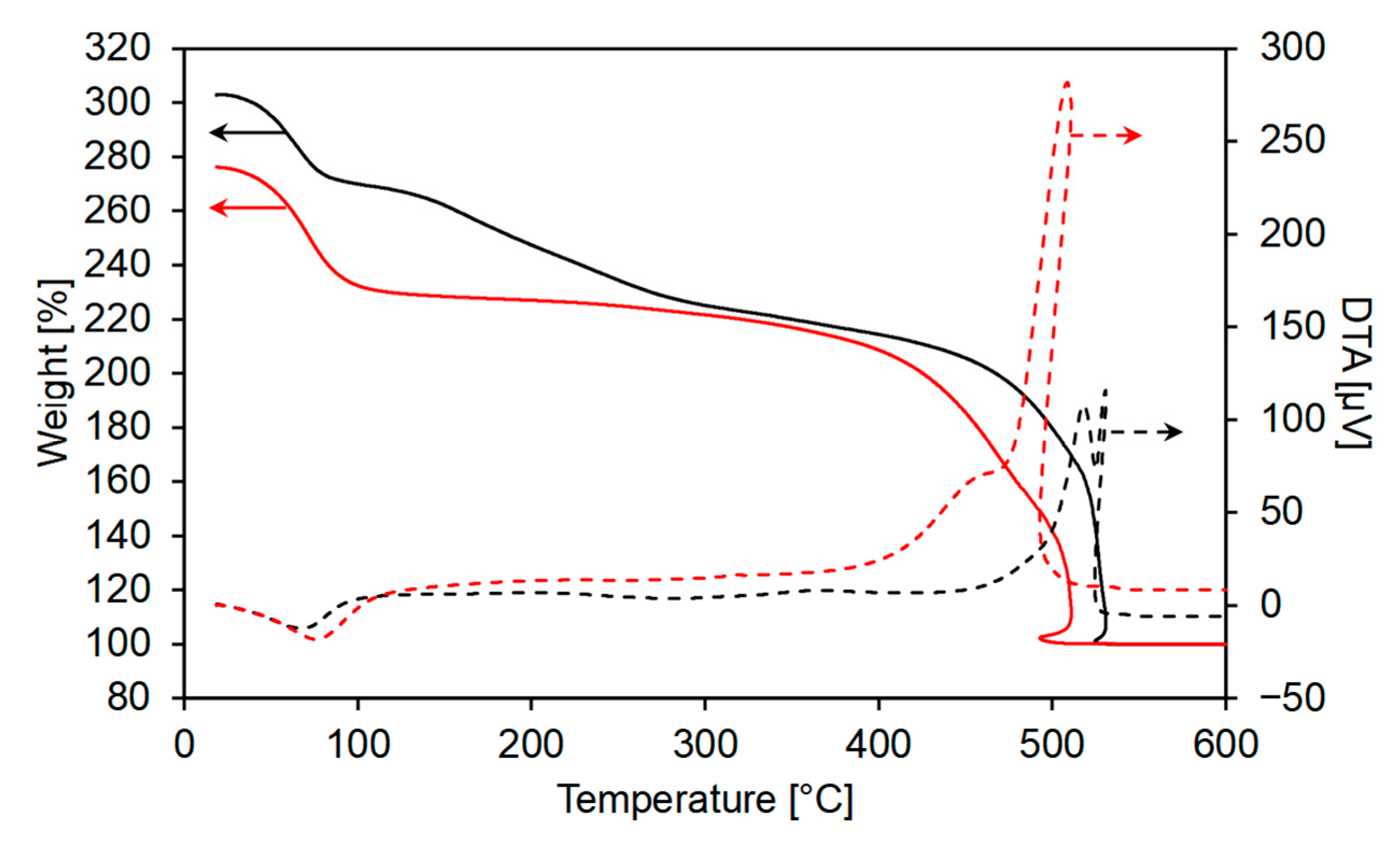
2.5. Characterizations
3. Results
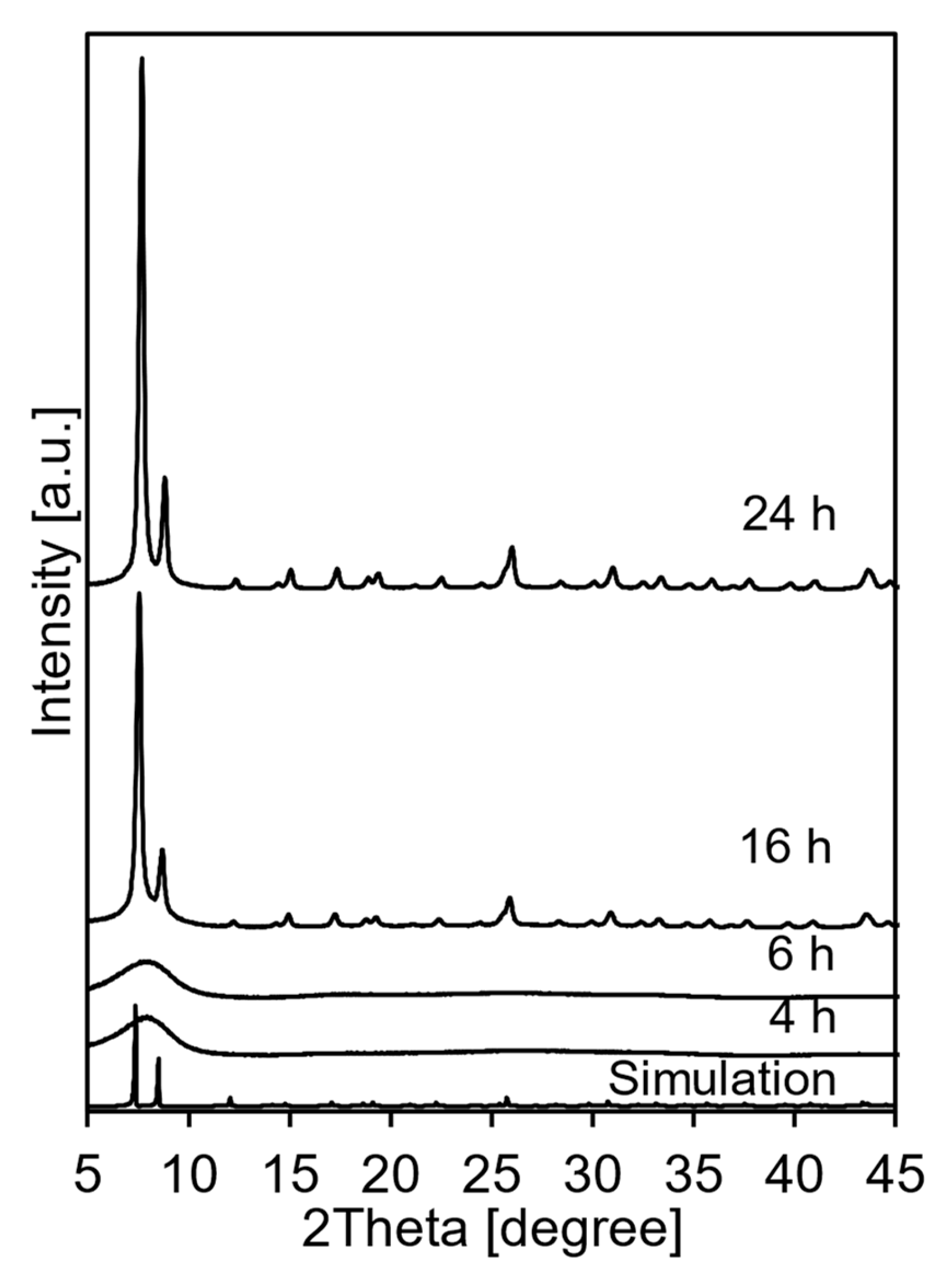
3.1. Spray Synthesis of UiO-66 Using ZrCl4 as a Zr Source
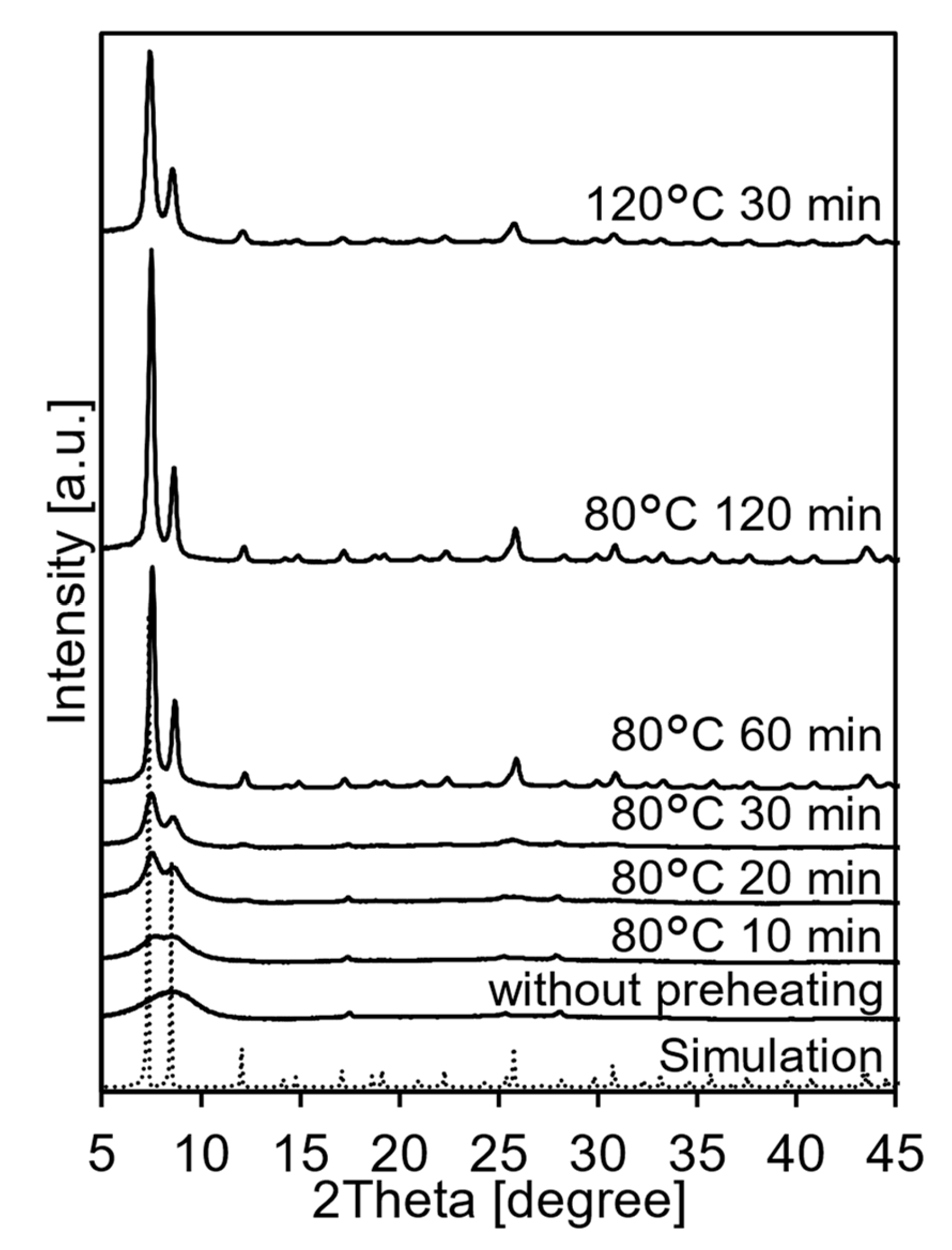
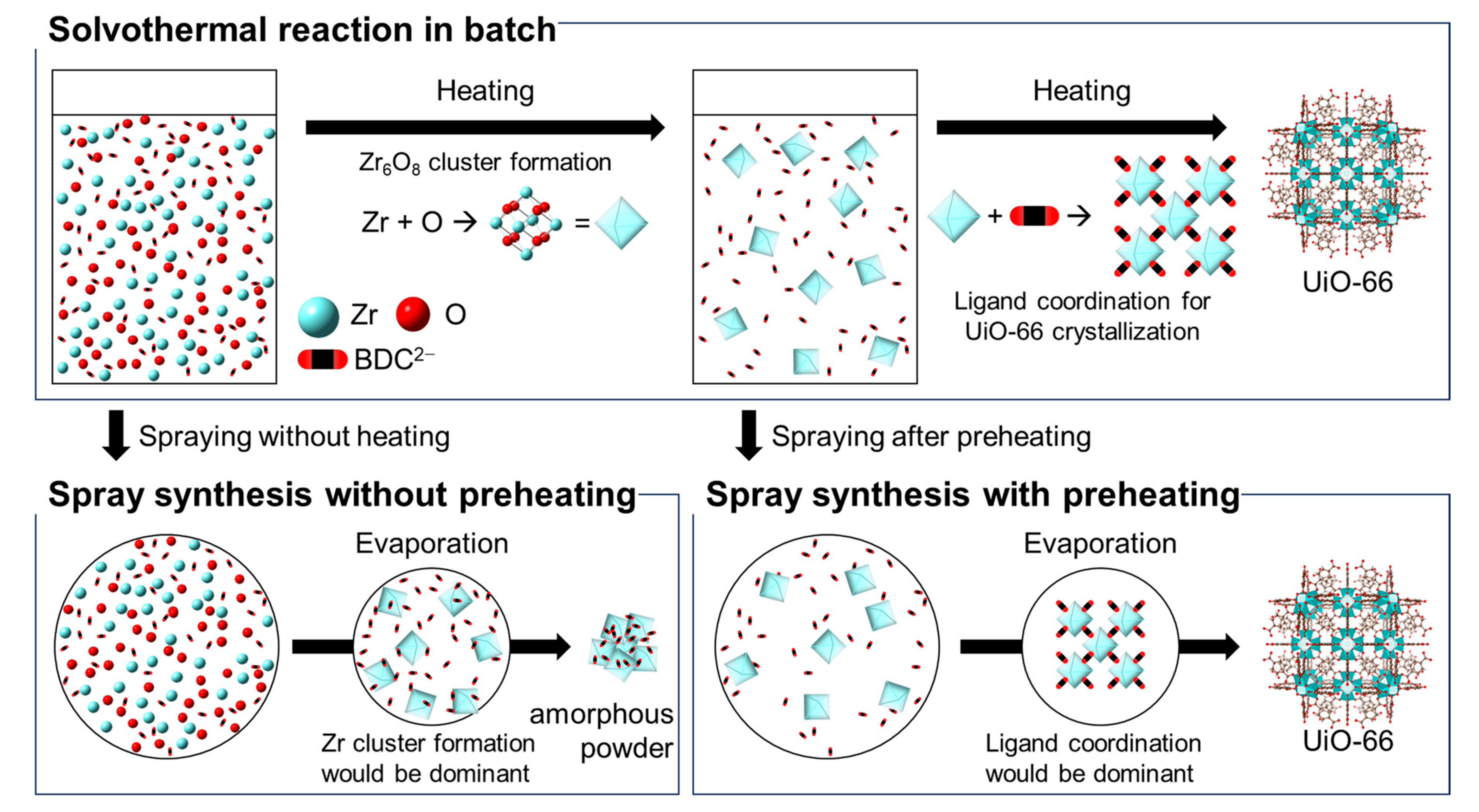
3.2. Spray Synthesis of UiO-66 Using Zr(OnPr)4 as a Zr Source without Preheating
3.3. Spray Synthesis of UiO-66-NH2 Using Zr(OnPr)4 as a Zr Source without Preheating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bradshaw, D.; Garai, A.; Huo, J. Metal-Organic Framework Growth at Functional Interfaces: Thin Films and Composites for Diverse Applications. Chem. Soc. Rev. 2012, 41, 2344–2381. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C.J. Application of Metal and Metal Oxide Nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, e1704303. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kirlikovali, K.O.; Li, P.; Farha, O.K. Reticular Chemistry for Highly Porous Metal-Organic Frameworks: The Chemistry and Applications. Acc. Chem. Res. 2022, 55, 579–591. [Google Scholar] [CrossRef]
- Rabiee, N.; Atarod, M.; Tavakolizadeh, M.; Asgari, S.; Rezaei, M.; Akhavan, O.; Pourjavadi, A.; Jouyandeh, M.; Lima, E.C.; Hamed Mashhadzadeh, A.; et al. Green Metal-Organic Frameworks (MOFs) for Biomedical Applications. Microporous Mesoporous Mater. 2022, 335, 111670. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-Based Metal-Organic Frameworks: Design, Synthesis, Structure, and Applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal–Organic Framework. Cryst. Growth Des. 2020, 20, 1347–1362. [Google Scholar] [CrossRef]
