CaH2-Assisted Molten Salt Synthesis of Zinc-Rich Intermetallic Compounds of RhZn13 and Pt3Zn10 for Catalytic Selective Hydrogenation Application
Abstract
1. Introduction
| Active Alloys | Hydrogenation Reactions | Ref. |
|---|---|---|
| RhZn | Selective hydrogenation of nitroarene | [19] |
| Selective hydrogenation of dienes to monoenes | [20] | |
| PtZn | Selective hydrogenation of crotonaldehyde | [21,22,23] |
| Selective hydrogenation of 4-nitrophenylacetylene | [24,25] | |
| Selective hydrogenation of halonitrobenzene to haloaniline | [26] | |
| Hydrogenation of diphenylacetylene | [27] | |
| PdZn | Semi-hydrogenation of Alkyne | [28,29,30] |
| Semi-hydrogenation of acetylene | [31,32,33,34,35,36,37,38,39,40,41,42,43] | |
| Semi-hydrogenation of alkynols | [44,45,46,47] | |
| Hydrogenation of 1-Butene and 1,3-Butadiene Mixtures | [48,49] | |
| Hydrogenation of 2-methyl-3-butyn-2-ol | [50,51,52] | |
| Selective hydrogenation of nitrostyrene to aminostyrene | [53] | |
| Hydrogenation of cinnamaldehyde to hydrocinnamyl alcohol | [54] | |
| Selective hydrogenative rearrangement of furan compounds | [55] |
2. Materials and Methods
3. Results and Discussion
3.1. Preparation of Zinc-Rich Intermetallic Compounds
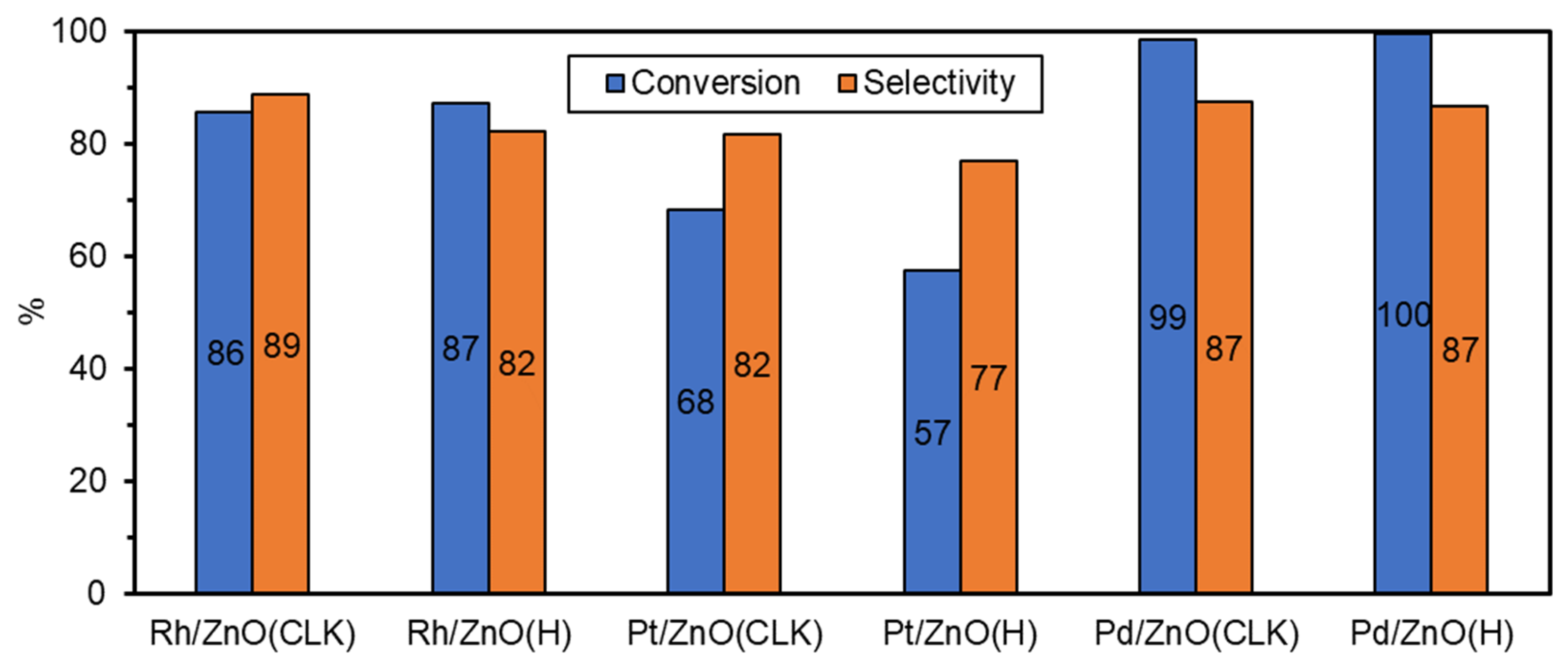
3.2. Catalytic Tests with the Prepared Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartholomew, C.H.; Farrauto, R.J. Hydrogenation and Dehydrogenation of Organic Compounds. In Fundamentals of Industrial Catalytic Processes, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 487–559. [Google Scholar]
- Soma-Noto, Y.; Sachtler, W.M.H. Infrared spectra of carbon monoxide adsorbed on supported palladium and palladium-silver alloys. J. Catal. 1974, 32, 315–324. [Google Scholar] [CrossRef]
- Boudart, M. Catalysis by Supported Metals. Adv. Catal. 1969, 20, 153–166. [Google Scholar] [CrossRef]
- Vargheese, V.; Ghampson, I.T.; Yun, G.-N.; Kobayashi, Y.; Takagaki, A.; Oyama, S.T. A New One-Pot Sequential Reduction-Deposition Method for the synthesis of Silica-supported NiPt and CuPt Bimetallic Catalysts. Appl. Catal. A 2020, 591, 117371. [Google Scholar] [CrossRef]
- Liao, F.; Lo, T.W.B.; Tsang, S.C.E. Recent Developments in Palladium-Based Bimetallic Catalysts. ChemCatChem 2015, 7, 1998–2014. [Google Scholar] [CrossRef]
