Optimal Doping Concentrations of Nd3+ Ions in CYGA Laser Crystals
Abstract
:1. Introduction
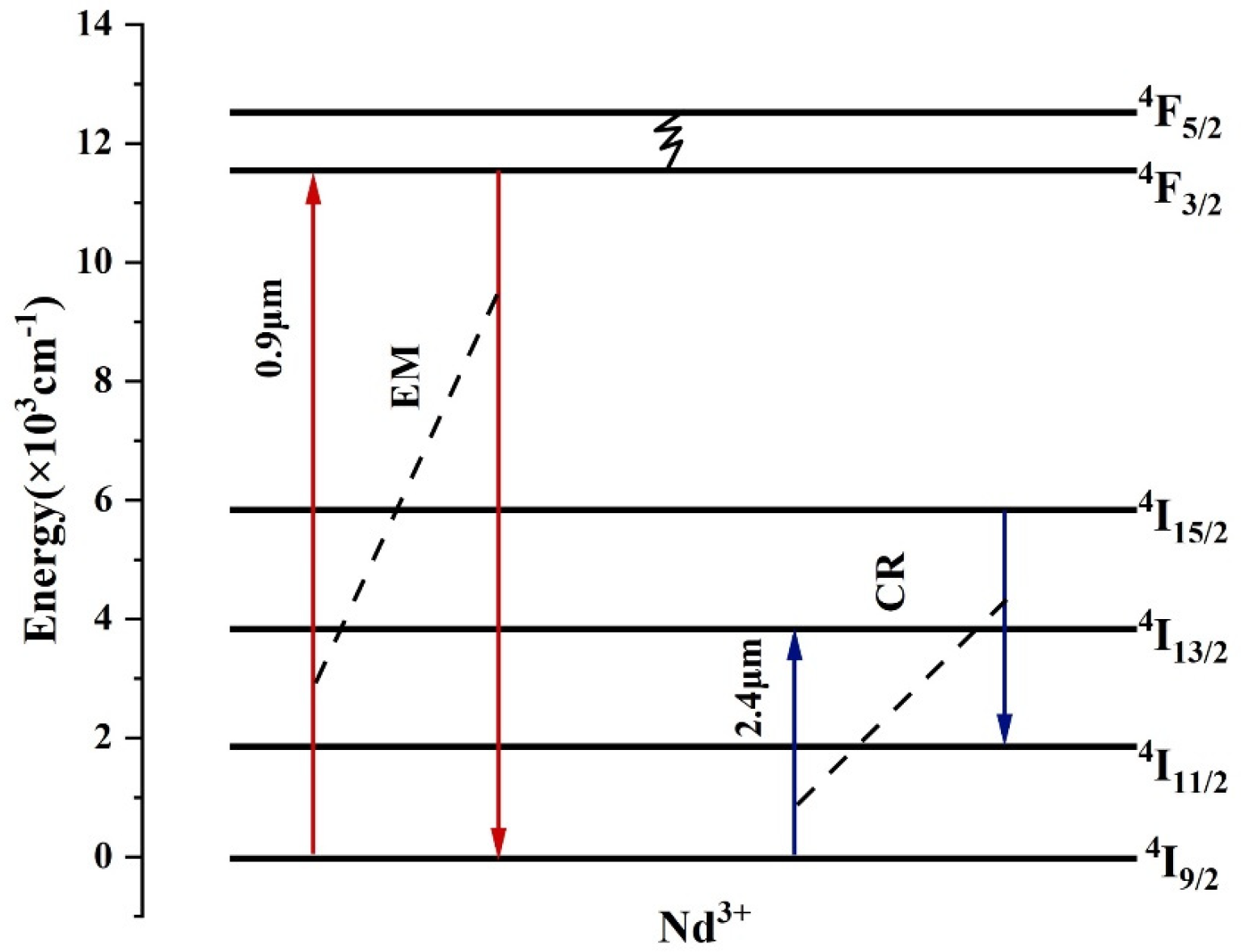
2. Theoretical Analysis
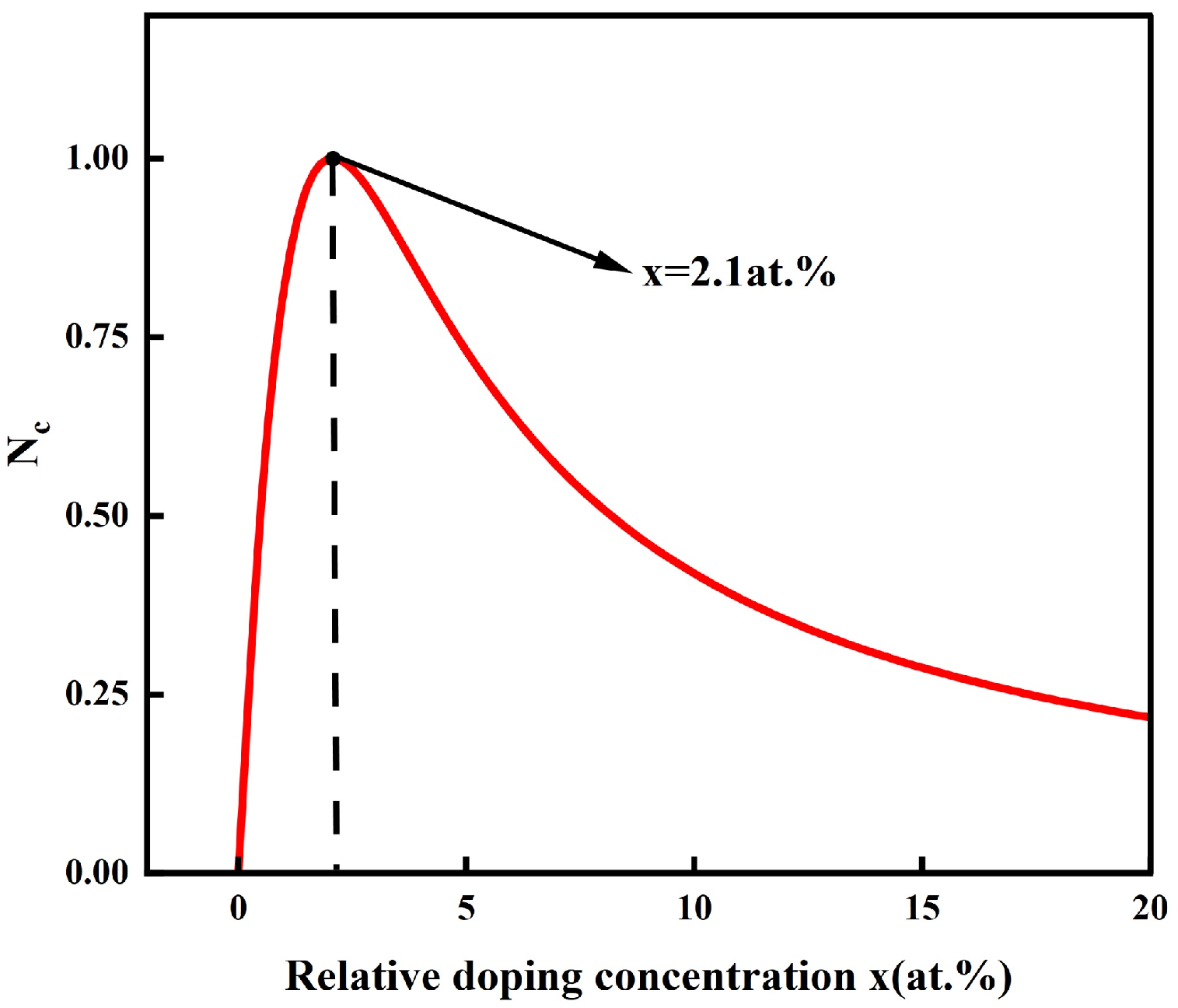
3. Results and Discussion
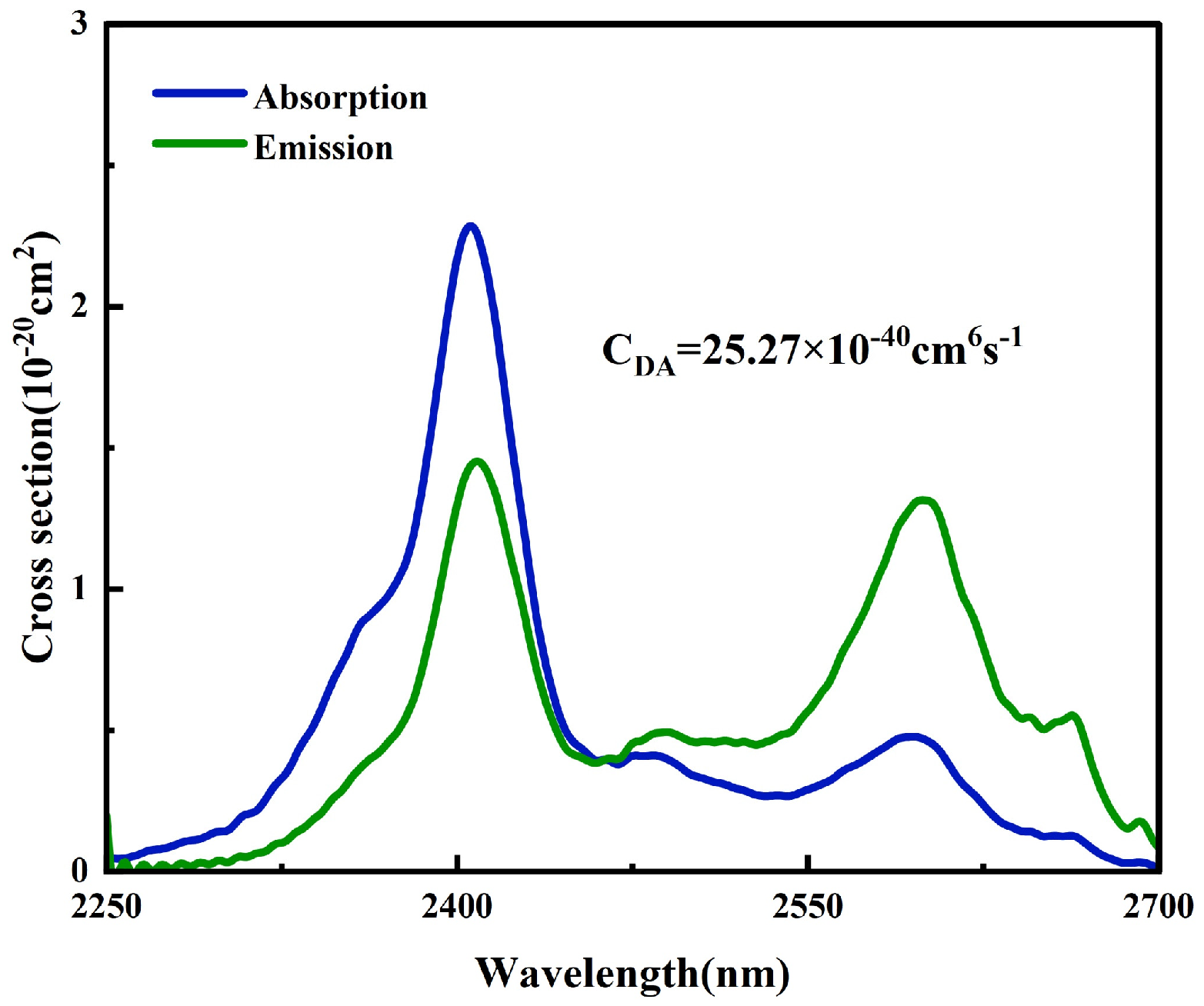
3.1. Spectral Overlap Model
3.2. Experiment Validation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Semwal, K.; Bhatt, S.C. Study of Nd3+ ion as a dopant in YAG and glass laser. Int. J. Phys. 2013, 1, 15–21. [Google Scholar]
- Reddy, C.M.; Vijaya, N.; Raju, B.D.P. NIR fluorescence studies of neodymium ions doped sodium fluoroborate glasses for 1.06 μm laser applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, S.A.; Dantas, N.O.; Serqueira, E.O.; Ayta, W.E.F.; Andrade, A.A.; Filadelpho, M.C.; Sampaio, J.A.; Bell, M.J.V.; Pereira-da-Silva, M.A. Eu3+ photoluminescence enhancement due to thermal energy transfer in Eu2O3-doped SiO2–B2O3–PbO2 glasses system. J. Lumin. 2011, 131, 850–855. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, B.J.; Tian, B.J.; Hua, R.N.; Sun, J.S.; Cheng, L.H.; Zhong, H.Y.; Li, X.P.; Zhang, J.S.; Zheng, Y.F.; et al. Concentration-dependent luminescence and energy transfer of flower-like Y2 (MoO4)3: Dy3+ phosphor. J. Alloys Compd. 2011, 509, 6096–6101. [Google Scholar]
- Trupke, T.; Shalav, A.; Richards, B.S.; Würfel, P.; Green, M.A. Efficiency enhancement of solar cells by luminescent up-conversion of sunlight. Sol. Energy Mater. Sol. Cells 2006, 90, 3327–3338. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, M.; Zhang, P.X.; Yin, H.; Zhu, S.Q.; LI, Z.; Chen, Z.Q. Passively Q-switched Nd: GYAP laser at 1.3 μm with bismuthene nanosheets as a saturable absorber. Infrared Phys. Technol. 2022, 121, 104023. [Google Scholar] [CrossRef]
