Phosphors and Scintillators in Biomedical Imaging
Abstract
1. Background
2. Use of Scintillators and Phosphors in Medical Imaging
- (a)
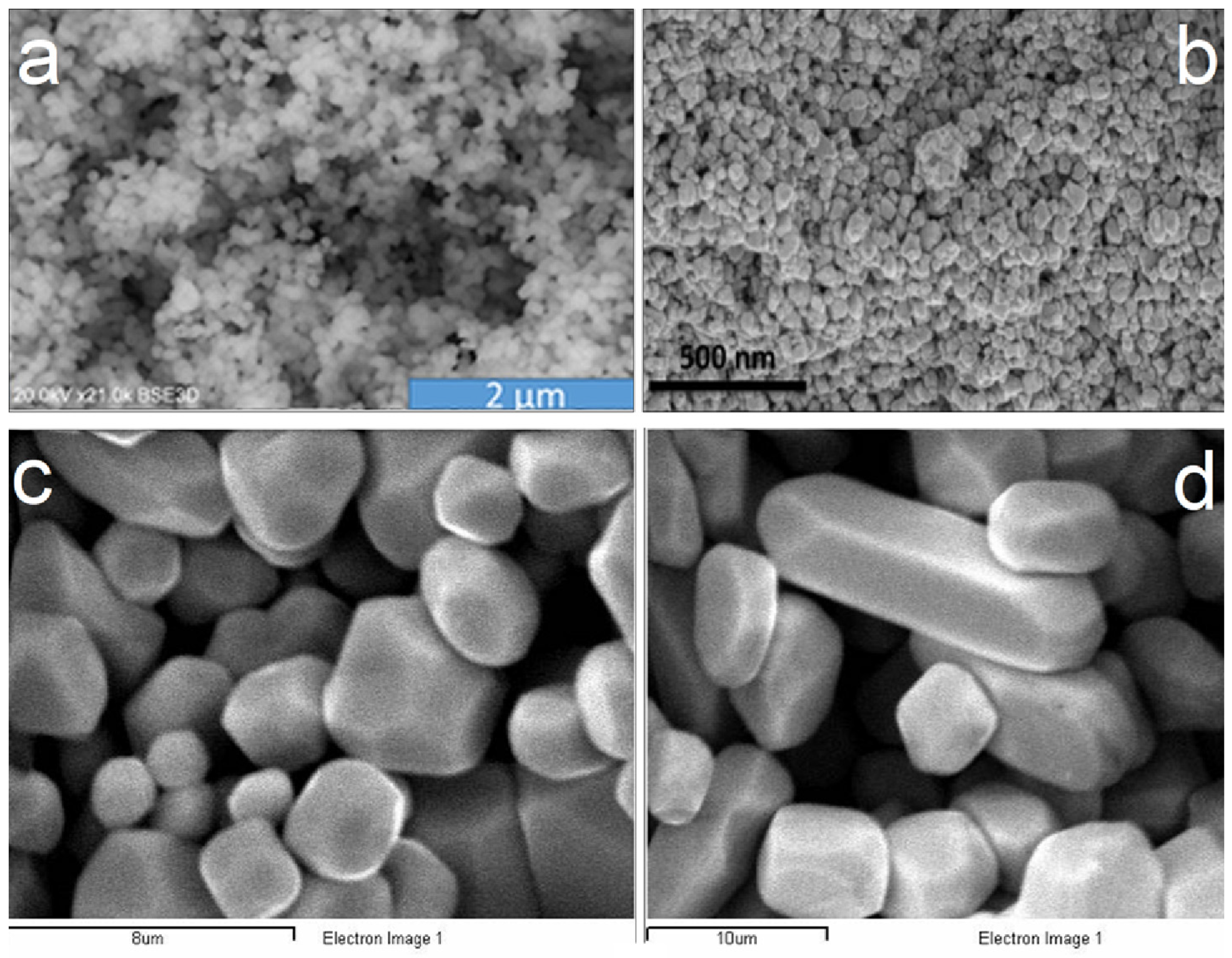
- Thin screens (approximately 150–300 μm thick) containing phosphor grains of various sizes (in the range from nano to some micrometers) manufactured in large areas (e.g., 18 × 24 cm2, 35 × 43 cm2, etc.), which are employed in traditional X-ray projection imaging as energy-integrating detectors, morphological imaging including X-ray screen-film radiography, active matrix flat panel arrays in digital radiography and fluoroscopy, and portal imaging (therapeutic imaging). Materials in this form are often referred to as phosphors, although the term scintillating screen has also been used. In this type of large-area detector, light spread within the material’s mass is deleterious for accurate position determination and image quality. Grains limit lateral spread effects by scattering and attenuating the laterally directed light photons. Grain size and shape affect both the emission efficiency and the imaging performance of the whole imaging system, i.e., small grains improve spatial resolution, while larger grains ameliorate screen sensitivity. An additional factor required to take into account is the thickness of the particular screen. Thick screens absorb larger fractions of incident radiation, while thin screens improve spatial resolution. Traditionally, rare earth phosphors (e.g., Gd2O2S:Tb) are the most popular for such applications, although many new materials are actually investigated. Detectors in digital radiography and mammography may contain large numbers of optical sensor elements (e.g., up to 4288 × 4288 photodiode arrays with pixel pitch between 50 and 200 μm, or even 20 μm with CMOS technology, covered by a large-area phosphor screen). The drawbacks of projection X-ray imaging, creating image blurring effects, are (i) the oblique incidence of X-rays since they are emitted by a point source, and (ii) their absorption at various depths within the screen, resulting in slight variations in the fluorescence intensity [6,14,17,19,24,25,32,36,37,38,39]. Figure 1 shows SEM images of various granular phosphors.
- (b)
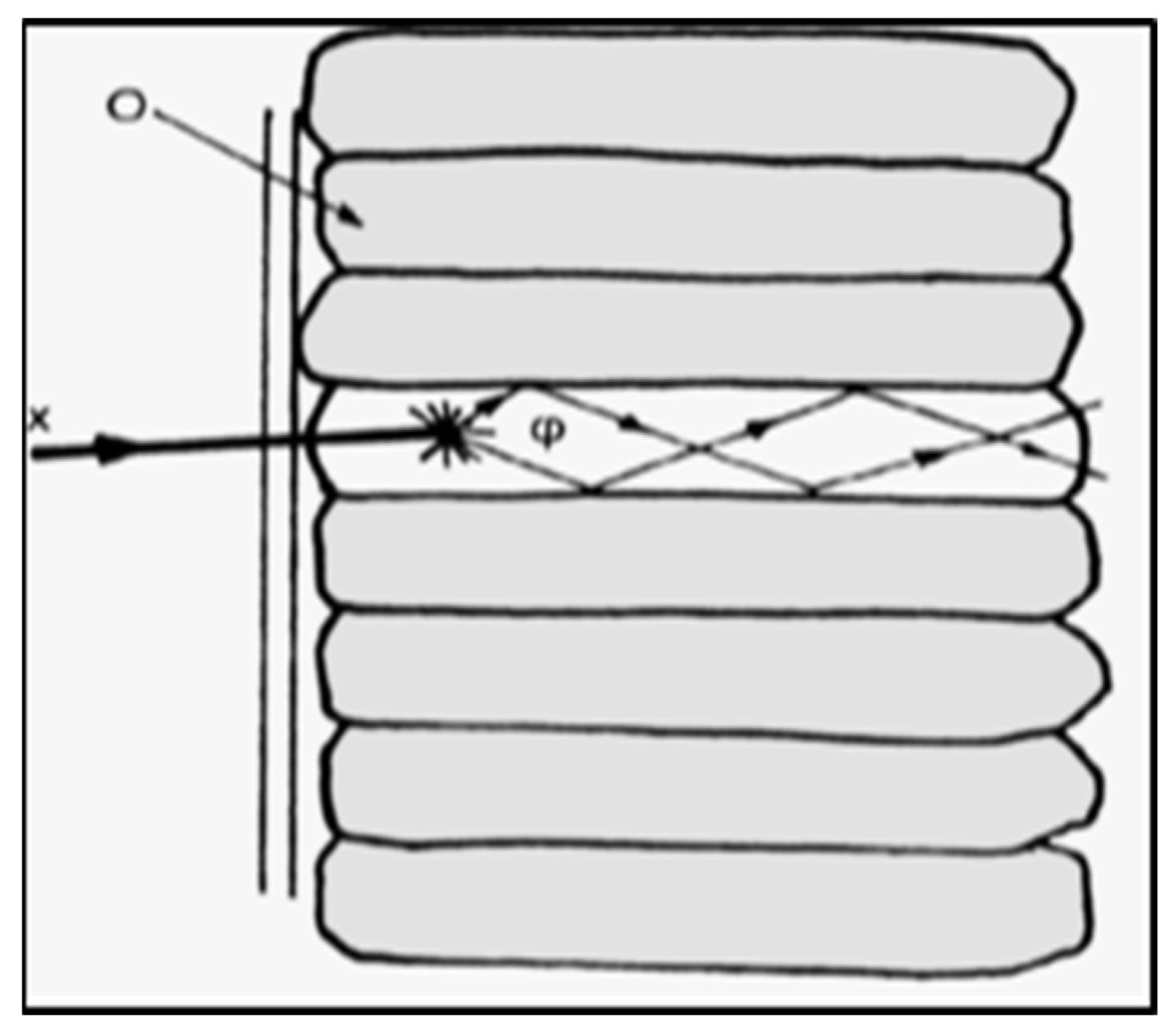
- Structured (needle-like as shown in Figure 2) crystals that are used as large-area thin screens in various digital imaging detectors (i.e., digital radiography, mammography, portal imaging active-matrix flat panels, etc.). Needle-like crystals (cracks) significantly improve spatial resolution due to limited lateral propagation and the spread of light within the material’s mass. Thick screens for improving radiation absorption can be prepared from such materials without degrading spatial resolution. A very popular scintillator in this form that has been used for a long time in image intensifiers (I.Is.) of conventional fluoroscopy systems is CsI:Na (referred to as phosphor in some literature). Another similar material is CsI:Tl. Both CsI:Na and CsI:Tl have been largely used under this form of crystal. The thickness of such screens ranges from 140 μm for digital mammography to 3000 μm for nuclear medicine. The optical sensor part of X-ray digital radiography and mammography detectors, based on CsI, is identical to that used with granular screens (i.e., AMFPI, CCD, CMOS) [17,19,25,37,38,39,40,41,42,43,44,45].