- Julien, P.A.; Mottillo, C.; Friščić, T. Metal–Organic Frameworks Meet Scalable and Sustainable Synthesis. Green Chem. 2017, 19, 2729–2747. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New Synthetic Routes towards MOF Production at Scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- Ameloot, R.; Vermoortele, F.; Vanhove, W.; Roeffaers, M.B.J.; Sels, B.F.; De Vos, D.E. Interfacial Synthesis of Hollow Metal-Organic Framework Capsules Demonstrating Selective Permeability. Nat. Chem. 2011, 3, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Fabra, M.; Munn, A.S.; Stevens, L.A.; Drage, T.C.; Grant, D.M.; Kashtiban, R.J.; Sloan, J.; Lester, E.; Walton, R.I. Instant MOFs: Continuous Synthesis of Metal-Organic Frameworks by Rapid Solvent Mixing. Chem. Commun. 2012, 48, 10642–10644. [Google Scholar] [CrossRef]
- He, B.; Sadiq, M.M.; Batten, M.P.; Suzuki, K.; Rubio-Martinez, M.; Gardiner, J.; Hill, M.R. Continuous Flow Synthesis of a Zr Magnetic Framework Composite for Post-Combustion CO2 Capture. Chemistry 2019, 25, 13184–13188. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Martinez, M.; Batten, M.P.; Polyzos, A.; Carey, K.-C.; Mardel, J.I.; Lim, K.-S.; Hill, M.R. Versatile, High Quality and Scalable Continuous Flow Production of Metal-Organic Frameworks. Sci. Rep. 2014, 4, 5443. [Google Scholar] [CrossRef]
