Changes in Functional Groups and Crystal Structure of Coal Tar Pitch with Respect to Carbonization Temperature
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Raw Materials and TG-DTG
2.2. Carbonization
2.3. X-ray Diffraction
2.4. Fourier Transform Infrared Spectroscopy
2.5. Optical Microscopy
2.6. Raman Spectroscopy
3. Results and Discussion
3.1. Carbonization Yield Measured Using TGA and a Tube Furnace
3.2. Crystallinity of CTP as a Function of Temperature
3.3. Variations in Functional Groups of CTP with Respect to Carbonization Temperature
3.4. Microstructure of CTP
3.5. Crystallinity of Mesophase Using Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Edwards, I.A.S.; Marsh, H.; Menendez, R. Introduction to Carbon Science; Butterworths-Heinemann: Oxford, UK, 2009; p. 12. [Google Scholar]
- Kim, J.H. Control of the properties of a binder pitch to enhance the density and strength of graphite blocks. Carbon Lett. 2023, 33, 1757–1766. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, Y.J.; Im, J.S.; Jo, A.; Lee, K.B.; Bai, B.C. Study of activation mechanism for dual model pore structured carbon based on effects of molecular weight of petroleum pitch. J. Ind. Eng. Chem. 2020, 88, 251–259. [Google Scholar] [CrossRef]
- Kremer, H.A. Recent developments in electrode pitch and coal tar technology. Chem. Ind. 1983, 18, 702–703. [Google Scholar]
- Brooks, J.D.; Taylor, G.H. The formation of graphitizing carbons from the liquid phase. Carbon 1965, 3, 185–186. [Google Scholar] [CrossRef]
- Marković, V. Use of coal tar pitch in carboncarbon composites. Fuel 1987, 66, 1512–1515. [Google Scholar] [CrossRef]