- Palatnikov, M.N.; Biryukova, I.V.; Sidorov, N.V.; Denisov, A.V.; Kalinnikov, V.T.; Smith, P.G.R.; Shur, V.Y. Growth and concentration dependencies of rare earth doped lithium niobate single crystals. J. Cryst. Growth 2006, 291, 390–397. [Google Scholar] [CrossRef]
- Cerrato, E.; Gionco, C.; Berruti, I.; Sordello, F.; Calza, P.; Paganini, M.C. Rare earth ions doped ZnO: Synthesis, characterization and preliminary photoactivity assessment. J. Solid State Chem. 2018, 264, 42–47. [Google Scholar] [CrossRef]
- Ma, F.K.; Zhang, P.X.; Su, L.B.; Yin, H.; Li, Z.; Lv, Q.T.; Chen, Z.Q. The host driven local structures modulation towards broadband photoluminescence in neodymium-doped fluorite crystal. Opt. Mater. 2021, 119, 111322. [Google Scholar]
- Duan, Y.M.; Zhu, H.Y.; Xu, C.W.; Yang, H.; Luo, D.W.; Lin, H.; Zhang, J.; Tang, D.Y. Comparison of the 1319 and 1338 nm dual-wavelength emission of neodymium-doped yttrium aluminum garnet ceramic and crystal lasers. Appl. Phys. Express 2012, 6, 012701. [Google Scholar] [CrossRef]
- Zhang, P.X.; Wang, R.; Huang, X.B.; Li, Z.; Yin, H.; Zhu, S.Q.; Chen, Z.Q.; Hang, Y. Sensitization and deactivation effects to Er3+ at ~2.7 μm mid-infrared emission by Nd3+ ions in Gd0.1Y0.9AlO3 crystal. J. Alloys Compd. 2018, 750, 147–152. [Google Scholar] [CrossRef]
- Zhou, H.Q.; Zhu, S.Q.; Li, Z.; Yin, H.; Zhang, P.X.; Chen, Z.Q.; Fu, S.H.; Zhang, Q.M.; Lv, Q.T. Investigation on 1.0 and 1.3 µm laser performance of Nd3+: GYAP crystal. Opt. Laser Technol. 2019, 119, 105601. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, Q.D.; Zhang, P.X.; Liao, J.Y.; Hong, H.; Chen, H.L.; Yin, H.; Hang, Y.; Li, Z.; Chen, Z.Q. Fabrication of Sb2O3 by an improved chemical reaction assisted vertical micro sublimation method and its saturable absorber performance. Opt. Mater. Express 2022, 12, 1337–1346. [Google Scholar] [CrossRef]