- McKinstry, C.; Cathcart, R.J.; Cussen, E.J.; Fletcher, A.J.; Patwardhan, S.V.; Sefcik, J. Scalable Continuous Solvothermal Synthesis of Metal Organic Framework (MOF-5) Crystals. Chem. Eng. J. 2016, 285, 718–725. [Google Scholar] [CrossRef]
- Carné-Sánchez, A.; Imaz, I.; Cano-Sarabia, M.; Maspoch, D. A Spray-Drying Strategy for Synthesis of Nanoscale Metal-Organic Frameworks and Their Assembly into Hollow Superstructures. Nat. Chem. 2013, 5, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Tovar, L.; Cano-Sarabia, M.; Carné-Sánchez, A.; Carbonell, C.; Imaz, I.; Maspoch, D. A Spray-Drying Continuous-Flow Method for Simultaneous Synthesis and Shaping of Microspherical High Nuclearity MOF Beads. React. Chem. Eng. 2016, 1, 533–539. [Google Scholar] [CrossRef]
- Kubo, M.; Saito, T.; Shimada, M. Evaluation of the Parameters Utilized for the Aerosol-Assisted Synthesis of HKUST-1. Microporous Mesoporous Mater. 2017, 245, 126–132. [Google Scholar] [CrossRef]
- Kubo, M.; Kitano, T.; Shimada, M. Spray Synthesis of Hierarchical Porous Metal-Organic Framework HKUST-1 with Soft Templates and Methylene Blue Adsorption Performance. Adv. Powder Technol. 2024, 35, 104280. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated Synthesis of Zr-Based Metal-Organic Frameworks: From Nano to Single Crystals. Chemistry 2011, 17, 6643–6651. [Google Scholar] [CrossRef]
