Near-Infrared Spectroscopic Study of Biotite–Phlogopite (Mg# = 30~99): OH-Stretching Modes and Mg# Content Prediction Equation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
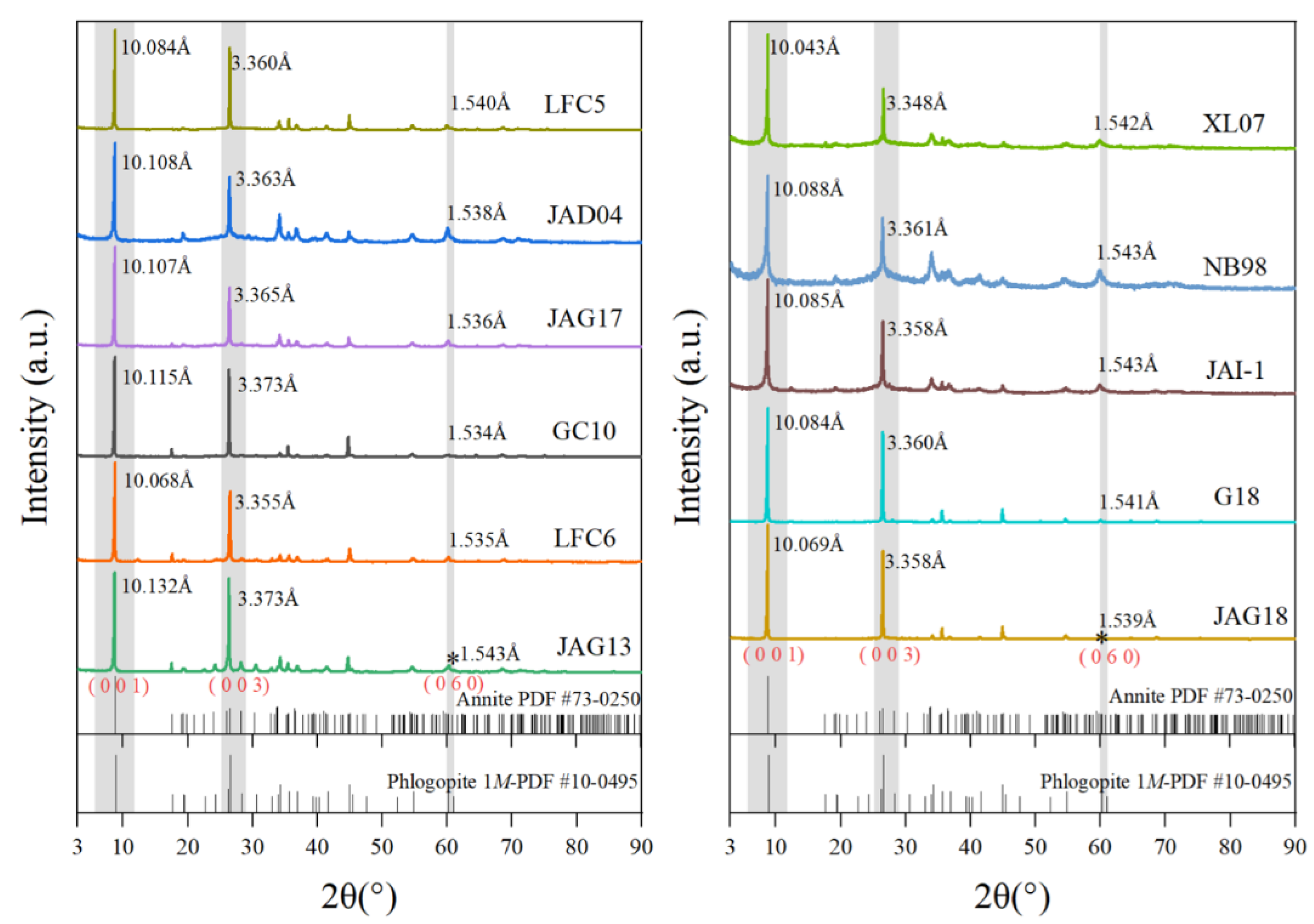