- Tsai, A.P.; Kameoka, S.; Nozawa, K.; Shimoda, M.; Ishii, Y. Intermetallic: A Pseudoelement for Catalysis. Acc. Chem. Res. 2017, 50, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Yang, J.; Wen, M.; Wu, Q. Nanoalloy Materials for Chemical Catalysis. Adv. Mater. 2018, 30, 1705698. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, C.; Yun, Q.; Han, S.; Lv, Y.; Lu, Q.; Chen, J. Recent advances of Rh-based intermetallic nanomaterials for catalytic applications. Chin. Chem. Lett. 2023, 34, 108515. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, Z.; Feng, Y.; Xie, S.; Liu, Y.; Dai, H.; Deng, J. In situ molten salt derived iron oxide supported platinum catalyst with high catalytic performance for o-xylene elimination. Catal. Today 2020, 351, 30–36. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Lacerda, R.A.; Leão, J.P.B.; Scholten, J.D.; Neto, B.A.D.; Suarez, P.A.Z. In situ generated palladium nanoparticles in imidazolium-based ionic liquids: A versatile medium for an efficient and selective partial biodiesel hydrogenation. Catal. Sci. Technol. 2011, 1, 480–488. [Google Scholar] [CrossRef]
- Lacarbonara, G.; Chini, S.; Ratso, S.; Kruusenberg, I.; Arbizzani, C. A MnOx–graphitic carbon composite from CO2 for sustainable Li-ion battery anodes. Mater. Adv. 2022, 3, 7087–7097. [Google Scholar] [CrossRef]
- Najafli, E.; Ratso, S.; Ivanov, Y.P.; Gatalo, M.; Pavko, L.; Yörük, C.R.; Walke, P.; Divitini, G.; Hodnik, N.; Kruusenberg, I. Sustainable CO2-Derived Nanoscale Carbon Support to a Platinum Catalyst for Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2023, 6, 5772–5780. [Google Scholar] [CrossRef]
- Kobayashi, Y. Synthesis of Porous Ni3Al Intermetallic Nano-compounds in a Molten LiCl with Assistance of CaH2 as a Structure-controlling Agent. Chem. Lett. 2019, 48, 1496–1499. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tada, S.; Mizoguchi, H. Chemical route to prepare nickel supported on intermetallic Ti6Si7Ni16 nanoparticles catalyzing CO methanation. Nanoscale 2021, 13, 16533–16542. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Yokoyama, S.; Shoji, R. Molten salt synthesis of intermetallic compound TiNi nanopowder passivated by TiOx shell prepared from NiTiO3 for catalytic hydrogenation. Materials 2022, 15, 8536. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tada, S.; Kondo, M.; Fujiwara, K.; Mizoguchi, H. Intermetallic YIr2 nanoparticles with negatively charged Ir active sites for catalytic hydrogenation of cyclohexanone to cyclohexanol. Catal. Sci. Technol. 2022, 12, 3088–3093. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tada, S.; Kondo, M.; Fujiwara, K.; Mizoguchi, H. Superior catalytic performance of intermetallic CaPt2 nanoparticles supported on titanium group oxides in hydrogenation of ketones to alcohols. Chem. Commun. 