- Kubo, M.; Ishimura, M.; Shimada, M. Improvement of Production Efficiency of Spray-Synthesized HKUST-1. Adv. Powder Technol. 2021, 32, 2370–2378. [Google Scholar] [CrossRef]
- Kubo, M.; Matsumoto, T.; Shimada, M. Spray Synthesis of Pd Nanoparticle Incorporated HKUST-1, and Its Catalytic Activity for 4-Nitrophenol Reduction. Adv. Powder Technol. 2022, 33, 103701. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Fu, Q.; Qin, H.; Lv, J.; Yang, K.; Zhang, Q.; Zhang, H.; Wang, M. An Efficient Modulated Synthesis of Zirconium Metal-Organic Framework UiO-66. RSC Adv. 2022, 12, 6083–6092. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Abdullah, M.; Wuled Lenggoro, I.; Iskandar, F. Preparation of Functional Nanostructured Particles by Spray Drying. Adv. Powder Technol. 2006, 17, 587–611. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Li, Y.; Zhang, R.; Lu, G. Large-Scale Synthesis of Monodisperse UiO-66 Crystals with Tunable Sizes and Missing Linker Defects via Acid/Base Co-Modulation. ACS Appl. Mater. Interfaces 2017, 9, 15079–15085. [Google Scholar] [CrossRef]
- Schoenecker, P.M.; Belancik, G.A.; Grabicka, B.E.; Walton, K.S. Kinetics Study and Crystallization Process Design for Scale-up of UiO-66-NH2 synthesis. AIChE J. 2013, 59, 1255–1262. [Google Scholar] [CrossRef]