3.1. X-ray Diffraction Analysis
3.2. Mineral Element Analysis
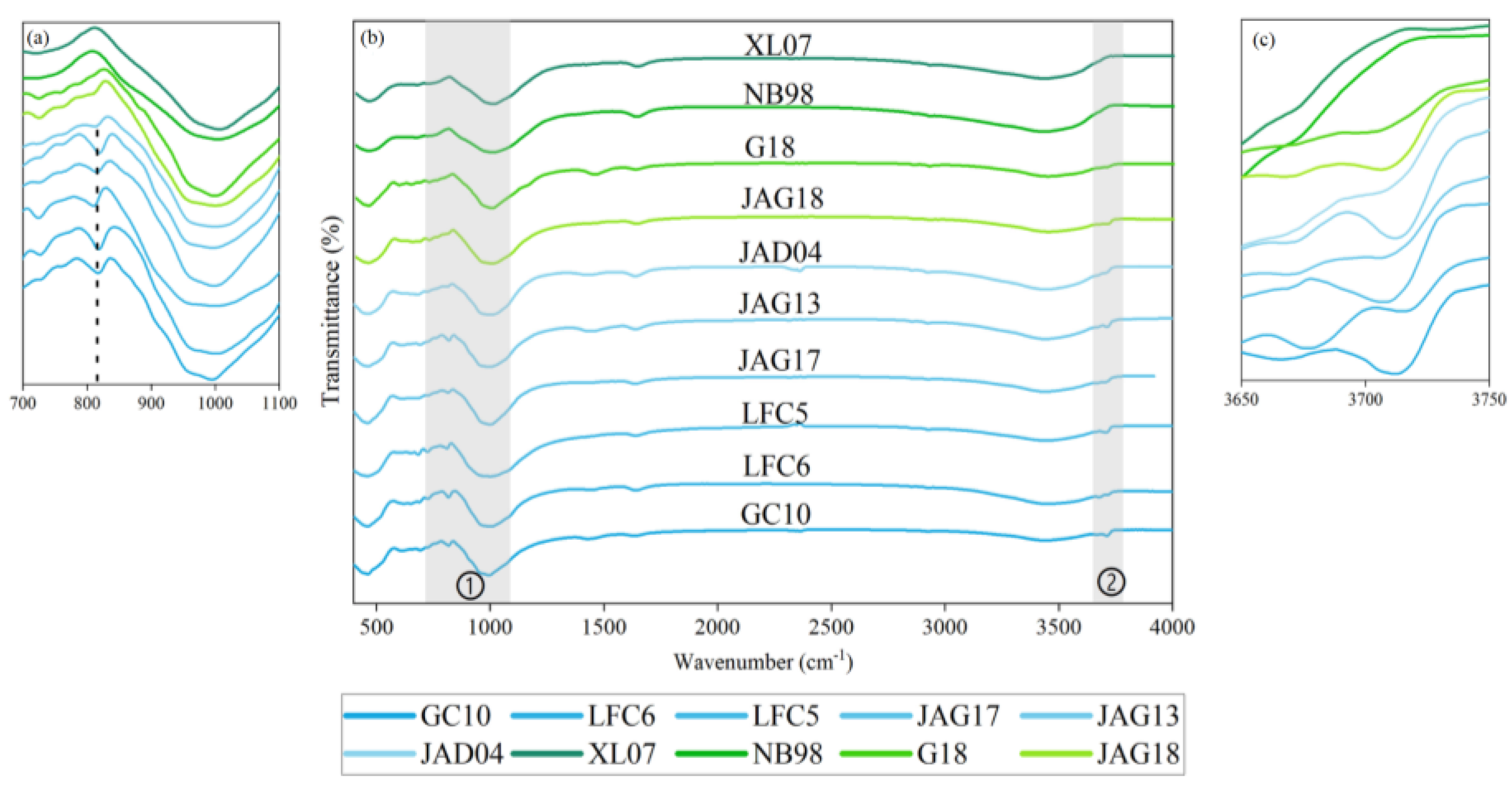