- Li, B.Z.; Chen, Q.D.; Zhang, P.X.; Tian, R.F.; Zhang, L.; Sai, Q.L.; Wang, B.; Pan, M.Y.; Liu, Y.C.; Xia, C.T.; et al. β-Ga2O3 Used as a Saturable Sbsorber to Realize Passively Q-Switched Laser Output. Crystals 2021, 11, 1501. [Google Scholar]
- Chen, Y.; Chen, Q.D.; Niu, X.C.; Zheng, W.B.; Zhang, P.X.; Li, Z.; Chen, Z.Q. Growth, spectroscopy properties and laser operation of a novel single crystal fiber: Nd3+-doped CaY0.9Gd0.1AlO4. Infrared Phys. Technol. 2023, 134, 104917. [Google Scholar] [CrossRef]
- Ikesue, A.; Kamata, K.; Yoshida, K. Effects of neodymium concentration on optical characteristics of polycrystalline Nd: YAG laser materials. J. Am. Ceram. Soc. 1996, 79, 1921–1926. [Google Scholar] [CrossRef]
- Denker, B.I.; Osiko, V.V.; Pashinin, P.P.; Prokhorov, A.M. Concentrated neodymium laser glasses. Sov. J. Quantum Electron. 1981, 11, 289. [Google Scholar] [CrossRef]
- Caird, J.A.; Ramponi, A.J.; Staver, P.R. Quantum efficiency and excited-state relaxation dynamics in neodymium-doped phosphate laser glasses. JOSA B 1991, 8, 1391–1403. [Google Scholar] [CrossRef]
- Fernandez, J.; Oleaga, A.; Azkargorta, J.; Iparraguirre, I.; Balda, R.; Voda, M.; Kaminskii, A.A. Nd3+ laser spectral dynamics in CaF2–YF3–NdF3 crystals. Opt. Mater. 1999, 13, 9–16. [Google Scholar] [CrossRef]
- Yu, H.H.; Zhang, H.J.; Wang, Z.P.; Wang, J.Y.; Yu, Y.G.; Shi, Z.B.; Zhang, X.Y.; Jiang, M.H. Continuous-wave and passively Q-switched laser performance with a disordered Nd: CLNGG crystal. Opt. Express 2009, 17, 19015–19020. [Google Scholar]
- Wang, Y.G.; Qu, Z.S.; Liu, J.; Tsang, Y.H. Graphene oxide absorbers for watt-level high-power passive mode-locked Nd: GdVO4 laser operating at 1 μm. J. Light. Technol. 2012, 30, 3259–3262. [Google Scholar] [CrossRef]