- Xu, H.; Sommer, S.; Broge, N.L.N.; Gao, J.; Iversen, B.B. The Chemistry of Nucleation: In Situ Pair Distribution Function Analysis of Secondary Building Units During UiO-66 MOF Formation. Chemistry 2019, 25, 2051–2058. [Google Scholar] [CrossRef]
- Butova, V.V.; Budnyk, A.P.; Charykov, K.M.; Vetlitsyna-Novikova, K.S.; Lamberti, C.; Soldatov, A.V. Water as a Structure-Driving Agent between the UiO-66 and MIL-140A Metal-Organic Frameworks. Chem. Commun. 2019, 55, 901–904. [Google Scholar] [CrossRef]
- Ordoubadi, M.; Gregson, F.K.A.; Melhem, O.; Barona, D.; Miles, R.E.H.; D’Sa, D.; Gracin, S.; Lechuga-Ballesteros, D.; Reid, J.P.; Finlay, W.H.; et al. Multi-Solvent Microdroplet Evaporation: Modeling and Measurement of Spray-Drying Kinetics with Inhalable Pharmaceutics. Pharm. Res. 2019, 36, 100. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A Facile Synthesis of UiO-66, UiO-67 and Their Derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Tulig, K.; Walton, K.S. An Alternative UiO-66 Synthesis for HCl-Sensitive Nanoparticle Encapsulation. RSC Adv. 2014, 4, 51080–51083. [Google Scholar] [CrossRef]
- DeStefano, M.R.; Islamoglu, T.; Garibay, S.J.; Hupp, J.T.; Farha, O.K. Room-Temperature Synthesis of UiO-66 and Thermal Modulation of Densities of Defect Sites. Chem. Mater. 2017, 29, 1357–1361. [Google Scholar] [CrossRef]