2022, 58, 4795–4798. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sohmiya, M.; Tada, S.; Kikuchi, R. Low-temperature Synthesis of Single Phase Intermetallic NiZn Bulk Nanopowder in Molten LiCl–KCl with CaH2 Reducing Agent. J. Japan Pet. Inst. 2020, 63, 380–387. [Google Scholar] [CrossRef]
- Furukawa, S.; Takahashi, K.; Komatsu, T. Well-structured bimetallic surface capable of molecular recognition for chemoselective nitroarene hydrogenation. Chem. Sci. 2016, 7, 4476–4484. [Google Scholar] [CrossRef]
- Miyazaki, M.; Furukawa, S.; Komatsu, T. Regio- and chemoselective hydrogenation of dienes to monoenes governed by a well-structured bimetallic surface. J. Am. Chem. Soc. 2017, 139, 18231–18239. [Google Scholar] [CrossRef]
- Silvestre-Albero, J.; Serrano-Ruiz, J.C.; Sepúlveda-Escribano, A.; Rodríguez-Reinoso, F. Modification of the catalytic behavior of platinum by zinc in crotonaldehyde hydrogenation and iso-butane dehydrogenation. Appl. Catal. A Gen. 2005, 292, 244–251. [Google Scholar] [CrossRef]
- Silvestre-Albero, J.; Coloma, F.; Sepúlveda-Escribano, A.; Rodríguez-Reinoso, F. Effect of the presence of chlorine in bimetallic PtZn/CeO2 catalysts for the vapor-phase hydrogenation of crotonaldehyde. Appl. Catal. A Gen. 2006, 304, 159–167. [Google Scholar] [CrossRef][Green Version]
- Galloway, E.; Armbrüster, M.; Kovnir, K.; Tikhov, M.S.; Lambert, R.M. Bromine-promoted PtZn is very effective for the chemoselective hydrogenation of crotonaldehyde. J. Catal. 2009, 261, 60–65. [Google Scholar] [CrossRef]
- Han, A.; Zhang, J.; Sun, W.; Chen, W.; Zhang, S.; Han, Y.; Feng, Q.; Zheng, L.; Gu, L.; Chen, C.; et al. Isolating contiguous Pt atoms and forming Pt-Zn intermetallic nanoparticles to regulate selectivity in 4-nitrophenylacetylene hydrogenation. Nat. Comm. 2019, 10, 3787. [Google Scholar] [CrossRef]
- Jin, Y.; Ren, G.; Feng, Y.; Geng, S.; Li, L.; Zhu, X.; Guo, J.; Shao, Q.; Xu, Y.; Huang, X.; et al. A top-down strategy to realize the synthesis of small-sized L10-platinum-based intermetallic compounds for selective hydrogenation. Nano Res. 2022, 15, 9631–9638. [Google Scholar] [CrossRef]
- Iihama, S.; Furukawa, S.; Komatsu, T. Efficient catalytic system for chemoselective hydrogenation of halonitrobenzene to haloaniline using PtZn intermetallic compound. ACS Catal. 2016, 6, 742–746. [Google Scholar] [CrossRef]