- (c)
- Single crystals, usually of large-area transparent layers, are traditionally employed in nuclear medicine (photon counting functional imaging). This form of luminescent material is traditionally referred to as a scintillator. In this case, the light spread is not a problem since position accuracy is assured by position-sensitive optical sensor arrays (e.g., PMTs, etc.). NaI:Tl is the most popular in gamma camera and SPECT systems, with a thickness ranging from 6.7 to 12.4 mm. CsI:Tl single crystals are also incorporated in some camera systems. For PET arrays, small single crystals are mostly used, arranged in ring-shaped detectors, with crystal thickness ranging from 10 to 30 mm. The number of crystals may be on the order of tens to hundreds of thousands in total body scanners, with a cross-section of 2 × 2 mm2 to 4 × 4 mm2 coupled to PSPMT, APDs, or SiPM optical sensors. In the case of X-ray CT detectors, where single crystals are often used and arranged in a 2D array in an arc-shaped configuration, the thickness ranges from 0.3 to 3 mm, and the cross-section is on the order of 1 mm. In PET systems, BGO, LSO:Ce, LYSO:Ce, and LGSO:Ce single crystals are considered the most appropriate, principally due to their fast response and high effective atomic number. For CT, CdWO4 as well as Gd2O2S with various activators, i.e., Pr, Ce, and mixtures such as (Y, Gd)2O3:Eu are mostly preferred. However, a large variety of crystalline materials are currently under investigation [6,18,20,21,26,27,31,34,46,47,48,49,50,51,52,53,54,55,56,57].
- (d)
- Transparent ceramics of various scintillators are now produced and incorporated into CT detectors. This form of material in polycrystalline form is produced by various techniques (e.g., sintering of grains of nano-sized powders, generally followed by hot pressure), is of lower cost, and sometimes shows superior light yield and overall better performance than traditional single crystals [31,51,58].
- (e)
- Storage phosphors (photostimulable materials) are employed in the form of X-ray imaging plates for use in computed radiography. After irradiation, a fraction of the electron/hole pairs do not recombine to transfer their energy to a luminescent center, as in traditional phosphors. In these phosphors, electrons are trapped in metastable states. Only if red wavelength photons interact with these states do the electrons escape to produce luminescence effects. Storage phosphor plates contain grains similar to those used in conventional film–screen radiography and digital radiography. These plates are enclosed in a light-tight cassette, and after being exposed to X-rays, they are read in raster scanning by a laser beam to emit light photons. Computed radiography systems are very successful applications; they can be used with conventional radiography systems and are widely used in hospital radiology departments. The commercially used materials are BaFBr:Eu2+ and CsBr:Eu2+ [7,59,60,61,62].
- (f)
- Organic scintillators have been investigated for imaging applications mainly due to their flexibility. Although their absorption efficiency (see below) is generally low, due to their low effective atomic number, light yield on the order of 80,000 photons per MeV has recently been reported for organic powders tested within X-ray imaging settings [63,64,65,66].
- (i)
- Radiation absorption and detection efficiency (depending on the effective atomic number, atomic K-shell absorption edge, density of materials, thickness of the particular sample, and energy of incident photons). An index indicating this efficiency is the product (: density, : effective atomic number). Absorption and detection efficiency are very crucial for capturing a significant fraction of the incident radiation (input signal) as well as for reducing the radiation dose burden to the patient (less radiation for a given level of output signal).
- (ii)
- The intrinsic conversion efficiency of radiation energy into light within the material’s mass or light yield (depending on the forbidden energy gap and other intrinsic material parameters) [35,67,68]. This parameter expresses the capability of a material to use efficiently the absorbed radiation energy.
- (iii)
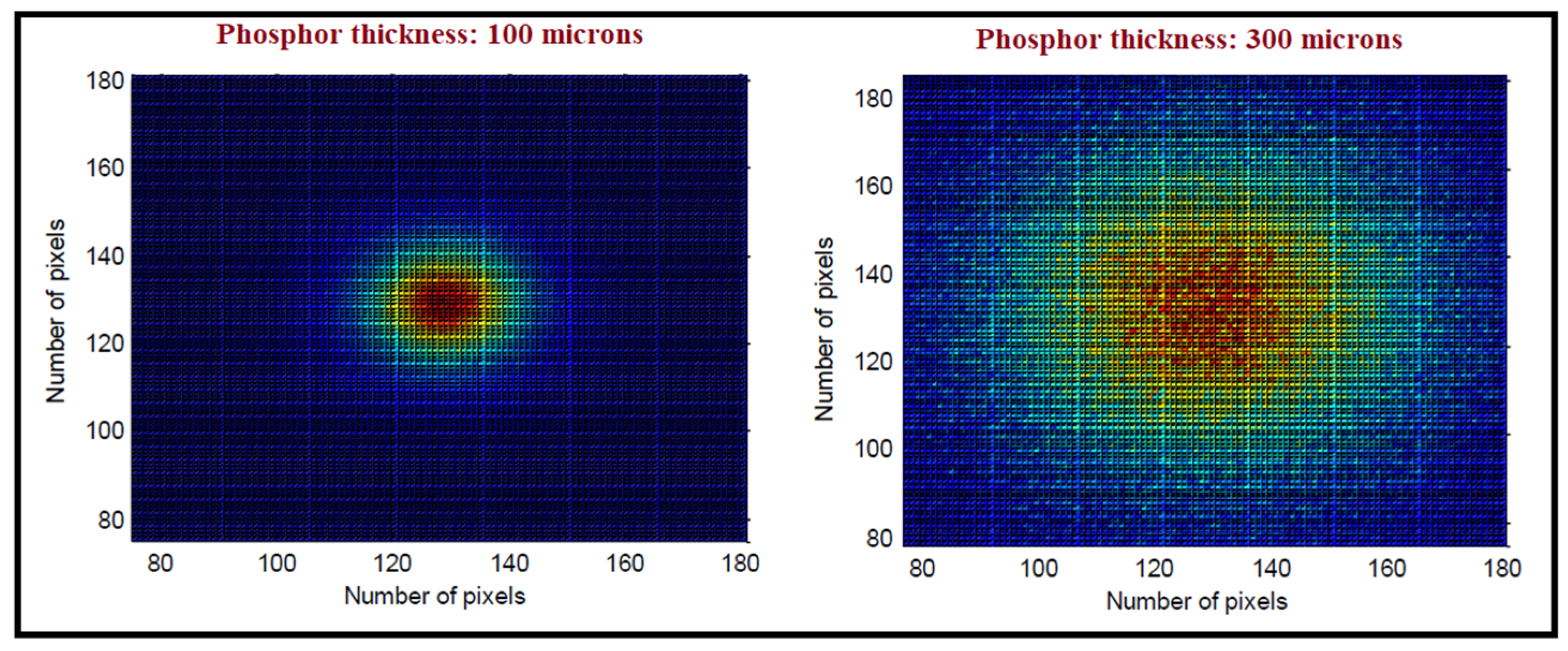
- Light transmission efficiency and the distribution of light at the material’s emitting surface (depending on the transparency, index of refraction, and light scattering properties of the material as well as on the thickness of the particular scintillator sample). In the case of granular phosphor screens, light scattering can be useful since it may reduce the extent of light spreading that deteriorates the point-by-point projection in X-ray radiographic and fluoroscopic imaging. On the other hand, in large-area transparent single crystals, usually employed in nuclear medicine, light is distributed in the whole scintillator mass. However, this is not a serious problem, due to the one-by-one photon counting and the use of position-sensitive optical sensors. In these techniques, high transparency is important since it improves light collection by the optical sensors. In some cases (e.g., in X-ray radiography), the thickness of the scintillating layer may decrease the transparency as well as the light transmission and emission, similarly affecting the quality of the final image. Additionally, thick scintillating layers (screens) contribute to a widening of the spread of the light photons that manage to reach their surface, thus reducing spatial resolution. This is shown in Figure 3, which depicts the distribution of light photons at the rear (emitting) surface of two granular phosphor layers of different thicknesses of 100 and 300 μm, respectively. These data were obtained by Monte Carlo methods (see Section 3.1.), assuming a pencil-like monoenergetic ionizing radiation beam absorbed at a single point within the scintillator layer. This distribution of light photons defines the so-called point spread function (PSF), which is an expression of the spatial resolution of an imaging system.