- Puchberger, M.; Kogler, F.R.; Jupa, M.; Gross, S.; Fric, H.; Kickelbick, G.; Schubert, U. Can the Clusters Zr6O4(OH)4(OOCR)12 and [Zr6O4(OH)4(OOCR)12]2 Be Converted into Each Other? Eur. J. Inorg. Chem. 2006, 2006, 3283–3293. [Google Scholar] [CrossRef]
- Karadeniz, B.; Howarth, A.J.; Stolar, T.; Islamoglu, T.; Dejanović, I.; Tireli, M.; Wasson, M.C.; Moon, S.-Y.; Farha, O.K.; Friščić, T.; et al. Benign by Design: Green and Scalable Synthesis of Zirconium UiO-Metal–Organic Frameworks by Water-Assisted Mechanochemistry. ACS Sustain. Chem. Eng. 2018, 6, 15841–15849. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the Complex Structure of UiO-66 Metal Organic Framework: A Synergic Combination of Experiment and Theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Ethiraj, J.; Vitillo, J.G.; Svelle, S.; Olsbye, U.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. Tuned to Perfection: Ironing out the Defects in Metal–Organic Framework UiO-66. Chem. Mater. 2014, 26, 4068–4071. [Google Scholar] [CrossRef]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and Highly Tunable Missing-Linker Defects in Zirconium Metal-Organic Framework UiO-66 and Their Important Effects on Gas Adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.; Qin, H.; Yuan, B.; Zhang, M.; Wang, L.; Yang, B.; Tao, C.-A.; Zhang, S. Defective UiO-66-NH2 Monoliths for Optimizing CO2 Capture Performance. Chem. Eng. J. 2023, 467, 143394. [Google Scholar] [CrossRef]