- Noom, D.W.E.; Witte, S.; Morgenweg, J.; Altmann, R.K.; Eikema, K.S.E. High-energy, high-repetition-rate picosecond pulses from a quasi-CW diode-pumped Nd: YAG system. Opt. Lett. 2013, 38, 3021–3023. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Kausas, A.; Taira, T. >MW peak power at 266 nm, low jitter kHz repetition rate from intense pumped microlaser. Opt. Express 2016, 24, 28748–28760. [Google Scholar]
- Chen, H.L.; Zhang, P.X.; Song, J.W.; Yin, H.; Hang, Y.; Yang, Q.G.; Li, Z.; Chen, Z.Q. Spectral broadening of a mixed Nd: CYGA crystal with tunable laser operation beyond 1100 nm. Opt. Express 2022, 30, 21943–21951. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Q.; Zhang, P.X.; Zhou, H.G.; Zhu, S.Q.; Zhang, Y.Q.; Li, Z.; Yin, H.; Chen, Z.Q.; Hang, Y. Crystal growth, optical properties and laser performance of new mixed Nd3+ doped Gd0.1Y0.9AlO3 crystal. J. Alloys Compd. 2019, 789, 664–669. [Google Scholar]
- Avanesov, A.G.; Denker, B.I.; Osiko, V.V.; Pirumov, S.S.; Sakun, V.P.; Smirnov, V.A.; Shcherbakov, I.A. Kinetics of nonradiative relaxation from the upper active level of neodymium in a Y3Al5O12 crystal. Sov. J. Quantum Electron. 1982, 12, 744. [Google Scholar] [CrossRef]
- Kaiser, W.; Garrett, C.G.B. Two-photon excitation in CaF2: Eu2+. Phys. Rev. Lett. 1961, 7, 229. [Google Scholar]
- Inokuti, M.; Hirayama, F. Influence of energy transfer by the exchange mechanism on donor luminescence. J. Chem. Phys. 1965, 43, 1978–1989. [Google Scholar] [CrossRef]
- Artamonova, M.V.; Briskina, C.M.; Burshtein, A.I.; Zusman, L.D.; Skleznev, A.G. Time variation of Nd3+ ion luminescence and an estimation of electron excitation migration along the ions in glass. Sov. Phys. JETP 1972, 35, 457–461. [Google Scholar]