- (iv)
- The spectrum of emitted light (determined by the energy levels of the activator). This spectrum should match, as closely as possible, the quantum spectral sensitivity distribution of the optical sensor.
- (v)
- The emission of K-characteristic X-ray fluorescence after photoelectric absorption. This kind of emission may be a disadvantage in planar projection imaging if its absorption occurs within the phosphor–scintillator mass at a point different from that of X-ray or gamma-ray initial incidence.
- (vi)
- Decay time (determined by the probability of electric dipole transitions and being proportional to the light photon wavelength) [67]. Detectors employed in X-ray fluoroscopy, CT, and in nuclear medicine (particularly in PET) should be as fast as possible, thus requiring short or very short decay time. This can be accomplished by phosphors–scintillators emitting in the blue or ultraviolet spectral region.
- (vii)
- Energy resolution (X- and gamma-ray spectral resolution depending on light yield and non-proportionality effects in the response of some materials below 100 keV) [31].
- (viii)
- Afterglow (i.e., non-principal emission components with a relatively long decay time).
- (ix)
- Hygroscopicity.
- (x)
- Fragility.
2.1. Detector Signal
2.2. Noise and the Signal-to-Noise Ratio
3. Methodology of Research
3.1. Theories
3.2. Definitions of Quality Metrics
3.2.1. Luminescence Emission Efficiency
3.2.2. Modulation Transfer Efficiency
3.2.3. Noise and Signal-to-Noise in the Spatial Frequency Domain
3.3. Experimental Techniques
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blasse, G. The Luminescence Efficiency of Scintillators for Several Applications: State-of-the-Art. J. Lumin. 1994, 60–61, 930–935. [Google Scholar] [CrossRef]
- Yaffe, M.J.; Rowlands, J.A. X-ray Detectors for Digital Radiography. Phys. Med. Biol. 1997, 42, 1. [Google Scholar] [CrossRef]
- Moy, J.-P. Recent Developments in X-ray Imaging Detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2000, 442, 26–37. [Google Scholar] [CrossRef]
- Eijk, C.W.E. van Inorganic Scintillators in Medical Imaging. Phys. Med. Biol. 2002, 47, R85–R106. [Google Scholar] [CrossRef]
- Nikl, M. Scintillation Detectors for X-rays. Meas. Sci. Technol. 2006, 17, R37–R54. [Google Scholar] [CrossRef]
- Kandarakis, I.S. Luminescence in Medical Image Science. J. Lumin. 2016, 169, 553–558. [Google Scholar] [CrossRef]
- Rowlands, J.A. The Physics of Computed Radiography. Phys. Med. Biol. 2002, 47, R123. [Google Scholar] [CrossRef] [PubMed]
- Gruner, S.M.; Tate, M.W.; Eikenberry, E.F. Charge-Coupled Device Area x-Ray Detectors. Rev. Sci. Instrum. 2002, 73, 2815–2842. [Google Scholar] [CrossRef]
- Hoheisel, M. Review of Medical Imaging with Emphasis on X-ray Detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 563, 215–224. [Google Scholar] [CrossRef]
- Milbrath, B.D.; Peurrung, A.J.; Bliss, M.; Weber, W.J. Radiation Detector Materials: An Overview. J. Mater. Res. 2008, 23, 2561–2581. [Google Scholar] [CrossRef]
- Kubrin, R. Nanophosphor Coatings: Technology and Applications, Opportunities and Challenges. KONA Powder Part. J. 2014, 31, 22–52. [Google Scholar] [CrossRef]
- Ronda, C.; Wieczorek, H.; Khanin, V.; Rodnyi, P. Review—Scintillators for Medical Imaging: A Tutorial Overview. ECS J. Solid State Sci. Technol. 2015, 5, R3121. [Google Scholar] [CrossRef]
- Luo, Z.; Moch, G.J.; Johnson, S.S.; Chen, C.C. A Review on X-ray Detection Using Nanomaterials. Curr. Nanosci. 2017, 13, 364–372. [Google Scholar] [CrossRef]
- Yanagida, T. Inorganic Scintillating Materials and Scintillation Detectors. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018, 94, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kang, H.; Seo, B.; Cheong, J.; Roh, C.; Hong, S. A Review of Nanomaterial Based Scintillators. Energies 2021, 14, 7701. [Google Scholar] [CrossRef]
- Yanagida, T.; Kato, T.; Nakauchi, D.; Kawaguchi, N. Fundamental Aspects, Recent Progress and Future Prospects of Inorganic Scintillators. Jpn. J. Appl. Phys. 2022, 62, 010508. [Google Scholar] [CrossRef]
- Rowlands, J.A.; Neitzel, U. Receptors for Projection Radiography. In Diagnostic Radiology Physics: A Handbook for Teachers and Students; Christofides, S., Dance, D.R., Maidment, A.D.A., McLean, I.D., Ng, K.-H., Eds.; International Atomic Energy Agency: Vienna, Austria, 2014; pp. 145–182. [Google Scholar]
- Baily, D.L.; Humm, J.L.; Todd-Pokropek, A.; van Eijk, C.W.E. (Eds.) Basic Radiation Detectors. In Nuclear Medicine Physics: A Handbook for Teachers and Students; STI/PUB; International Atomic Energy Agency: Vienna, Austria, 2014; pp. 196–213. ISBN 978-92-0-143810-2. [Google Scholar]
- Rowlands, J.A.; Yorkston, J. Flat Panel Detectors for Digital Radiography. In Handbook of Medical Imaging Physics and Psychophysics; Beutel, J., Kundel, H., Van Metter, R., Eds.; SPIE: Bellingham, WA, USA, 2000; Volume 1, pp. 223–328. ISBN 978-0-8194-7772-9. [Google Scholar]
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. Physics in Nuclear Medicine, 4th ed.; Elsevier/Saunders: Philadelphia, PA, USA, 2012. [Google Scholar]
- Lecomte, R. Biomedical Imaging: SPECT and PET. AIP Conf. Proc. 2007, 958, 115–122. [Google Scholar] [CrossRef]
- Boone, J.M. X-ray Production, Interaction, and Detection in Diagnostic Imaging. In Handbook of Medical Imaging. Volume 1: Physics and Psychophysics; Beutel, J., Kundel, H.L., Van Metter, R.L., Eds.; SPIE Press Book: Bellingham, WA, USA, 2000; Volume 1, pp. 1–79. ISBN 978-0-8194-7772-9. [Google Scholar]
- Poletti, J.L. Projection Radiography. In Diagnostic Radiology Physics: A Handbook for Teachers and Students; Christofides, S., Dance, D.R., Maidment, A.D.A., McLean, I.D., Ng, K.-H., Eds.; International Atomic Energy Agency: Vienna, Austria, 2014; pp. 117–144. [Google Scholar]
- Taibi, A. Conventional Radiology. In Ionizing Radiation Detectors for Medical Imaging; World Scientific: Singapore, 2004; pp. 17–52. ISBN 978-981-238-674-8. [Google Scholar]
- Russo, P. Detectors for Digital Radiography. In Ionizing Radiation Detectors for Medical Imaging; World Scientific: Singapore, 2004; pp. 53–124. ISBN 978-981-238-674-8. [Google Scholar]