- Smirnova, N.S.; Khramov, E.V.; Stolarov, I.P.; Yakushev, I.A.; Baeva, G.N.; Bragina, G.O.; Belova, E.V.; Ishchenko, A.V.; Popova, A.S.; Zubavichus, Y.V.; et al. Nanostructured PtZn intermetallic compound: Controlled formation from PtZn(CH3COO)4 molecular precursor and tests of catalytic properties. Intermetallics 2021, 132, 107160. [Google Scholar] [CrossRef]
- Tew, M.W.; Emerich, H.; van Bokhoven, J.A. Formation and Characterization of PdZn Alloy: A Very Selective Catalyst for Alkyne Semihydrogenation. J. Phys. Chem. C 2011, 115, 8457–8465. [Google Scholar] [CrossRef]
- Mashkovsky, I.S.; Markov, P.V.; Bragina, G.O.; Rassolov, A.V.; Baeva, G.N.; Stakheev, A.Y. Intermetallic Pd1–Zn1 nanoparticles in the selective liquid-phase hydrogenation of substituted alkynes. Kinet. Catal. 2017, 58, 480–491. [Google Scholar] [CrossRef]
- Mashkovsky, I.S.; Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Rassolov, A.V.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Stakheev, A.Y. PdZn/α-Al2O3 catalyst for liquid-phase alkyne hydrogenation: Effect of the solid-state alloy transformation into intermetallics. Mendeleev Commun. 2018, 28, 152–154. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, X.; Wang, A.; Miao, S.; Liu, X.; Pan, X.; Su, Y.; Li, L.; Tan, Y.; Zhang, T. Pd/ZnO catalysts with different origins for high chemoselectivity in acetylene semi-hydrogenation. Chin. J. Catal. 2016, 37, 692–699. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, X.; Li, L.; Liu, X.; Huang, Y.; Pan, X.; Wang, A.; Li, J.; Zhang, T. PdZn intermetallic nanostructure with Pd–Zn–Pd ensembles for highly active and chemoselective semi-hydrogenation of acetylene. ACS Catal. 2016, 6, 1054–1061. [Google Scholar] [CrossRef]
- Glyzdova, D.V.; Smirnova, N.S.; Leont’eva, N.N.; Gerasimov, E.Y.; Prosvirin, I.P.; Vershinin, V.I.; Shlyapina, D.A.; Tsyrul’nikov, P.G. Synthesis and characterization of Sibunit-supported Pd–Ga, Pd–Zn, and Pd–Ag catalysts for liquid-phase acetylene hydrogenation. Kinet. Catal. 2017, 58, 140–146. [Google Scholar] [CrossRef]
- Glyzdova, D.V.; Smirnova, N.S.; Temerev, V.L.; Khramov, E.V.; Gulyaeva, T.I.; Trenikhin, M.V.; Shlyapin, D.A.; Tsyrul’nikov, P.G. Study of the Influence Exerted by Zinc Additive on the Structure and Catalytic Properties of Pd/Al2O3 Catalysts for Liquid-Phase Hydrogenation of Acetylene. Russ. J. Appl. Chem. 2017, 90, 1908–1917. [Google Scholar] [CrossRef]