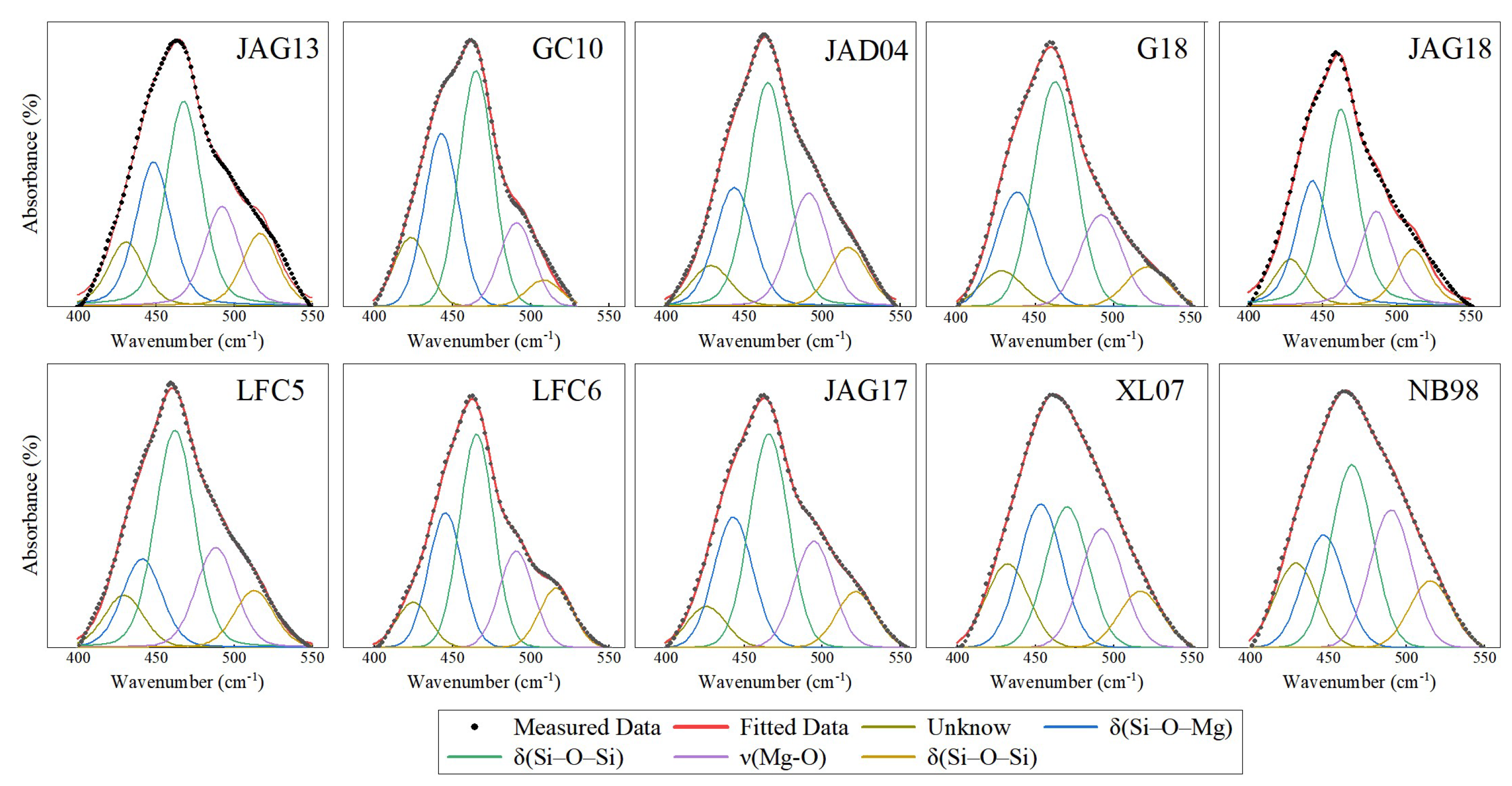
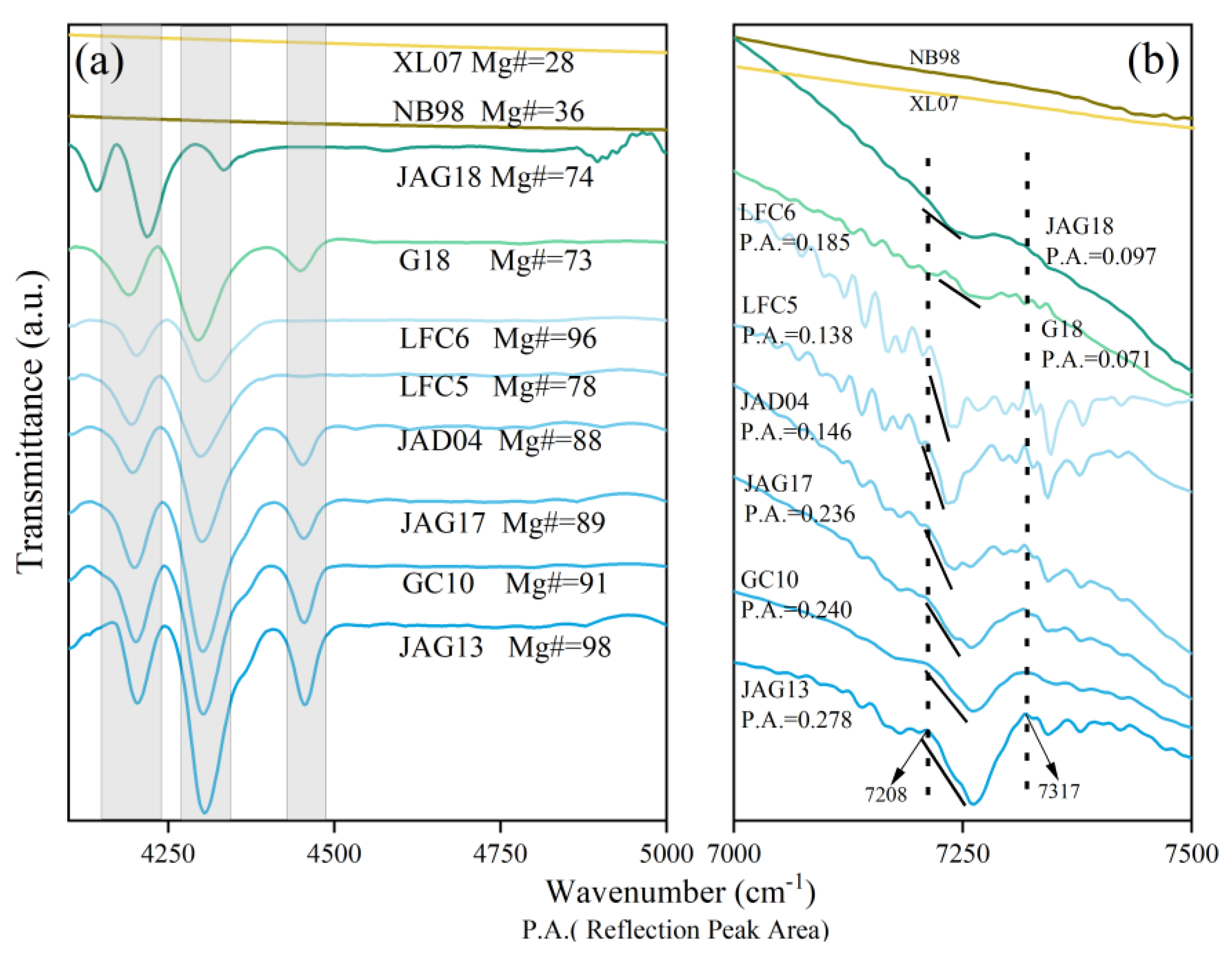
3.3. Characteristics