- Passeri, A.; Formiconi, A.R. SPECT and Planar Imaging in Nuclear Medicine. In Ionizing Radiation Detectors for Medical Imaging; World Scientific: Singapore, 2004; pp. 235–285. ISBN 978-981-238-674-8. [Google Scholar]
- Del Guerra, A.; Motta, A. Positron Emission Tomography. In Ionizing Radiation Detectors for Medical Imaging; World Scientific: Singapore, 2004; pp. 287–358. ISBN 978-981-238-674-8. [Google Scholar]
- Ou, X.; Chen, X.; Xu, X.; Xie, L.; Chen, X.; Hong, Z.; Bai, H.; Liu, X.; Chen, Q.; Li, L.; et al. Recent Development in X-ray Imaging Technology: Future and Challenges. Research 2021, 2021, 9892152. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sun, M.; Lu, Q.; Wu, T.; Huang, B. High Energy X-ray Radiation Sensitive Scintillating Materials for Medical Imaging, Cancer Diagnosis and Therapy. Nano Energy 2020, 79, 105437. [Google Scholar] [CrossRef]
- Danielsson, M.; Persson, M.; Sjölin, M. Photon-Counting x-Ray Detectors for CT. Phys. Med. Biol. 2021, 66, 03TR01. [Google Scholar] [CrossRef]
- Lecoq, P. Development of New Scintillators for Medical Applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2016, 809, 130–139. [Google Scholar] [CrossRef]
- Tyrrell, G.C. Phosphors and Scintillators in Radiation Imaging Detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2005, 546, 180–187. [Google Scholar] [CrossRef]
- Antonuk, L.E. Electronic Portal Imaging Devices: A Review and Historical Perspective of Contemporary Technologies and Research. Phys. Med. Biol. 2002, 47, R31. [Google Scholar] [CrossRef]
- Jana, A.; Cho, S.; Patil, S.A.; Meena, A.; Jo, Y.; Sree, V.G.; Park, Y.; Kim, H.; Im, H.; Taylor, R.A. Perovskite: Scintillators, Direct Detectors, and X-ray Imagers. Mater. Today 2022, 55, 110–136. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mao, Y. Recent Advances, Challenges, and Opportunities of Inorganic Nanoscintillators. Front. Optoelectron. 2020, 13, 156–187. [Google Scholar] [CrossRef] [PubMed]
- Lindström, J.; Alm Carlsson, G.; Wåhlin, E.; Carlsson Tedgren, Å.; Poludniowski, G. Experimental Assessment of a Phosphor Model for Estimating the Relative Extrinsic Efficiency in Radioluminescent Detectors. Phys. Medica 2020, 76, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bohndiek, S.E.; Cook, E.J.; Arvanitis, C.D.; Olivo, A.; Royle, G.J.; Clark, A.T.; Prydderch, M.L.; Turchetta, R.; Speller, R.D. A CMOS Active Pixel Sensor System for Laboratory- Based x-Ray Diffraction Studies of Biological Tissue. Phys. Med. Biol. 2008, 53, 655–672. [Google Scholar] [CrossRef]
- Konstantinidis, A.C.; Szafraniec, M.B.; Speller, R.D.; Olivo, A. The Dexela 2923 CMOS X-ray Detector: A Flat Panel Detector Based on CMOS Active Pixel Sensors for Medical Imaging Applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2012, 689, 12–21. [Google Scholar] [CrossRef]
- Yorkston, J. Recent Developments in Digital Radiography Detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 580, 974–985. [Google Scholar] [CrossRef]
- Rocha, J.G.; Lanceros-Mendez, S. Review on X-ray Detectors Based on Scintillators and CMOS Technology. Recent Pat. Electr. Eng. 2011, 4, 16–41. [Google Scholar] [CrossRef]
- Kim, H.-K.; Cunningham, I.A.; Yin, Z.; Cho, G.-S. On the Development of Digital Radiography Detectors: A Review. Int. J. Precis. Eng. Manuf. 2008, 9, 86–100. [Google Scholar]
- Cowen, A.R.; Kengyelics, S.M.; Davies, A.G. Solid-State, Flat-Panel, Digital Radiography Detectors and Their Physical Imaging Characteristics. Clin. Radiol. 2008, 63, 487–498. [Google Scholar] [CrossRef]
- Miller, S.R.; Gaysinskiy, V.; Shestakova, I.; Nagarkar, V.V. Recent Advances in Columnar CsI(Tl) Scintillator Screens. In Proceedings of the Penetrating Radiation Systems and Applications VII, San Diego, CA, USA, 1–4 August 2005; SPIE: Bellingham, WA, USA, 2005; Volume 5923, pp. 99–108. [Google Scholar]
- Rocha, J.G.; Dias, R.A.; Goncalves, L.; Minas, G.; Ferreira, A.; Costa, C.M.; Lanceros-Mendez, S. X-ray Image Detector Based on Light Guides and Scintillators. IEEE Sens. J. 2009, 9, 1154–1159. [Google Scholar] [CrossRef]
- Martinazzoli, L.; Kratochwil, N.; Gundacker, S.; Auffray, E. Scintillation Properties and Timing Performance of State-of-the-Art Gd3Al2Ga3O12 Single Crystals. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2021, 1000, 165231. [Google Scholar] [CrossRef]
- Shefer, E.; Altman, A.; Behling, R.; Goshen, R.; Gregorian, L.; Roterman, Y.; Uman, I.; Wainer, N.; Yagil, Y.; Zarchin, O. State of the Art of CT Detectors and Sources: A Literature Review. Curr. Radiol. Rep. 2013, 1, 76–91. [Google Scholar] [CrossRef]
- Makek, M.; Bosnar, D.; Kožuljević, A.M.; Pavelić, L. Investigation of GaGG:Ce with TOFPET2 ASIC Readout for Applications in Gamma Imaging Systems. Crystals 2020, 10, 1073. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, X.; Zhang, Y.; Ying, G.; Huang, Q.; Xu, J.; Peng, Q. Evaluation of Various Scintillator Materials in Radiation Detector Design for Positron Emission Tomography (PET). Crystals 2020, 10, 869. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, X.; Zhang, H.; Peng, H.; Ren, Q.; Xu, J.; Peng, Q.; Xie, S. Requirements of Scintillation Crystals with the Development of PET Scanners. Crystals 2022, 12, 1302. [Google Scholar] [CrossRef]
- Zatcepin, A.; Ziegler, S.I. Detectors in Positron Emission Tomography. Z. Für Med. Phys. 2023, 33, 4–12. [Google Scholar] [CrossRef]
- Zhu, D.; Nikl, M.; Chewpraditkul, W.; Li, J. Development and Prospects of Garnet Ceramic Scintillators: A Review. J. Adv. Ceram. 2022, 11, 1825–1848. [Google Scholar] [CrossRef]
- Nikl, M.; Yoshikawa, A. Recent R&D Trends in Inorganic Single-Crystal Scintillator Materials for Radiation Detection. Adv. Opt. Mater. 2015, 3, 463–481. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, F.; Lu, Y.; Sun, Q.; Xu, Y.; Zhang, B.-B.; Jie, W.; Kanatzidis, M.G. High-Sensitivity X-ray Detectors Based on Solution-Grown Caesium Lead Bromide Single Crystals. J. Mater. Chem. C 2020, 8, 1248–1256. [Google Scholar] [CrossRef]
- Tyagi, M.; Rawat, S.; Kumar, G.A.; Gadkari, S.C. A Novel Versatile Phoswich Detector Consisting of Single Crystal Scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 951, 162982. [Google Scholar] [CrossRef]