- Mochida, I.; Korai, Y.; Ku, C.H.; Watanabe, F.; Sakai, Y. Chemistry of synthesis, structure, preparation and application of aromatic-derived mesophase pitch. Carbon 2000, 38, 305–328. [Google Scholar] [CrossRef]
- Lim, C.; Ko, Y.; Kwak, C.H.; Kim, S.; Lee, Y.S. Mesophase pitch production aided by the thermal decomposition of polyvinylidene fluoride. Carbon Lett. 2022, 32, 1329–1335. [Google Scholar] [CrossRef]
- Oberlin, A. Carbonization and graphitization. Carbon 1984, 22, 521–541. [Google Scholar] [CrossRef]
- Yang, J.Y.; Park, S.H.; Park, S.J.; Seo, M.K. Preparation and characteristic of Carbon/Carbon composites with Coa-tar and petroleum binder pitches. Appl. Chem. Eng. 2015, 26, 406–412. [Google Scholar] [CrossRef]
- Zhang, W.; Li, T.; Lu, M.; Hou, C. A comparative study of the characteristics and carbonization behaviors of three modified coal tar pitches. New Carbon Mater. 2013, 28, 140–144. [Google Scholar] [CrossRef]
- Guillén, M.D.; Iglesias, M.J.; Domínguez, A.; Blanco, C.G. Fourier transform infrared study of coal tar pitches. Fuel 1995, 74, 1595–1598. [Google Scholar] [CrossRef]
- Lin, Q.; Su, W.; Xie, Y. Effect of rosin to coal-tar pitch on carbonization behavior and optical texture of resultant semi-cokes. J. Anal. Appl. Pyrolysis 2009, 86, 8–13. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Pasa, V.M.D.; Molhallem, N.D.S.; Otani, C.; Otani, S.; Pardini, L.C. Structural evolution of Eucalyptus tar pitch-based carbons during carbonization. Biomass Bioenergy 2005, 28, 53–61. [Google Scholar] [CrossRef]