of MIR Spectra
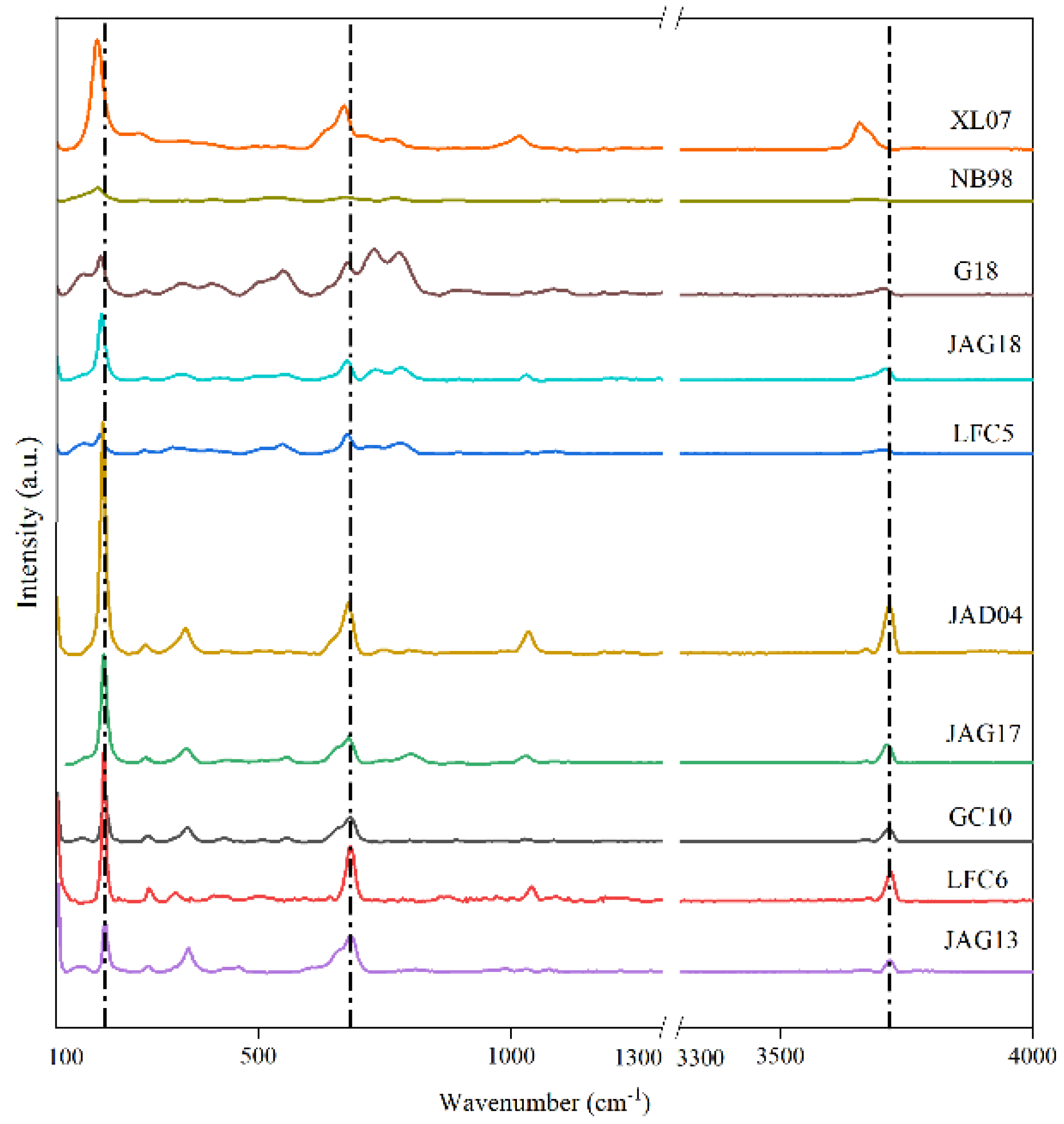
3.4. OH-Stretching Vibration Characteristics of Raman Spectra