- Jin, T.; Hao, S.; Shang, Y.; Lei, Z.; Yang, C. Recent Trends in Elpasolite Single Crystal Scintillators for Radiation Detection. Crystals 2022, 12, 887. [Google Scholar] [CrossRef]
- Piotrowski, W.M.; Bolek, P.; Brik, M.G.; Zych, E.; Marciniak, L. Frontiers of Deep-Red Emission of Mn4+ Ions with Ruddlesden–Popper Perovskites. Inorg. Chem. 2023, 62, 21164–21172. [Google Scholar] [CrossRef]
- Bartosiewicz, K.; Fritz, V.; der Heggen, D.V.; Szymanski, D.; Zeler, J.; Pejchal, J.; Yamaji, A.; Kucerkova, R.; Beitlerova, A.; Kurosawa, S.; et al. Towards Deliberate Design of Persistent Phosphors: A Study of La–Ga Admixing in LuAG:Ce Crystals to Engineer Elemental Homogeneity and Carrier Trap Depths. J. Mater. Chem. C 2023, 11, 8850–8865. [Google Scholar] [CrossRef]
- Elzbieciak-Piecka, K.; Sójka, M.; Tian, F.; Li, J.; Zych, E.; Marciniak, L. The Comparison of the Thermometric Performance of Optical Ceramics, Microcrystals and Nanocrystals of Cr3+- Doped Gd3Ga5O12Garnets. J. Alloys Compd. 2023, 963, 171284. [Google Scholar] [CrossRef]
- Vedantham, S.; Karellas, A. Modeling the Performance Characteristics of Computed Radiography (CR) Systems. IEEE Trans. Med. Imaging 2010, 29, 790–806. [Google Scholar] [CrossRef]
- Leblans, P.; Vandenbroucke, D.; Willems, P. Storage Phosphors for Medical Imaging. Materials 2011, 4, 1034–1086. [Google Scholar] [CrossRef] [PubMed]
- Rivetti, S.; Lanconelli, N.; Bertolini, M.; Nitrosi, A.; Burani, A.; Acchiappati, D. Comparison of Different Computed Radiography Systems: Physical Characterization and Contrast Detail Analysis. Med. Phys. 2010, 37, 440–448. [Google Scholar] [CrossRef]
- Sójka, M.; Piotrowski, W.; Marciniak, L.; Zych, E. Co-Doping to Extend the Operating Range of Luminescence Thermometers. The Case of Y2SiO5:Pr3+,Tb3+. J. Alloys Compd. 2024, 970, 172662. [Google Scholar] [CrossRef]
- Xu, L.-J.; Lin, X.; He, Q.; Worku, M.; Ma, B. Highly Efficient Eco-Friendly X-ray Scintillators Based on an Organic Manganese Halide. Nat. Commun. 2020, 11, 4329. [Google Scholar] [CrossRef]
- Blakesley, J.C.; Speller, R. Modeling the Imaging Performance of Prototype Organic X-ray Imagers. Med. Phys. 2008, 35, 225–239. [Google Scholar] [CrossRef]
- Shao, W.; Zhu, G.; Wang, X.; Zhang, Z.; Lv, H.; Deng, W.; Zhang, X.; Liang, H. Highly Efficient, Flexible, and Eco-Friendly Manganese(II) Halide Nanocrystal Membrane with Low Light Scattering for High-Resolution X-ray Imaging. ACS Appl. Mater. Interfaces 2023, 15, 932–941. [Google Scholar] [CrossRef]
- Chen, M.; Sun, L.; Ou, X.; Yang, H.; Liu, X.; Dong, H.; Hu, W.; Duan, X. Organic Semiconductor Single Crystals for X-ray Imaging. Adv. Mater. 2021, 33, 2104749. [Google Scholar] [CrossRef]
- Rodnyi, P.A. Physical Processes in Inorganic Scintillators; CRC Press: London, UK, 2020; ISBN 978-0-13-874335-2. [Google Scholar]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994; ISBN 978-3-540-58019-5. [Google Scholar]
- Bunch, P.C.; Huff, K.E.; Metter, R.V. Analysis of the Detective Quantum Efficiency of a Radiographic Screen–Film Combination. J. Opt. Soc. Am. A 1987, 4, 902–909. [Google Scholar] [CrossRef]
- Ludwig, G.W. X-ray Efficiency of Powder Phosphors. J. Electrochem. Soc. 1971, 118, 1152. [Google Scholar] [CrossRef]
- Nishikawa, R.M.; Yaffe, M.J. Model of the Spatial-Frequency-Dependent Detective Quantum Efficiency of Phosphor Screens. Med. Phys. 1990, 17, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.; Shaw, R.; Metter, R.V. Detective Quantum Efficiency of Imaging Systems with Amplifying and Scattering Mechanisms. J. Opt. Soc. Am. A 1987, 4, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, I.A. Applied Linear-Systems Theory. In Handbook of Medical Imaging, Volume 1, Physics and Psychophysics; Beutel, J., Kundel, H., Van Metter, R., Eds.; SPIE: Bellingham, WA, USA, 2000; Volume 1, pp. 79–156. ISBN 978-0-8194-7772-9. [Google Scholar]
- Kim, H.K.; Yun, S.M.; Ko, J.S.; Cho, G.; Graeve, T. Cascade Modeling of Pixelated Scintillator Detectors for X-ray Imaging. IEEE Trans. Nucl. Sci. 2008, 55, 1357–1366. [Google Scholar] [CrossRef]
- Zhao, C.; Kanicki, J.; Konstantinidis, A.C.; Patel, T. Large Area CMOS Active Pixel Sensor X-ray Imager for Digital Breast Tomosynthesis: Analysis, Modeling, and Characterization. Med. Phys. 2015, 42, 6294–6308. [Google Scholar] [CrossRef]
- Swank, R.K. Calculation of Modulation Transfer Functions of X-ray Fluorescent Screens. Appl. Opt. AO 1973, 12, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, J.T. Image Quality Metrics for Digital Systems. In Handbook of Medical Imaging, Volume 1. Physics and Psychophysics; Beutel, J., Kundel, H., Van Metter, R., Eds.; SPIE: Bellingham, WA, USA, 2000; pp. 161–223. [Google Scholar] [CrossRef]
- Marshall, N.W. The Practical Application of Signal Detection Theory to Image Quality Assessment in X-ray Image Intensifier-TV Fluoroscopy. Phys. Med. Biol. 2001, 46, 1631. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, A.; Montalto, L.; Scalise, L.; Rinaldi, D.; Mengucci, P.; Michail, C.; Fountos, G.; Martini, N.; Koukou, V.; Valais, I.; et al. Luminescence and Structural Characterization of Gd2O2S Scintillators Doped with Tb3+, Ce3+, Pr3+ and F for Imaging Applications. Crystals 2022, 12, 854. [Google Scholar] [CrossRef]
- Lubberts, G. Random Noise Produced by X-ray Fluorescent Screens. J. Opt. Soc. Am. 1968, 58, 1475–1483. [Google Scholar] [CrossRef]
- Howansky, A.; Lubinsky, A.R.; Ghose, S.K.; Suzuki, K.; Zhao, W. Direct Measurement of Lubberts Effect in CsI:Tl Scintillators Using Single x-Ray Photon Imaging. In Proceedings of the Medical Imaging 2017: Physics of Medical Imaging, Orlando, FL, USA, 13–16 February 2017; SPIE: Bellingham, WA, USA, 2017; Volume 10132, pp. 61–71. [Google Scholar]
- Marshall, N.W. A Comparison between Objective and Subjective Image Quality Measurements for a Full Field Digital Mammography System. Phys. Med. Biol. 2006, 51, 2441. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, R. XMuDat: Photon Attenuation Data on PC, International Atomic Energy Agency. Available online: https://www-nds.iaea.org/publications/iaea-nds/iaea-nds-0195.htm (accessed on 31 March 2021).