- Meunier, F.; Maffre, M.; Schuurman, Y.; Colussi, S.; Trovarelli, A. Acetylene semi-hydrogenation over Pd-Zn/CeO2: Relevance of CO adsorption and methanation as descriptors of selectivity. Catal. Comm. 2018, 105, 52–55. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, S.; Liu, S.; Chen, C.; Chen, W.; Zhu, W.; Liang, C.; Cheong, W.C.; Wang, Y.; Yu, Y.; et al. MOF-Confined Sub-2 nm Atomically Ordered Intermetallic PdZn Nanoparticles as High-Performance Catalysts for Selective Hydrogenation of Acetylene. Adv. Mater. 2018, 30, 1801878. [Google Scholar] [CrossRef] [PubMed]
- Glyzdova, D.V.; Vedyagin, A.A.; Tsapina, A.M.; Kaichev, V.V.; Trigub, A.L.; Trenikhin, M.V.; Shlyapin, D.A.; Tsyrulnikov, P.G.; Lavrenov, A.V. A study on structural features of bimetallic Pd-M/C (M: Zn, Ga, Ag) catalysts for liquid-phase selective hydrogenation of acetylene. Appl. Catal. A Gen. 2018, 563, 18–27. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Y.; Long, J.; Xia, L.; Li, D.; Xiao, J.; Liu, H. PdZn alloy nanoparticles encapsulated within a few layers of graphene for efficient semi-hydrogenation of acetylene. Chem. Commun. 2019, 55, 14693–14696. [Google Scholar] [CrossRef]
- Glyzdova, D.V.; Afonasenko, T.N.; Khramov, E.V.; Leont’eva, N.N.; Trenikhin, M.V.; Prosvirin, I.P.; Bukhtiyarov, A.V.; Shlyapin, D.A. Zinc Addition Influence on the Properties of Pd/Sibunit Catalyst in Selective Acetylene Hydrogenation. Top. Catal. 2020, 63, 139–151. [Google Scholar] [CrossRef]
- Glyzdova, D.V.; Afonasenko, T.N.; Temerev, V.L.; Shlyapin, D.A. Acetylene Hydrogenation on Pd–Zn/Sibunit Catalyst: Effect of Solvent and Carbon Monoxide. Pet. Chem. 2021, 61, 490–497. [Google Scholar] [CrossRef]
- Cao, X.; Tong, R.; Tang, S.; Jang, B.W.-L.; Mirjalili, A.; Li, J.; Guo, X.; Zhang, J.; Hu, J.; Meng, X. Design of Pd–Zn Bimetal MOF Nanosheets and MOF-Derived Pd3.9Zn6.1/CNS Catalyst for Selective Hydrogenation of Acetylene under Simulated Front-End Conditions. Molecules 2022, 27, 5736. [Google Scholar] [CrossRef]
- Glyzdova, D.V.; Afonasenko, T.N.; Khramov, E.V.; Trenikhin, M.V.; Prosvirin, I.P.; Shlyapin, D.A. Effect of Reductive Treatment Duration on the Structure and Properties of Pd–Zn/C Catalysts for Liquid-Phase Acetylene Hydrogenation. ChemCatChem 2022, 14, e202200893. [Google Scholar] [CrossRef]
- Tiwari, G.; Sharma, G.; Verma, R.; Gakhad, P.; Singh, A.K.; Polshettiwar, V.; Jagirdar, B.R. Acetylene Semi-Hydrogenation at Room Temperature over Pd–Zn Nanocatalyst. Chem. Eur. J. 2023, 29, e202301932. [Google Scholar] [CrossRef]