- Tian, C.; Chen, X.; Yu, S.B. Concentration dependence of spectroscopic properties and energy transfer analysis in Nd3+ doped bismuth silicate glasses. Solid State Sci. 2015, 48, 171–176. [Google Scholar] [CrossRef]
- Sontakke, A.D.; Biswas, K.; Mandal, A.K.; Annapurna, K. Concentration quenched luminescence and energy transfer analysis of Nd3+ ion doped Ba-Al-metaphosphate laser glasses. Appl. Phys. B 2010, 101, 235–244. [Google Scholar] [CrossRef]
- Privis, Y.S.; Smirnov, V.A.; Shcherbakov, I.A. Determination of the optimal concentrations of active particles in laser media. Sov. J. Quantum Electron. 1983, 13, 868. [Google Scholar] [CrossRef]
- Matta, F.; Reichel, A. Uniform computation of the error function and other related functions. Math. Comput. 1971, 25, 339–344. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.Y.; Li, D.H.; Wei, Z.Y.; Zhang, Z.G.; Eichle, H.J.; Strohmaier, S. Characteristics of Nd: YGG laser operating at 4F3/2–4I9/2. Chin. Phys. Lett. 2008, 25, 3988. [Google Scholar]
- Braud, A.; Girard, S.; Doualan, J.L.; Moncorge, R. Spectroscopy and fluorescence dynamics of (Tm3+, Tb3+) and (Tm3+, Eu3+) doped LiYF4 single crystals for 1.5-μm laser operation. IEEE J. Quantum Electron. 1998, 34, 2246–2255. [Google Scholar] [CrossRef]
- Lupei, V.; Lupei, A.; Georgescu, S.; Ionescu, C. Energy transfer between Nd3+ ions in YAG. Opt. Commun. 1986, 60, 59–63. [Google Scholar] [CrossRef]
- Lupei, A.; Lupei, V.; Georgescu, S.; Yen, W.M. Mechanisms of energy transfer between Nd3+ Ions in YAG. J. Lumin. 1987, 39, 35–43. [Google Scholar] [CrossRef]