- Liu, S.; Xue, J.; Liu, X.; Chen, H.; Li, X. Pitch derived graphene oxides: Characterization and effect on pyrolysis and carbonization of coal tar pitch. J. Anal. Appl. Pyrolysis 2020, 145, 104746. [Google Scholar] [CrossRef]
- Mongem, J.A.; Amorós, D.C.; Solano, A.L. Characterisation of coal tar pitches by thermal analysis, infrared spectroscopy and solvent fractionation. Fuel 2001, 80, 41–48. [Google Scholar]
- Guillen, M.D.; Iglesias, M.J.; Dominguez, A.; Blanco, C.G. Semiquantitative FTIR analysis of coal tar pitch and its extracts and residues in several organic solvents. Energy Fuels 1992, 6, 518–525. [Google Scholar] [CrossRef]
- Li, Q.; Han, D.; Qiao, H.; Shi, W.; Zhang, Y.; Cao, Z. Structural evolution of the thermal conversion products of modified coal tar pitch. Carbon Lett. 2023, 33, 261–271. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, D.S.; Lee, S.M.; Roh, J.S. X-ray diffraction analysis of the effect of ball milling time on crystallinity of milled polyacrylonitrile-based carbon fiber. Carbon Lett. 2018, 26, 11–17. [Google Scholar]
- Fuente, E.; Menéndez, J.A.; Díez, M.A.; Suárez, D.; Montes-Morán, M.A. Infrared spectroscopy of carbon materials: A quantum chemical study of model compounds. J. Phys. Chem. B 2003, 107, 6350–6359. [Google Scholar] [CrossRef]
- Pérez, M.; Granda, M.; Santamaría, R.; Morgan, T.; Menéndez, R. A thermoanalytical study of the co-pyrolysis of coal-tar pitch and petroleum pitch. Fuel 2004, 83, 1257–1265. [Google Scholar] [CrossRef]