- Shen, L.; Mao, S.; Li, J.; Li, M.; Chen, P.; Li, H.; Chen, Z.; Wang, Y. PdZn intermetallic on a CN@ZnO hybrid as an efficient catalyst for the semihydrogenation of alkynols. J. Catal. 2017, 350, 13–20. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; Wang, X.-B.; Li, W.-Y.; Liang, C. Intermetallic PdZn nanoparticles catalyze the continuous-flow hydrogenation of alkynols to cis-enols. Comm. Chem. 2021, 4, 175. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, H.; Ma, H.; Li, B.; Kou, J.; Li, J.; Gao, M.; Zeng, G.; Fang, J.; Dong, Z. Ultrafine PdZn bimetallic nanoparticles anchored on sulfur-doped mesoporous carbon for the partial hydrogenation of alkynols. Catal. Today 2022, 405–406, 242–250. [Google Scholar] [CrossRef]
- Ye, C.; Chen, X.; Li, S.; Feng, B.; Fu, Y.; Zhang, F.; Chen, D.-L.; Zhu, W. PdZn intermetallic compound stabilized on ZnO/nitrogen-decorated carbon hollow spheres for catalytic semihydrogenation of alkynols. Nano Res. 2022, 15, 3090–3098. [Google Scholar] [CrossRef]
- Sarkany, A.; Zsoldos, Z.; Furlong, B.; Hightower, J.W.; Guczi, L. Hydrogenation of 1-Butene and 1,3-Butadiene Mixtures over Pd/ZnO Catalysts. J. Catal. 1993, 141, 566–582. [Google Scholar] [CrossRef]
- Castillejos-López, E.; Agostini, G.; Michel, M.D.; Iglesias-Juez, A.; Bachiller-Baeza, B. Synergy of contact between ZnO surface planes and PdZn nanostructures: Morphology and chemical property effects in the intermetallic sites for selective 1,3-butadiene hydrogenation. ACS Catal. 2017, 7, 796–811. [Google Scholar] [CrossRef]
- Vernuccio, S.; Goy, R.; von Rohr, P.R.; Medlock, J.; Bonrath, W. Hydrogenation of 2-methyl-3-butyn-2-ol over a Pd/ZnO catalyst: Kinetic model and selectivity study. React. Chem. Eng. 2016, 1, 445–453. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Kerzhentseva, M.A.; Ismagilov, Z.R. Internal Surface Coating of a Capillary Microreactor for the Selective Hydrogenation of 2-Methyl-3-Butyn-2-Ol Using a PdZn/TiO2 Catalyst. The Effect of the Catalyst’s Activation Conditions on Its Catalytic Properties. Kinet. Catal. 2018, 59, 347–356. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Cherepanova, S.V.; Prosvirin, I.P.; Kerzhentsev, M.A.; Ismagilov, Z.R. Semihydrogenation of 2-methyl-3-butyn-2-ol on Pd-Zn nanoalloys: Effect of composition and heterogenization. Appl. Catal. A Gen. 2018, 549, 245–253. [Google Scholar] [CrossRef]
- Furukawa, S.; Yoshida, Y.; Komatsu, T. Chemoselective hydrogenation of nitrostyrene to aminostyrene over Pd- and Rh-based intermetallic compounds. ACS Catal. 2014, 4, 1441–1450. [Google Scholar] [CrossRef]