- Tseremoglou, S.; Michail, C.; Valais, I.; Ninos, K.; Bakas, A.; Kandarakis, I.; Fountos, G.; Kalyvas, N. Optical Photon Propagation Characteristics and Thickness Optimization of LaCl3:Ce and LaBr3:Ce Crystal Scintillators for Nuclear Medicine Imaging. Crystals 2024, 14, 24. [Google Scholar] [CrossRef]
- Liaparinos, P.F. Optical Diffusion Performance of Nanophosphor-Based Materials for Use in Medical Imaging. JBO 2012, 17, 126013. [Google Scholar] [CrossRef] [PubMed]
- Karpetas, G.E.; Michail, C.M.; Fountos, G.P.; Kalyvas, N.I.; Valais, I.G.; Kandarakis, I.S.; Panayiotakis, G.S. Detective Quantum Efficiency (DQE) in PET Scanners: A Simulation Study. Appl. Radiat. Isot. 2017, 125, 154–162. [Google Scholar] [CrossRef]
- Cuplov, V.; Buvat, I.; Pain, F.; Jan, S. Extension of the GATE Monte-Carlo Simulation Package to Model Bioluminescence and Fluorescence Imaging. JBO 2014, 19, 026004. [Google Scholar] [CrossRef]
- Zarrini-Monfared, Z.; Karbasi, S.; Zamani, A.; Mosleh-Shirazi, M.A. Full Modulation Transfer Functions of Thick Parallel- and Focused-Element Scintillator Arrays Obtained by a Monte Carlo Optical Transport Model. Med. Phys. 2023, 50, 3651–3660. [Google Scholar] [CrossRef]
- Roncali, E.; Mosleh-Shirazi, M.A.; Badano, A. Modelling the Transport of Optical Photons in Scintillation Detectors for Diagnostic and Radiotherapy Imaging. Phys. Med. Biol. 2017, 62, R207. [Google Scholar] [CrossRef]
- Liaparinos, P.F.; Kandarakis, I.S.; Cavouras, D.A.; Delis, H.B.; Panayiotakis, G.S. Modeling Granular Phosphor Screens by Monte Carlo Methods. Med. Phys. 2006, 33, 4502–4514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dainty, J.C.; Shaw, R. Image Science; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Siewerdsen, J.H.; Antonuk, L.E.; El-Mohri, Y.; Yorkston, J.; Huang, W.; Boudry, J.M.; Cunningham, I.A. Empirical and Theoretical Investigation of the Noise Performance of Indirect Detection, Active Matrix Flat-Panel Imagers (AMFPIs) for Diagnostic Radiology. Med. Phys. 1997, 24, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, M.J.; Mainprize, J.G. Detectors for Digital Mammography. Technol. Cancer Res. Treat 2004, 3, 309–324. [Google Scholar] [CrossRef]
- Yun, S.; Kim, S.H.; Kim, D.W.; Kim, H.K. Detective Quantum Efficiency of a Phosphor-Coupled Photodiode Array Detector for Use in Digital X-ray Tomosynthesis Systems. NDT E Int. 2017, 92, 130–135. [Google Scholar] [CrossRef]
- Linardatos, D.; Konstantinidis, A.; Valais, I.; Ninos, K.; Kalyvas, N.; Bakas, A.; Kandarakis, I.; Fountos, G.; Michail, C. On the Optical Response of Tellurium Activated Zinc Selenide ZnSe:Te Single Crystal. Crystals 2020, 10, 961. [Google Scholar] [CrossRef]
- Tseremoglou, S.; Michail, C.; Valais, I.; Ninos, K.; Bakas, A.; Kandarakis, I.; Fountos, G.; Kalyvas, N. Evaluation of Cerium-Doped Lanthanum Bromide (LaBr3:Ce) Single-Crystal Scintillator’s Luminescence Properties under X-ray Radiographic Conditions. Appl. Sci. 2023, 13, 419. [Google Scholar] [CrossRef]
- Tseremoglou, S.; Michail, C.; Valais, I.; Ninos, K.; Bakas, A.; Kandarakis, I.; Fountos, G.; Kalyvas, N. Efficiency Properties of Cerium-Doped Lanthanum Chloride (LaCl3:Ce) Single Crystal Scintillator under Radiographic X-ray Excitation. Crystals 2022, 12, 655. [Google Scholar] [CrossRef]