- Dumont, M.; Dourges, M.A.; Bourrat, X.; Pailler, R.; Naslain, R.; Babot, O.; Birot, M.; Pillot, J.P. Carbonization behaviour of modified synthetic mesophase pitches. Carbon 2005, 43, 2277–2284. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Yoshii, T.; Nishihara, H. Quantitative study on catalysis of unpaired electrons in carbon edge sites. Carbon 2023, 210, 118069. [Google Scholar] [CrossRef]
- Ishii, T.; Kashihara, S.; Hoshikawa, Y.; Ozaki, J.; Kannari, N.; Takai, K.; Enoki, T.; Kyotani, T. A quantitative analysis of carbon edge sites and an estimation of graphene sheet size in high-temperature treated, non-porous carbons. Carbon 2014, 80, 135–145. [Google Scholar] [CrossRef]
- Honda, H.; Kimura, H.; Sanada, Y.; Sugawara, S.; Furuta, T. Optical mesophase texture and X-ray diffraction pattern of the early-stage carbonization of pitches. Carbon 1970, 8, 181–182. [Google Scholar] [CrossRef]
- Marsh, H.; Martínez-Escandell, M.; Rodríguez-Reinoso, F. Semicokes from pitch pyrolysis: Mechanisms and kinetics. Carbon 1999, 37, 363–390. [Google Scholar] [CrossRef]
- Cottinet, D.; Couderc, P.; Saint Romain, J.L. Raman microprobe study of heat-treated pitches. Carbon 1988, 26, 339–344. [Google Scholar] [CrossRef]


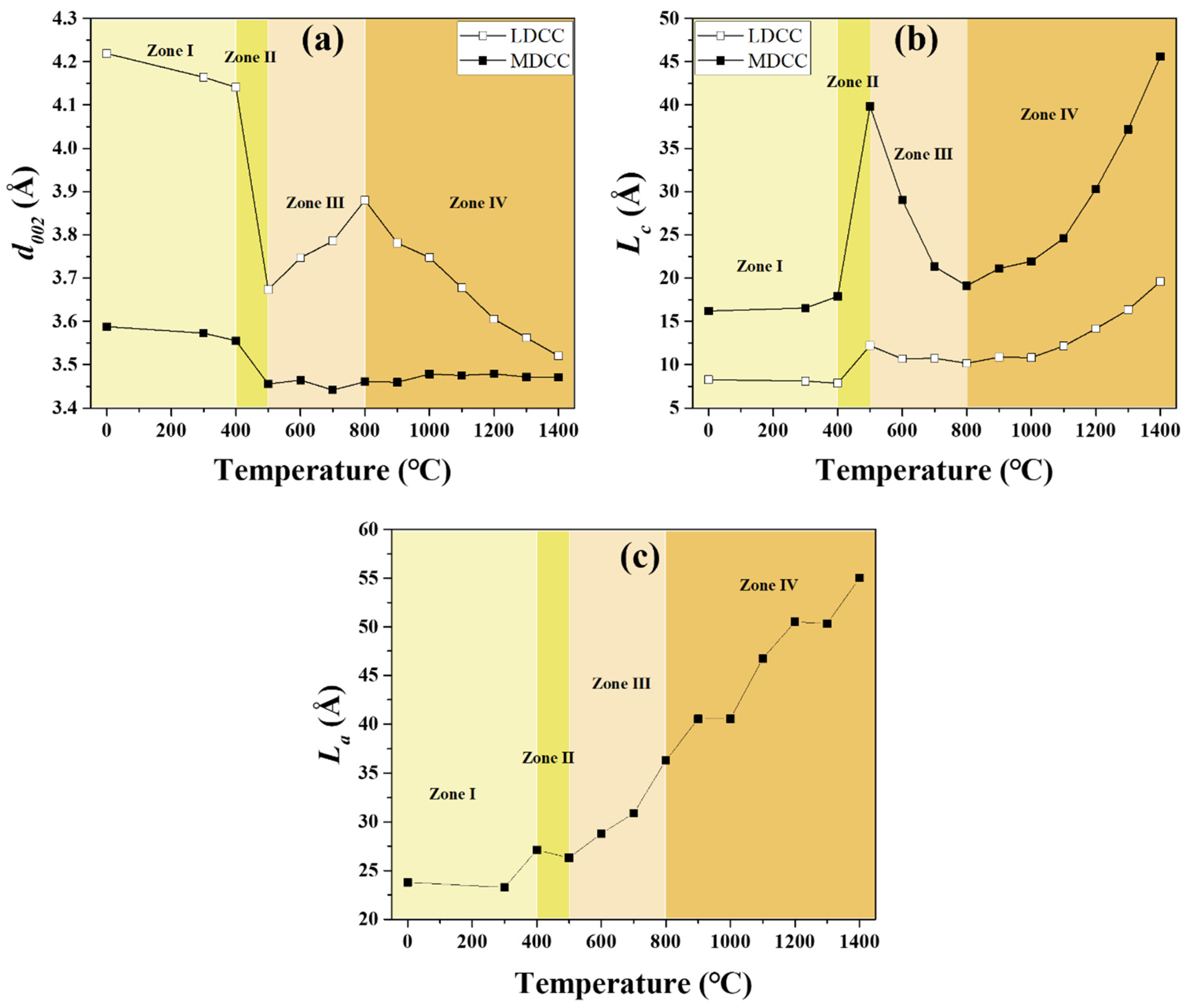
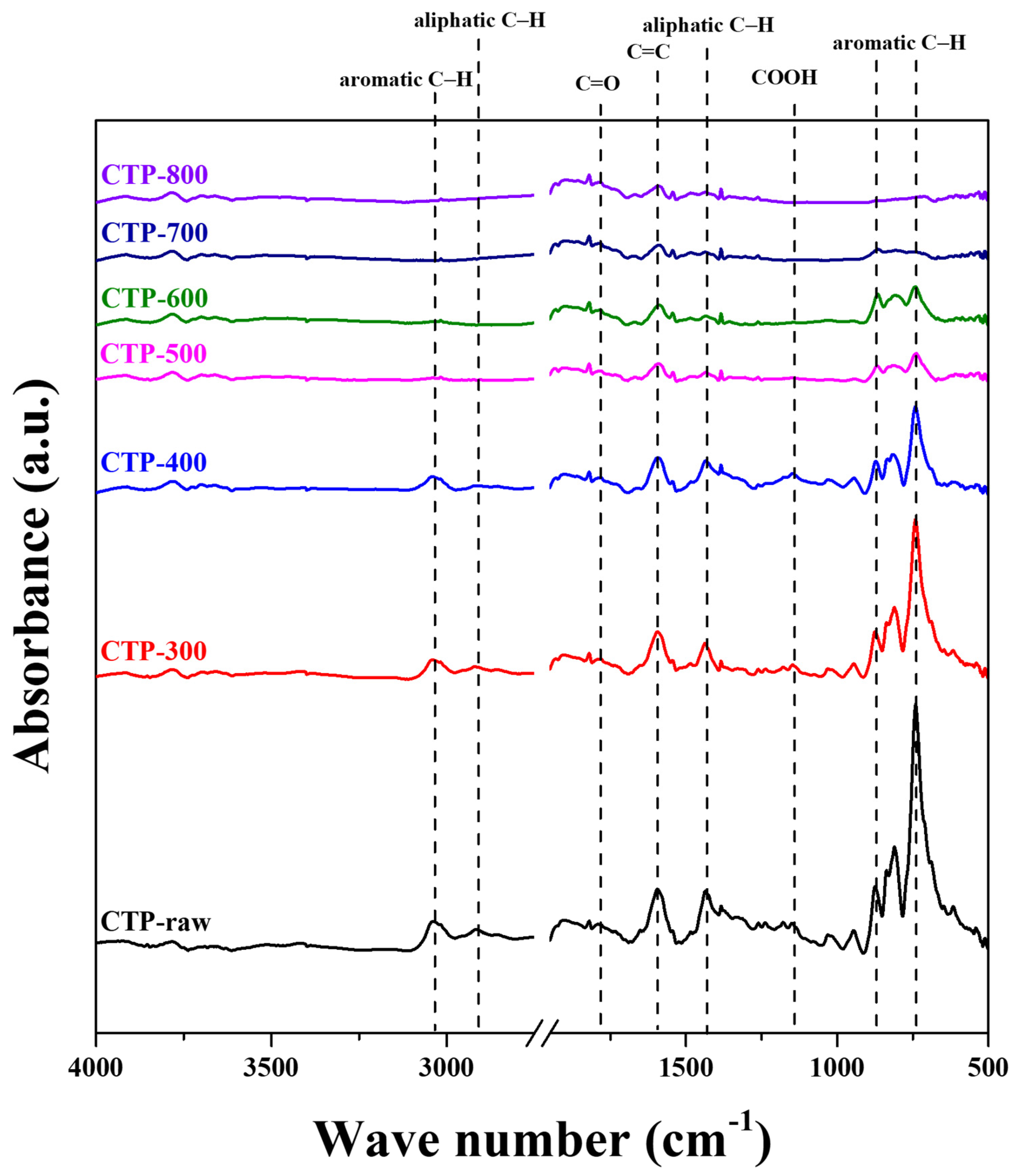
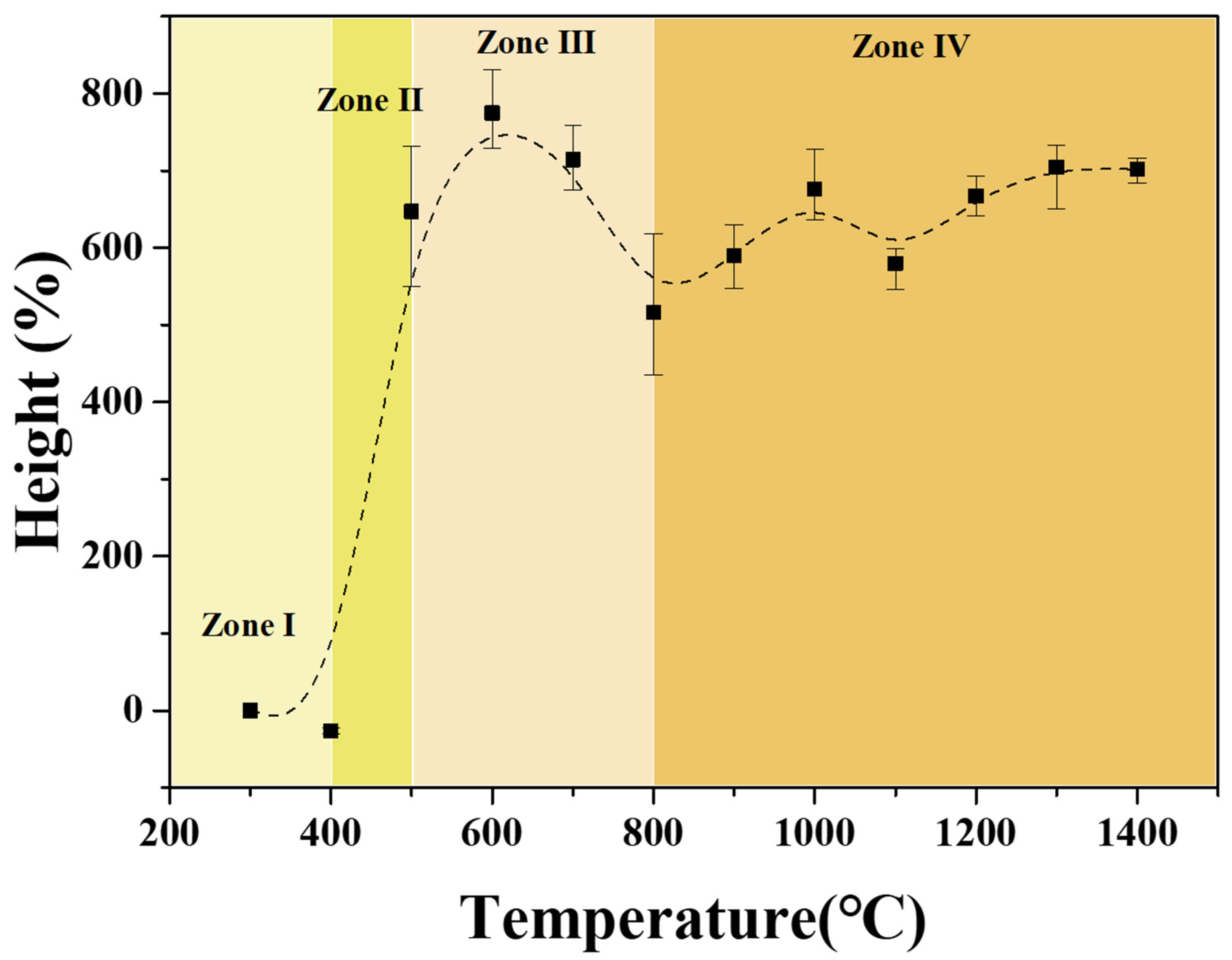


| Softening point (°C) | 115.3 |
| Toluene insoluble, TI (%) | 34.0 |
| Quinoline insoluble, QI (%) | 12.1 |
| Coking value (%) | 53.2 |
| Ash (%) | 0.6 |
| Wavenumber (cm−1) | Functional Group |
|---|---|
| 3000–3100 | Aromatic C-H bond stretching |
| 2800–2980 | Aliphatic C-H bond stretching |
| 1780 | C=O stretching |
| 1600 | Aromatic C=C stretching |
| 1440 | Aliphatic C-H bond bending |