- Fujita, S.; Mitani, H.; Zhang, C.; Li, K.; Zhao, F.; Arai, M. Pd and PdZn supported on ZnO as catalysts for the hydrogenation of cinnamaldehyde to hydrocinnamyl alcohol. Mol. Catal. 2017, 442, 12–19. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, Y.; Wang, J.; Zeng, Z.; Deng, S.; Zou, J.-J.; Deng, Q. Intermetallic Palladium–Zinc Nanoparticles for the Ultraselective Hydrogenative Rearrangement of Furan Compounds. ACS Catal. 2023, 13, 13205–13214. [Google Scholar] [CrossRef]
- Yanilkin, V.V.; Nastapova, N.V.; Nasretdinova, G.R.; Osin, Y.N.; Evtjugin, V.G.; Ziganshina, A.Y.; Gubaidullin, A.T. Structure and catalytic activity of ultrasmall Rh, Pd and (Rh + Pd) nanoparticles obtained by mediated electrosynthesis. New J. Chem. 2019, 43, 3931–3945. [Google Scholar] [CrossRef]
- Gregor, L.; Reilly, A.K.; Dickstein, T.A.; Mazhar, S.; Bram, S.; Morgan, D.G.; Losovyj, Y.; Pink, M.; Stein, B.D.; Matveeva, V.G.; et al. Facile synthesis of magnetically recoverable Pd and Ru catalysts for 4-nitrophenol reduction: Identifying key factors. ACS Omega 2018, 3, 14717–14725. [Google Scholar] [CrossRef]
- Kim, J.D.; Choi, M.Y.; Choi, H.C. Catalyst activity of carbon nanotube supported Pd catalysts for the hydrogenation of nitroarenes. Mater. Chem. Phys. 2016, 173, 404–411. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Y.; He, G.; Sun, X.; Wang, X. Catalytic hydrogenation of nitrophenols and nitrotoluenes over a palladium/graphene nanocomposite. Catal. Sci. Technol. 2014, 4, 1742–1748. [Google Scholar] [CrossRef]
- Sun, W.; Lu, X.; Tong, Y.; Zhang, Z.; Lei, J.; Nie, G.; Wang, C. Fabrication of highly dispersed palladium/graphene oxide nanocomposites and their catalytic properties for efficient hydrogenation of p-nitrophenol and hydrogen generation. Int. J. Hydrogen Energy 2014, 39, 9080–9086. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Catalytic reduction of p-nitrophenol by using platinum nanoparticles stabilised by guar gum. Carbohydr. Polym. 2014, 113, 525–531. [Google Scholar] [CrossRef]

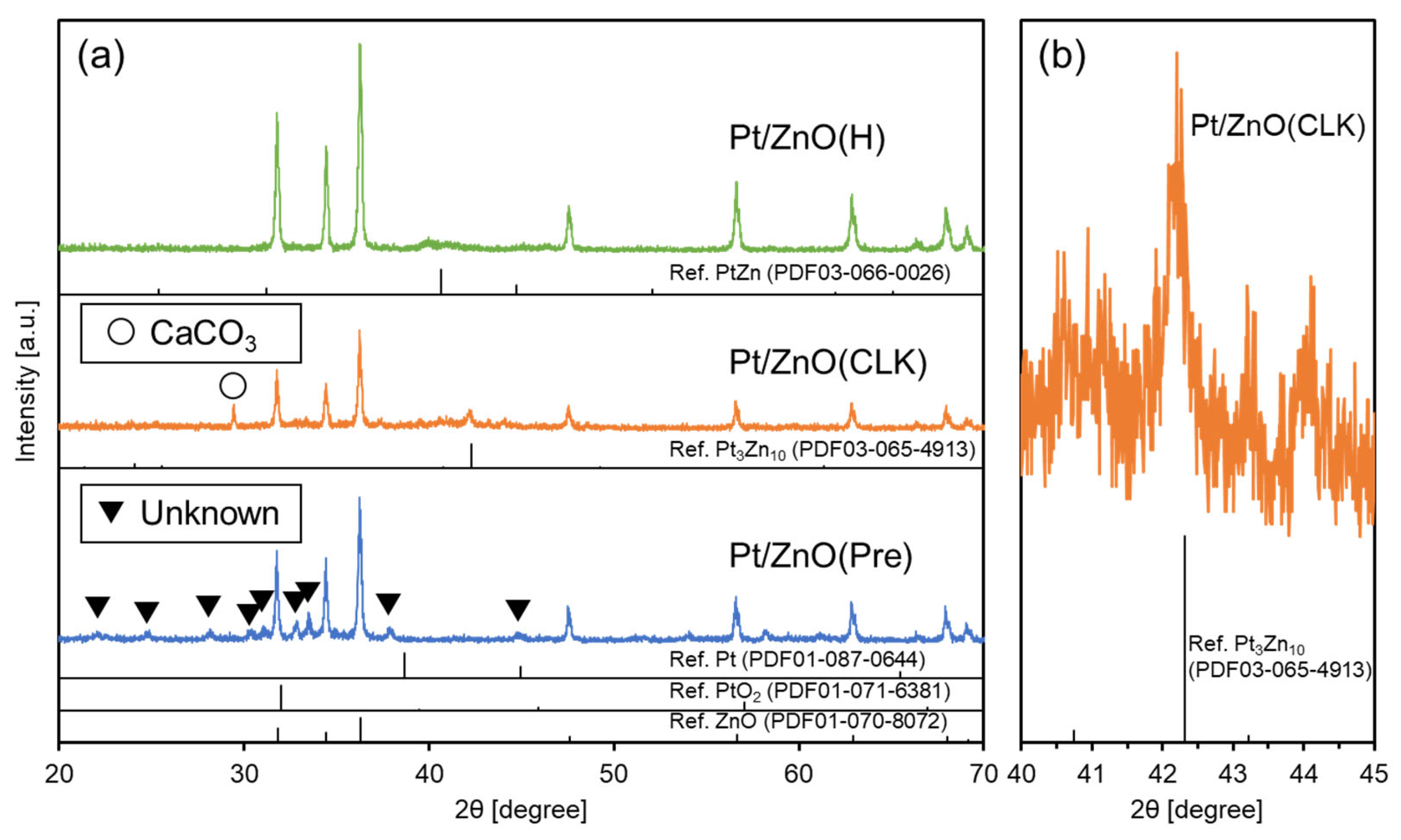


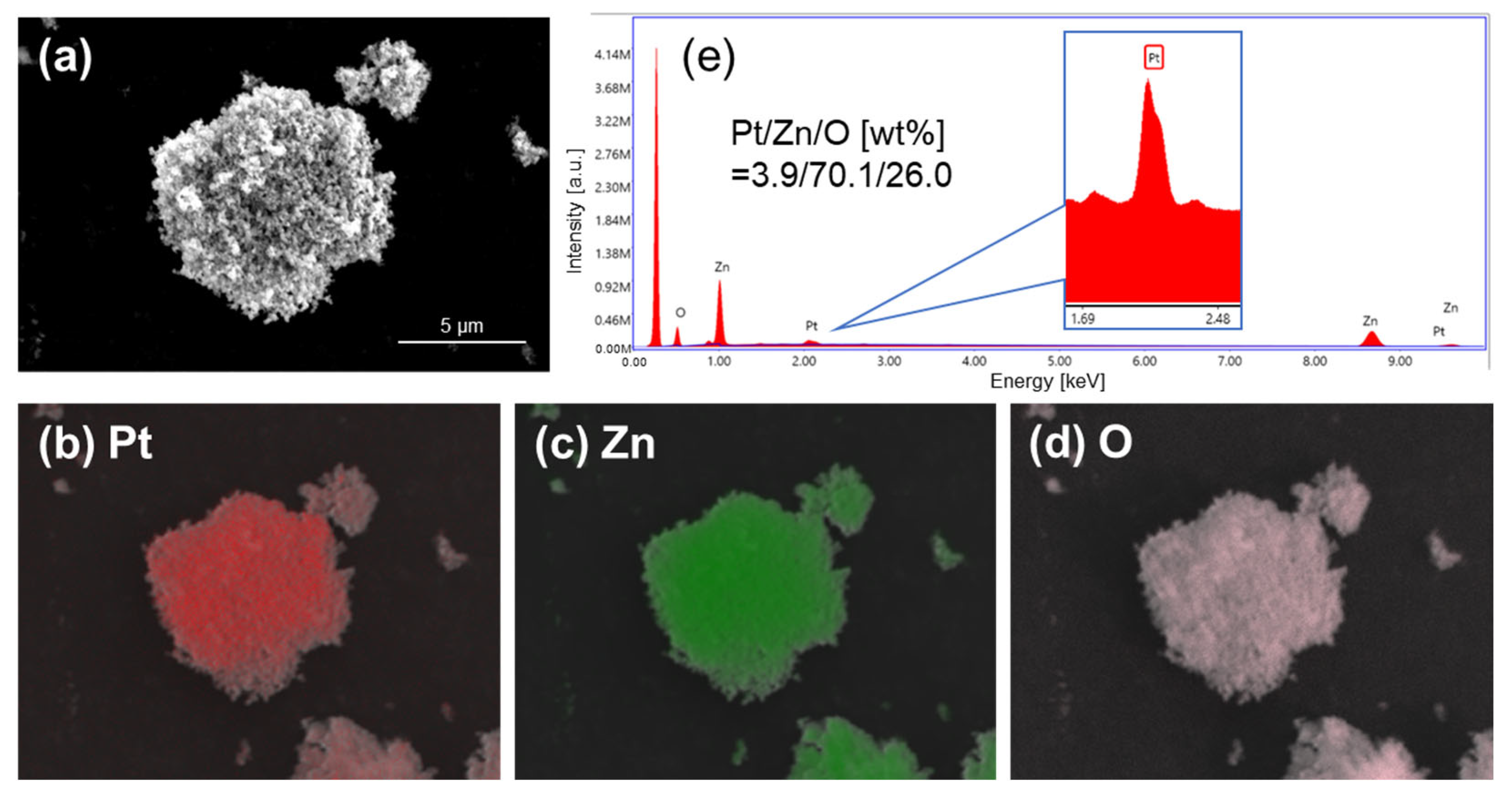
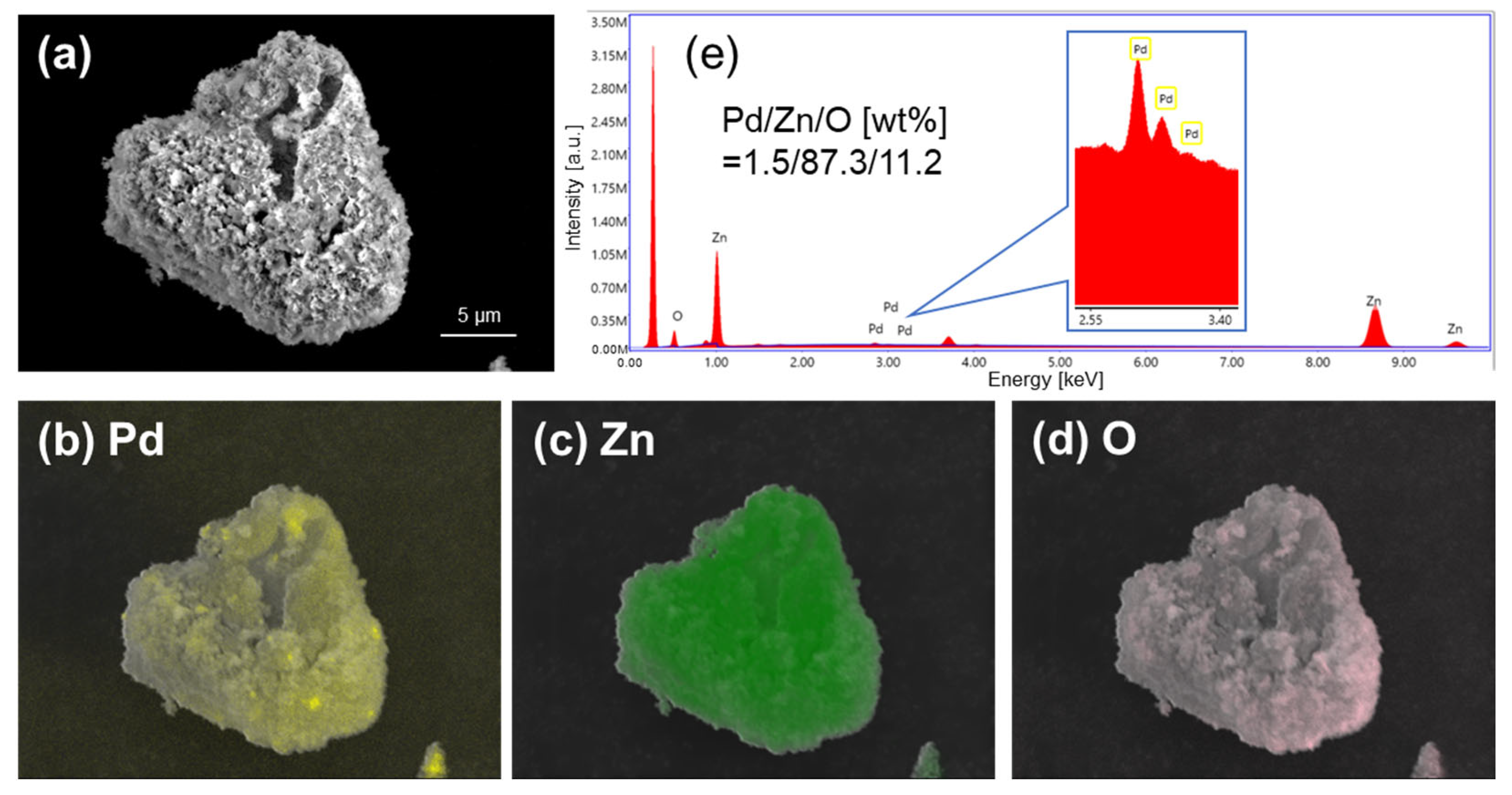
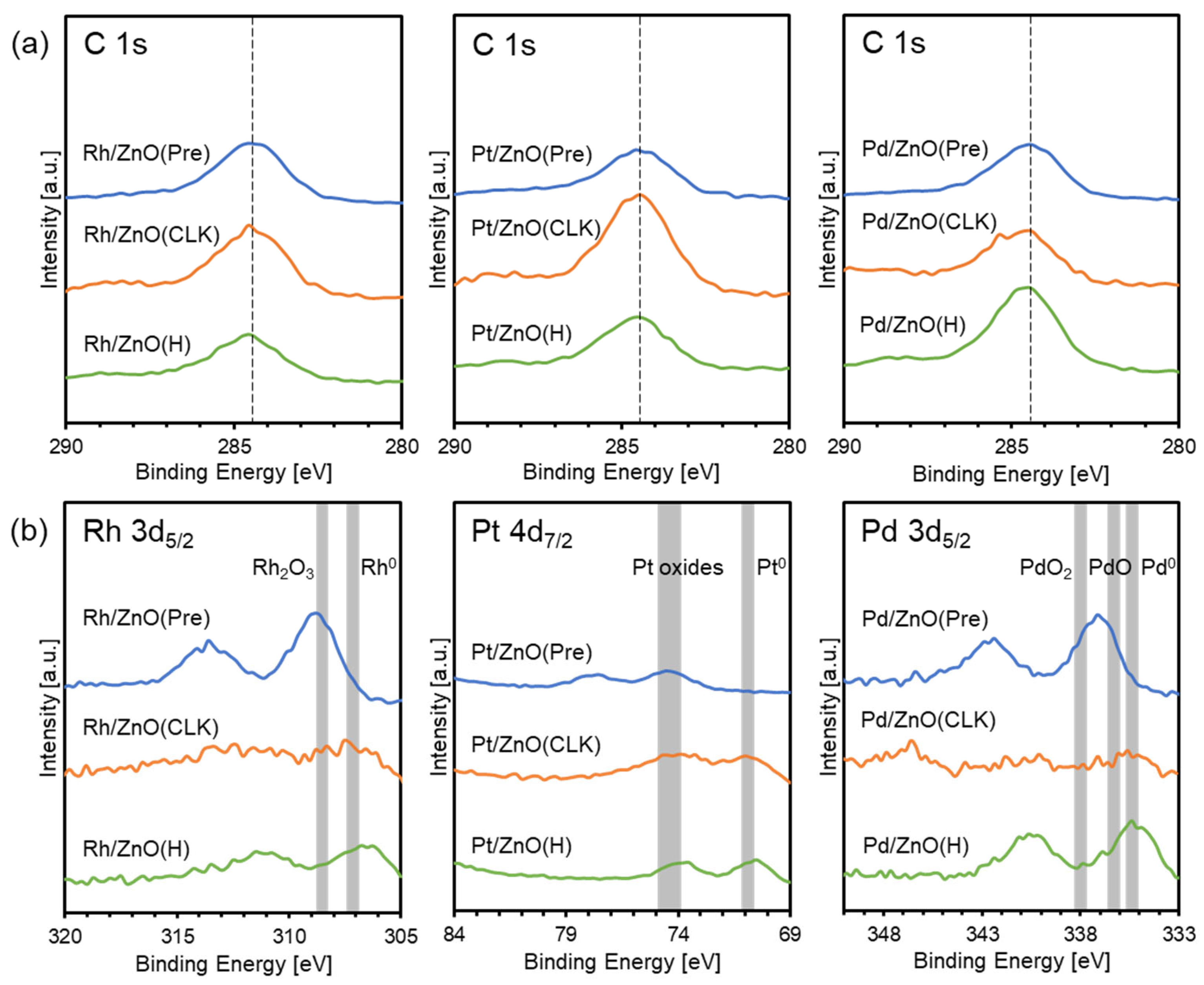
| Sample | Active Metal | BET S. A. [m2/g] | XRD Measurements | XPS Measurements | ||
|---|---|---|---|---|---|---|
| Zn Species | Metal Species | Main Chemical States of Metals | Molar Ratios of Metal/Zn [mol/mol] | |||
| ZnO | None | 12.7 | ZnO | None | None | None |
| Rh/ZnO(Pre) | Rh | 12.5 | ZnO | Rh2O3 | Rh(+3) | 0.101 |
| Rh/ZnO(CLK) | 20.4 | ZnO | RhZn13 | Rh(0) | 0.046 | |
| Rh/ZnO(H) | 4.8 | ZnO | RhZn | Rh(0) | 0.028 | |