- Voron’Ko, Y.K.; Mamedov, T.G.; Osiko, V.V.; Prokhorov, A.M.; Sakun, V.P.; Shcherbakov, I.A. Nature of nonradiative excitation-energy relaxation in condensed media with high activator concentrations. Sov. J. Exp. Theor. Phys. 1976, 44, 251. [Google Scholar]
- Glushkov, N.A. Kinetics of nonstationary migration-accelerated energy transfer in a solid body doped with rare earth and transition-metal ions. Opt. Spectrosc. 2014, 116, 700–705. [Google Scholar] [CrossRef]
- Miniscalco, W.J.; Quimby, R.S. General procedure for the analysis of Er3+ cross sections. Opt. Lett. 1991, 16, 258–260. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, J.J.; Yu, C.L.; Hu, L.L. A method for emission cross section determination of Tm3+ at 2.0 μm emission. J. Appl. Phys. 2010, 108, 103117. [Google Scholar] [CrossRef]
- Tian, F.; Chen, C.; Liu, Y.; Liu, Q.; Ivanov, M.; Wang, Q.Q.; Jiang, N.; Chen, H.H.; Yang, Z.X.; Xie, T.F.; et al. Fabrication of Nd: YAG transparent ceramics from co-precipitated powders by vacuum pre-sintering and HIP post-treatment. Opt. Mater. 2020, 101, 109728. [Google Scholar] [CrossRef]
- Kassab, L.R.P.; Fukumoto, M.E.; Gomes, L. Energy transfer in PbO-Bi2O3-Ga2O3 glasses codoped with Yb3+ and Er3+. JOSA B 2005, 22, 1255–1259. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Tan, J.; Li, Z.; Zhang, P.; Chen, Z. Optimal Doping Concentrations of Nd3+ Ions in CYGA Laser Crystals. Crystals 2024, 14, 168. https://doi.org/10.3390/cryst14020168
Lu S, Tan J, Li Z, Zhang P, Chen Z. Optimal Doping Concentrations of Nd3+ Ions in CYGA Laser Crystals. Crystals. 2024; 14(2):168. https://doi.org/10.3390/cryst14020168
Chicago/Turabian StyleLu, Siliang, Juncheng Tan, Zhen Li, Peixiong Zhang, and Zhenqiang Chen. 2024. "Optimal Doping Concentrations of Nd3+ Ions in CYGA Laser Crystals" Crystals 14, no. 2: 168. https://doi.org/10.3390/cryst14020168