- Ethiraj, J.; Albanese, E.; Civalleri, B.; Vitillo, J.G.; Bonino, F.; Chavan, S.; Shearer, G.C.; Lillerud, K.P.; Bordiga, S. Carbon Dioxide Adsorption in Amine-functionalized Mixed-ligand Metal–Organic Frameworks of UiO-66 Topology. ChemSusChem 2014, 7, 3382–3388. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, M.; Miyoshi, Y.; Uchitomi, Y.; Shimada, M. Insights into the Spray Synthesis of UiO-66 and UiO-66-NH2 Metal–Organic Frameworks: Effect of Zirconium Precursors and Process Parameters. Crystals 2024, 14, 116. https://doi.org/10.3390/cryst14020116
Kubo M, Miyoshi Y, Uchitomi Y, Shimada M. Insights into the Spray Synthesis of UiO-66 and UiO-66-NH2 Metal–Organic Frameworks: Effect of Zirconium Precursors and Process Parameters. Crystals. 2024; 14(2):116. https://doi.org/10.3390/cryst14020116
Chicago/Turabian StyleKubo, Masaru, Yusuke Miyoshi, Yushi Uchitomi, and Manabu Shimada. 2024. "Insights into the Spray Synthesis of UiO-66 and UiO-66-NH2 Metal–Organic Frameworks: Effect of Zirconium Precursors and Process Parameters" Crystals 14, no. 2: 116. https://doi.org/10.3390/cryst14020116
APA StyleKubo, M., Miyoshi, Y., Uchitomi, Y., & Shimada, M. (2024). Insights into the Spray Synthesis of UiO-66 and UiO-66-NH2 Metal–Organic Frameworks: Effect of Zirconium Precursors and Process Parameters. Crystals, 14(2), 116. https://doi.org/10.3390/cryst14020116