| 1135 | -COOH |
| 700–900 | Aromatic C-H out of plane bending |
| Temp. (°C) | Absorbance Peak Intensity | ||||||
|---|---|---|---|---|---|---|---|
| C-Har (3041 cm−1) | C-Hal (2918 cm−1) | C=O (1790 cm−1) | C=C (1590 cm−1) | C-Hal (1435 cm−1) | COOH (1149 cm−1) | C-Har (741 cm−1) | |
| Raw | 0.034 | 0.024 | 0.031 | 0.073 | 0.071 | 0.032 | 0.296 |
| 300 | 0.022 | 0.013 | 0.022 | 0.055 | 0.041 | 0.015 | 0.191 |
| 400 | 0.019 | 0.008 | 0.018 | 0.042 | 0.038 | 0.022 | 0.104 |
| 500 | N.D. | N.D. | 0.012 | 0.021 | 0.011 | 0.004 | 0.033 |
| 600 | N.D. | N.D. | 0.018 | 0.024 | 0.011 | N.D. | 0.046 |
| 700 | N.D. | N.D. | 0.020 | 0.019 | 0.010 | N.D. | 0.010 |
| 800 | N.D. | N.D. | 0.024 | 0.019 | 0.012 | N.D. | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Roh, J.-S. Changes in Functional Groups and Crystal Structure of Coal Tar Pitch with Respect to Carbonization Temperature. Crystals 2024, 14, 122. https://doi.org/10.3390/cryst14020122
Lee S-H, Roh J-S. Changes in Functional Groups and Crystal Structure of Coal Tar Pitch with Respect to Carbonization Temperature. Crystals. 2024; 14(2):122. https://doi.org/10.3390/cryst14020122
Chicago/Turabian StyleLee, Sang-Hye, and Jae-Seung Roh. 2024. "Changes in Functional Groups and Crystal Structure of Coal Tar Pitch with Respect to Carbonization Temperature" Crystals 14, no. 2: 122. https://doi.org/10.3390/cryst14020122
APA StyleLee, S.-H., & Roh, J.-S. (2024). Changes in Functional Groups and Crystal Structure of Coal Tar Pitch with Respect to Carbonization Temperature. Crystals, 14(2), 122. https://doi.org/10.3390/cryst14020122