- International Electrotechnical Commission International Electrotechnical Commission IEC 62220-1-1:2015 Medical Electrical Equipment: Characteristics of Digital X-ray Imaging Devices. Part 1-1. Available online: https://webstore.iec.ch/publication/21937 (accessed on 7 January 2024).
- Tzomakas, M.K.; Peppa, V.; Alexiou, A.; Karakatsanis, G.; Episkopakis, A.; Michail, C.; Valais, I.; Fountos, G.; Kalyvas, N.; Kandarakis, I.S. A Phantom Based Evaluation of the Clinical Imaging Performance of Electronic Portal Imaging Devices. Heliyon 2023, 9, e21116. [Google Scholar] [CrossRef]
- Michail, C.M.; Fountos, G.P.; Liaparinos, P.F.; Kalyvas, N.E.; Valais, I.; Kandarakis, I.S.; Panayiotakis, G.S. Light Emission Efficiency and Imaging Performance of Gd2O2S:Eu Powder Scintillator under X-ray Radiography Conditions. Med. Phys. 2010, 37, 3694–3703. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, W.; Su, Y.; Chen, Z.; Chen, X.; Ma, Y.; Bai, L.; Xiao, W.; Liu, T.; Zhu, H.; et al. Low-Dose Real-Time X-ray Imaging with Nontoxic Double Perovskite Scintillators. Light Sci. Appl. 2020, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, J. The Measurement and Evaluation of Standard DQE in Digital Radiography. In Proceedings of the 2015 International Conference on Mechatronics, Electronic, Industrial and Control Engineering, Shenyang, China, 1–3 April 2015; Atlantis Press: Amsterdam, The Netherlands, 2015; pp. 9–12. [Google Scholar]
- Rivetti, S.; Lanconelli, N.; Bertolini, M.; Nitrosi, A.; Burani, A. Characterization of a Clinical Unit for Digital Radiography Based on Irradiation Side Sampling Technology. Med. Phys. 2013, 40, 101902. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Nitrosi, A.; Rivetti, S.; Lanconelli, N.; Pattacini, P.; Ginocchi, V.; Iori, M. A Comparison of Digital Radiography Systems in Terms of Effective Detective Quantum Efficiency. Med. Phys. 2012, 39, 2617–2627. [Google Scholar] [CrossRef]
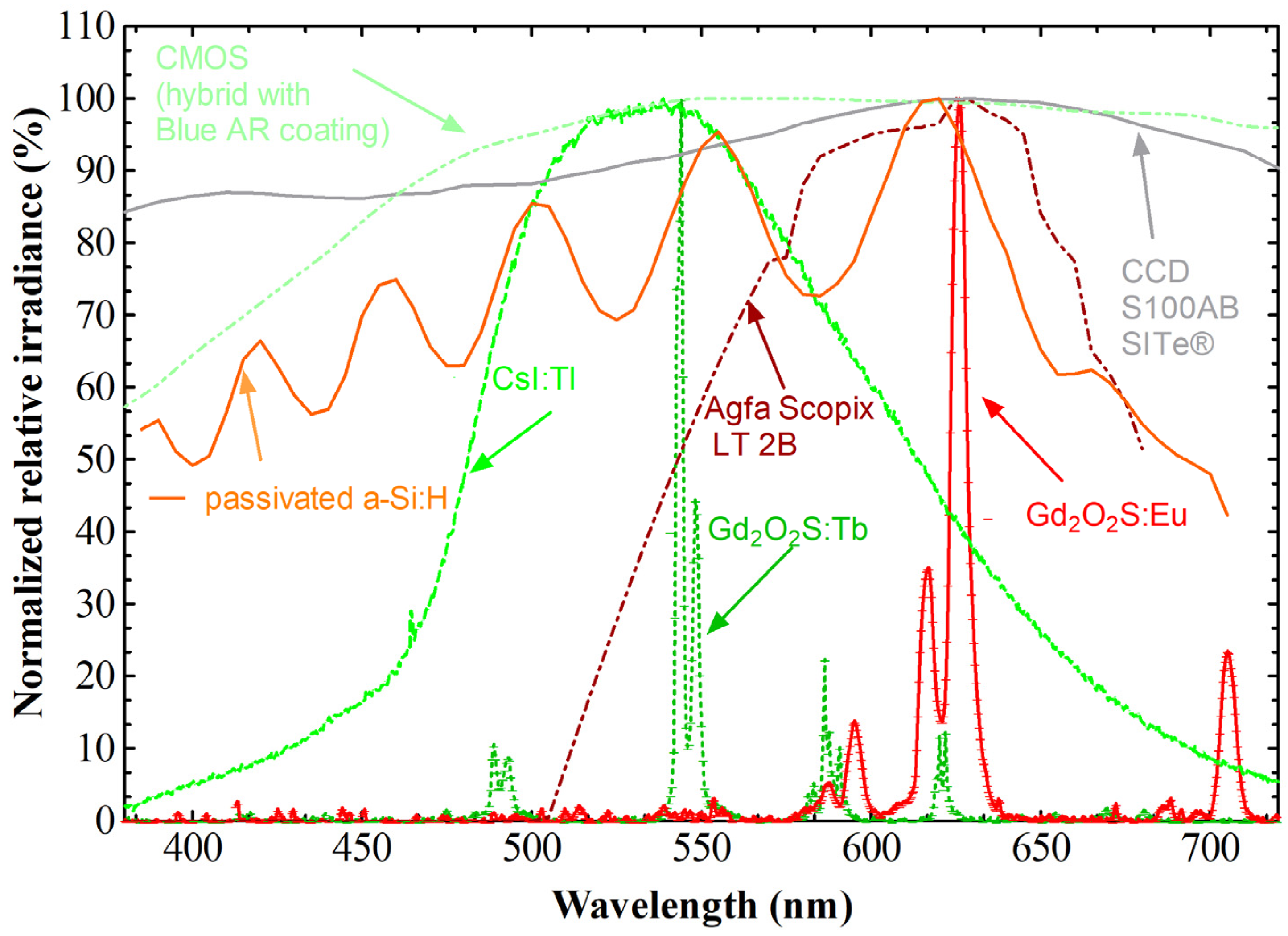

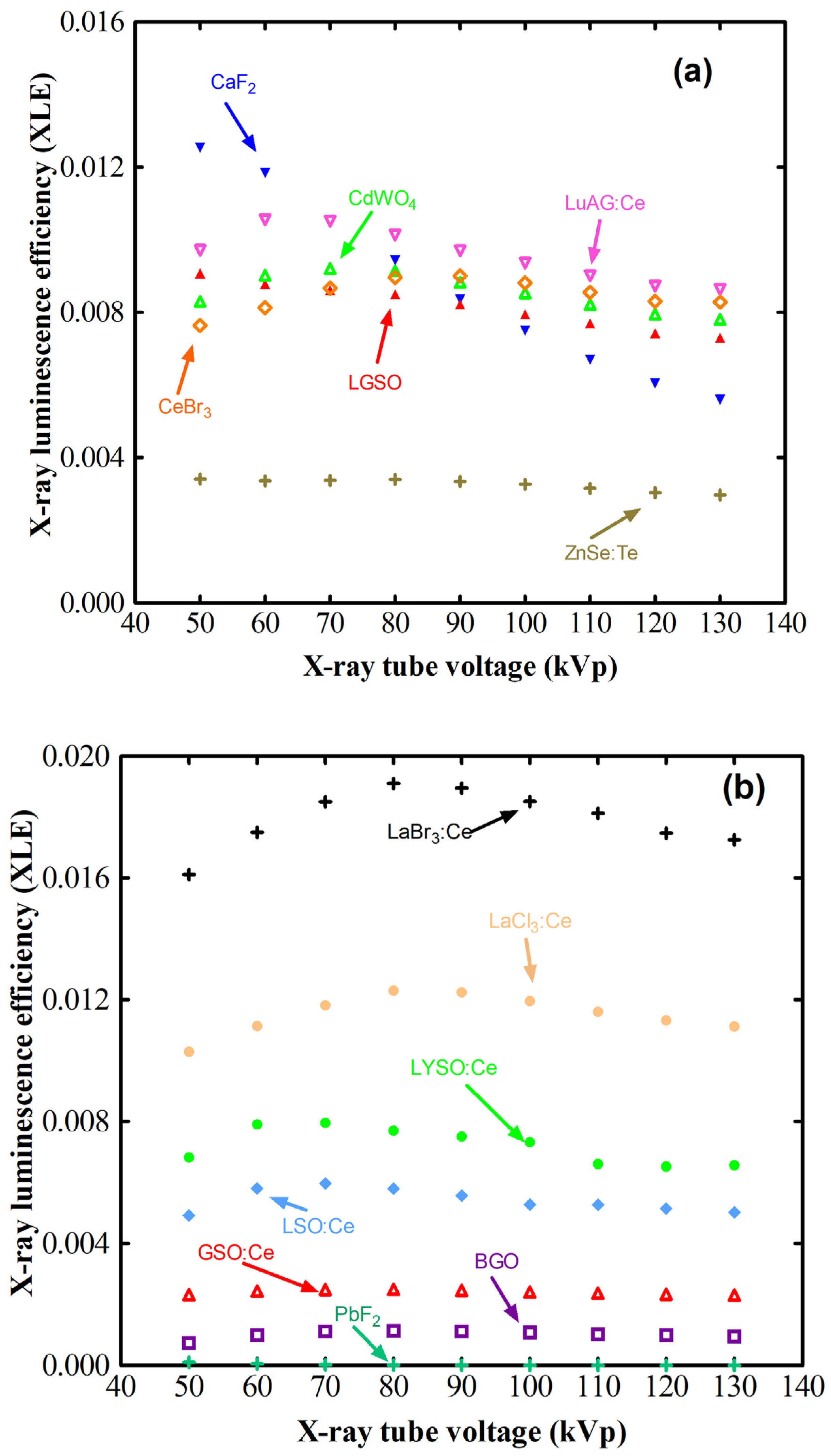

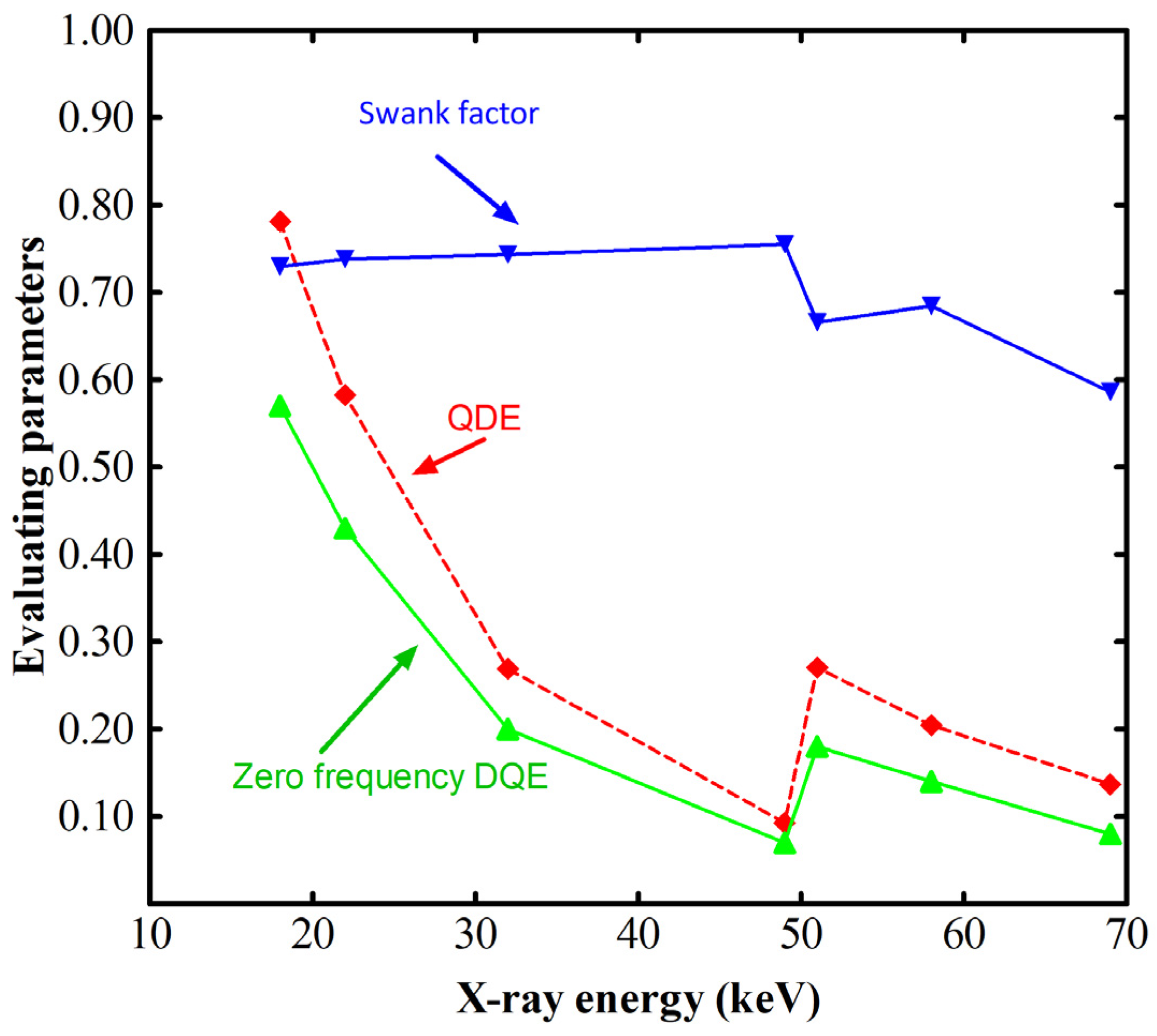
| Scintillator/Phosphor | (g/cm3) | LY (Light Photons per MeV) | Wavelength (nm) | Decay (ns) | Hygro | Afterglow | |
|---|---|---|---|---|---|---|---|
| CaWO4 | 6.1 | 89 | 20,000 | 420 | No | No | |
| Gd2O2S:Tb | 7.3 | 103 | 60,000 | 545 | 106 | No | No |
| CsI:Na | 4.5 | 38 | 40,000 | 420 | 630 | Yes | No |
| CsI:Tl | 4.51 | 38 | 54,000–66,000 | 550 | 1000 | Yes | Yes |
| CdWO4 | 7.9 | 134 | 28,000 | 495 | 2.15 × 103 | No | Slight |
| Gd2O2S:Pr, Ce, F | 7.3 | 103 | 35,000 | 510 | 4 × 103 | No | Slight |
| Gd2O2S:Pr (UFC) | 7.3 | 103 | 50,000 | 510 | 3 × 103 | No | Slight |
| NaI:Tl | 3.67 | 24.5 | 38,000 | 415 | 230 | Yes | No |
| Bi4Ge3O12(BGO) | 7.1 | 227 | 9000 | 480 | 300 | No | No |
| Lu2SiO5:Ce(LSO) | 7.4 | 143 | 26,000 | 420 | 40 | No | No |
| Gd2SiO5:Ce(GSO) | 6.7 | 84 | 8000 | 440 | 60 | No | No |
| YAlO3:Ce(YAP) | 5.5 | 7 | 21,000 | 350 | 30 | No | No |
| LaCl3:Ce | 3.86 | 23.2 | 46,000–50,000 | 330 | 24 (60%) | Yes | No |
| LaBr3:Ce | 5.03 | 25.6 | 61,000–70,000 | 358 | 16 | Yes | No |
| Optical Detectors | CaF2:Eu | CdWO4 | CeBr3 | ZnSe(Te) | LGSO:Ce | LuAG:Ce | LaBr3:Ce | LaCl3:Ce |
|---|---|---|---|---|---|---|---|---|
| CCD broadband AR coating | 0.94 | 0.97 | 0.76 | 0.91 | 0.94 | 0.97 | 0.65 | 0.22 |
| CCD infrared (IR) anti-reflection (AR) coating | 0.54 | 0.69 | 0.42 | 0.92 | 0.57 | 0.77 | 0.37 | 0.14 |