| Pt/ZnO(Pre) | Pt | 7.4 | ZnO | Unknown | Oxides | 0.049 |
| Pt/ZnO(CLK) | 19.2 | ZnO | Pt3Zn10 | Pt(0) | 0.128 | |
| Pt/ZnO(H) | 6.5 | ZnO | PtZn | Pt(0) | 0.017 | |
| Pd/ZnO(Pre) | Pd | 5.2 | ZnO | Pd | Pd(+2, +4) | 0.058 |
| Pd/ZnO(CLK) | 17.1 | ZnO | Distorted Pd5Zn8 | Pd(0) | 0.011 | |
| Pd/ZnO(H) | 4.5 | ZnO | PdZn | Pd(0) | 0.060 | |
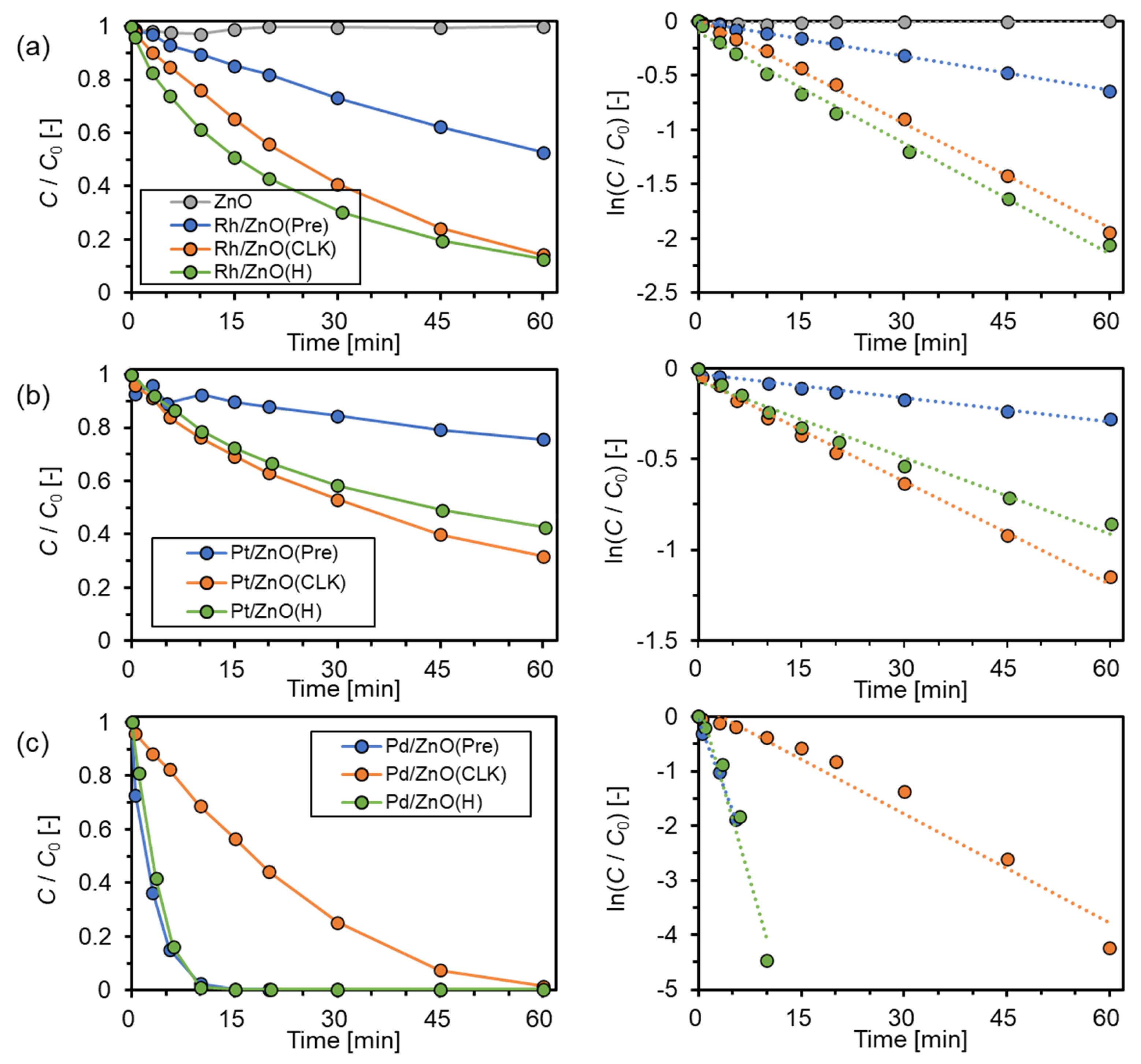
| Catalyst | PGM Amount [μg] | k [min−1] | Reaction Conditions | Ref. |
|---|---|---|---|---|
| Rh/ZnO(Pre) | 500 | 0.010 | 4-NP (1.6 mM) NaBH4 (47 mM) 10 mg-cat/9 mL | This study |
| Rh/ZnO(CLK) | 0.032 | |||
| Rh/ZnO(H) | 0.034 | |||
| Pt/ZnO(Pre) | 0.004 | |||
| Pt/ZnO(CLK) | 0.019 | |||
| Pt/ZnO(H) | 0.014 | |||
| Pd/ZnO(Pre) | 0.328 | |||
| Pd/ZnO(CLK) | 0.067 | |||
| Pd/ZnO(H) | 0.439 | |||
| Rh NPs stabilized by PVP or CTAC | 0.3 | 0.38–0.70 | 4-NP (0.1 mM) NaBH4 (0.005 M) 0.0032 μmol-cat/20 μL | [56] |
| Pd NPs stabilized by CTAC | 0.03–0.14 | |||
| 5.0–8.7 wt%Pd/Fe3O4 | 0.4–0.7 | 0.38–0.75 | 4-NP (10 mM) NaBH4 (0.1 M) 0.008 mg-cat/3 mL | [57] |
| Pd/CNT | n.a. | 0.376 | 4-NP (2 mM) NaBH4 (0.1 M) 0.3 mg-cat/45 mL | [58] |
| 5 wt%Pd/graphene | 200 | 2.19 | 4-NP (0.3 mM) NaBH4 (0.1 M) 4 mg-cat/40 mL | [59] |
| Pd/graphene oxide | 0.02 | 2.06 | 4-NP (7.4 mM) NaBH4 (0.41 M) 7.5 μg-cat/30 μL | [60] |
| Pt NPs stabilized by guar gum | 24 | 0.42 | 4-NP (1 mM) NaBH4 (0.1 M) 0.125 mol-cat/50 μL | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, Y.; Yamamoto, K.; Shoji, R. CaH2-Assisted Molten Salt Synthesis of Zinc-Rich Intermetallic Compounds of RhZn13 and Pt3Zn10 for Catalytic Selective Hydrogenation Application. Crystals 2024, 14, 278. https://doi.org/10.3390/cryst14030278
Kobayashi Y, Yamamoto K, Shoji R. CaH2-Assisted Molten Salt Synthesis of Zinc-Rich Intermetallic Compounds of RhZn13 and Pt3Zn10 for Catalytic Selective Hydrogenation Application. Crystals. 2024; 14(3):278. https://doi.org/10.3390/cryst14030278
Chicago/Turabian StyleKobayashi, Yasukazu, Koharu Yamamoto, and Ryo Shoji. 2024. "CaH2-Assisted Molten Salt Synthesis of Zinc-Rich Intermetallic Compounds of RhZn13 and Pt3Zn10 for Catalytic Selective Hydrogenation Application" Crystals 14, no. 3: 278. https://doi.org/10.3390/cryst14030278
APA StyleKobayashi, Y., Yamamoto, K., & Shoji, R. (2024). CaH2-Assisted Molten Salt Synthesis of Zinc-Rich Intermetallic Compounds of RhZn13 and Pt3Zn10 for Catalytic Selective Hydrogenation Application. Crystals, 14(3), 278. https://doi.org/10.3390/cryst14030278