| CMOS hybrid with blue anti-reflection (AR) coating | 0.60 | 0.74 | 0.51 | 0.94 | 0.63 | 0.81 | 0.31 | 0.05 |
| Hybrid CMOS blue | 0.79 | 0.93 | 0.63 | 0.99 | 0.82 | 0.98 | 0.38 | 0.06 |
| CMOS (monolithic 0.25 μm) | 0.64 | 0.84 | 0.32 | 0.96 | 0.67 | 0.92 | 0.20 | 0.02 |
| a-Si:H passivated | 0.63 | 0.75 | 0.55 | 0.77 | 0.66 | 0.80 | 0.24 | 0.02 |
| a-Si:H_non-passivated | 0.92 | 0.97 | 0.84 | 0.85 | 0.93 | 0.99 | 0.38 | 0.03 |
| CCD with indium tin oxide (ITO) gates with microlenses | 0.68 | 0.78 | 0.58 | 0.96 | 0.70 | 0.83 | 0.44 | 0.12 |
| CCD with indium tin oxide (ITO) gates | 0.51 | 0.68 | 0.39 | 0.90 | 0.55 | 0.75 | 0.30 | 0.08 |
| CCD with polygates | 0.18 | 0.46 | 0.03 | 0.78 | 0.24 | 0.59 | 0.02 | 0.00 |
| CCD no poly-gate LoD | 0.34 | 0.66 | 0.19 | 0.87 | 0.43 | 0.82 | 0.03 | 0.00 |
| CCD with traditional poly gates | 0.34 | 0.70 | 0.20 | 0.88 | 0.46 | 0.87 | 0.03 | 0.00 |
| CMOS (photogate array 0.5 μm) | 0.26 | 0.60 | 0.14 | 0.91 | 0.37 | 0.76 | 0.20 | 0.02 |
| CMOS RadEye HR | 0.68 | 0.82 | 0.05 | 0.97 | 0.75 | 0.89 | 0.00 | 0.00 |
| GaAs photocathode | 0.95 | 0.96 | 0.94 | 0.99 | 0.95 | 0.97 | 0.94 | 0.93 |
| GaAsP phosphor photocathode | 0.52 | 0.76 | 0.35 | 0.79 | 0.58 | 0.84 | 0.34 | 0.27 |
| Extended photocathode (E-S20) | 0.94 | 0.85 | 0.95 | 0.60 | 0.94 | 0.78 | 0.94 | 0.83 |
| Si PM MicroFC-30035-SMT | 0.94 | 0.72 | 0.86 | 0.34 | 0.88 | 0.59 | 0.85 | 0.66 |
| Si PM MicroFB-30035-SMT | 0.92 | 0.66 | 0.78 | 0.30 | 0.85 | 0.52 | 0.76 | 0.55 |
| Si PM MicroFM-10035 | 0.61 | 0.88 | 0.34 | 0.67 | 0.70 | 0.89 | 0.09 | 0.00 |
| Si PM S10985-050C | 0.95 | 0.87 | 0.86 | 0.55 | 0.95 | 0.79 | 0.85 | 0.67 |
| Si PM S10362-11-025U | 0.96 | 0.85 | 0.86 | 0.54 | 0.94 | 0.77 | 0.85 | 0.67 |
| Si PM S10362-11-050U | 0.95 | 0.87 | 0.82 | 0.55 | 0.95 | 0.79 | 0.81 | 0.62 |
| Si PM S10362-11-100U | 0.96 | 0.86 | 0.88 | 0.52 | 0.95 | 0.78 | 0.86 | 0.65 |
| Flat panel PS-PMT H8500C-03 | 0.91 | 0.56 | 0.99 | 0.08 | 0.82 | 0.38 | 0.98 | 0.93 |
| Flat panel PS-PMT H8500D-03 | 0.78 | 0.43 | 0.95 | 0.05 | 0.69 | 0.30 | 0.95 | 0.99 |
| Flat panel PS-PMT H10966A | 0.79 | 0.43 | 0.96 | 0.05 | 0.70 | 0.29 | 0.96 | 0.99 |
| Flat panel PS-PMT H8500C | 0.86 | 0.53 | 0.97 | 0.07 | 0.79 | 0.37 | 0.96 | 0.91 |
| Bialkali photocathode | 0.78 | 0.45 | 0.95 | 0.06 | 0.70 | 0.31 | 0.95 | 0.94 |
| Multialkali photocathode | 0.81 | 0.64 | 0.97 | 0.36 | 0.79 | 0.58 | 0.97 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michail, C.; Liaparinos, P.; Kalyvas, N.; Kandarakis, I.; Fountos, G.; Valais, I. Phosphors and Scintillators in Biomedical Imaging. Crystals 2024, 14, 169. https://doi.org/10.3390/cryst14020169
Michail C, Liaparinos P, Kalyvas N, Kandarakis I, Fountos G, Valais I. Phosphors and Scintillators in Biomedical Imaging. Crystals. 2024; 14(2):169. https://doi.org/10.3390/cryst14020169
Chicago/Turabian StyleMichail, Christos, Panagiotis Liaparinos, Nektarios Kalyvas, Ioannis Kandarakis, George Fountos, and Ioannis Valais. 2024. "Phosphors and Scintillators in Biomedical Imaging" Crystals 14, no. 2: 169. https://doi.org/10.3390/cryst14020169
APA StyleMichail, C., Liaparinos, P., Kalyvas, N., Kandarakis, I., Fountos, G., & Valais, I. (2024). Phosphors and Scintillators in Biomedical Imaging. Crystals, 14(2), 169. https://doi.org/10.3390/cryst14020169