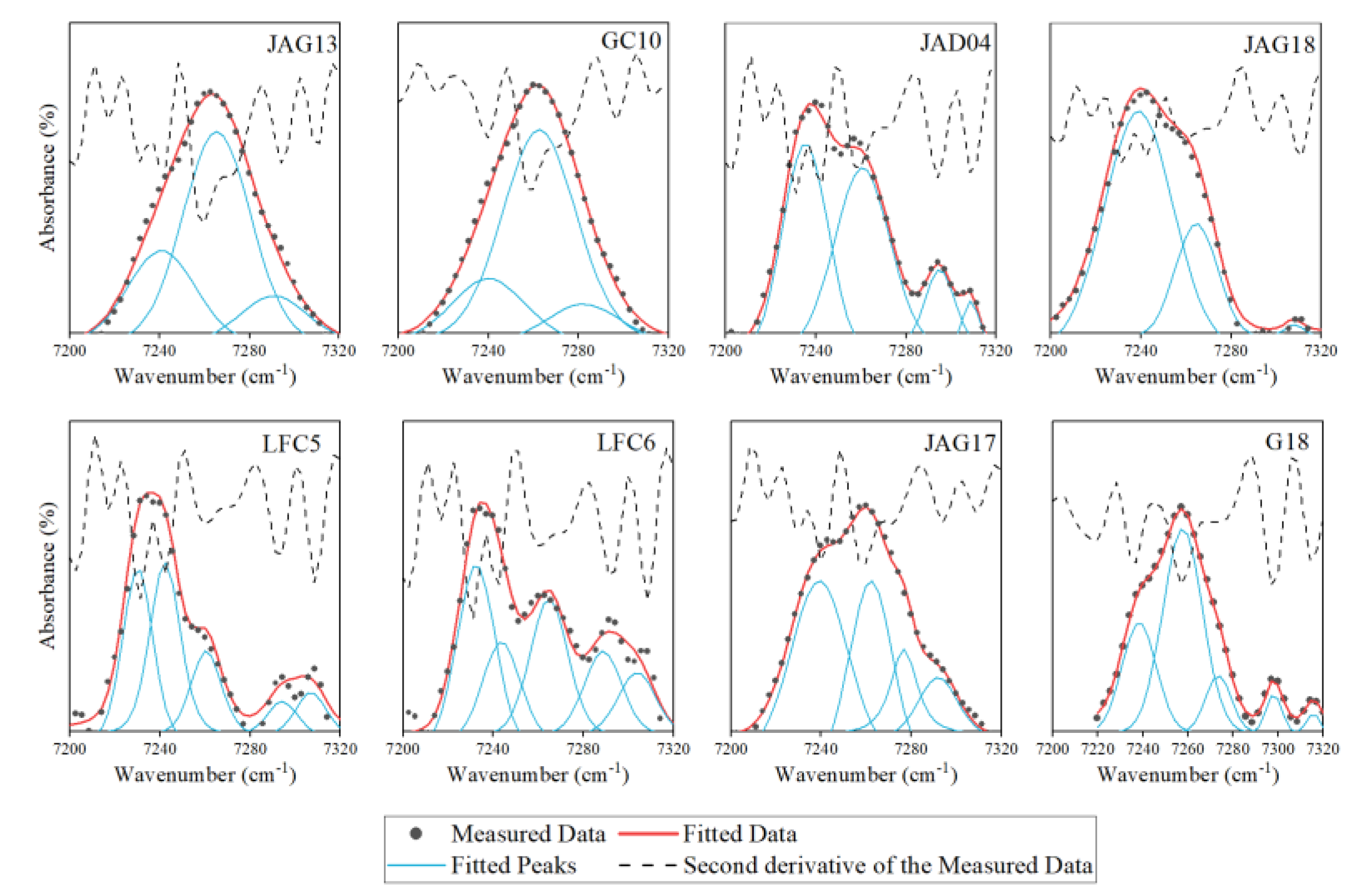
3.5. Characteristics of NIR Spectra
4. Discussion
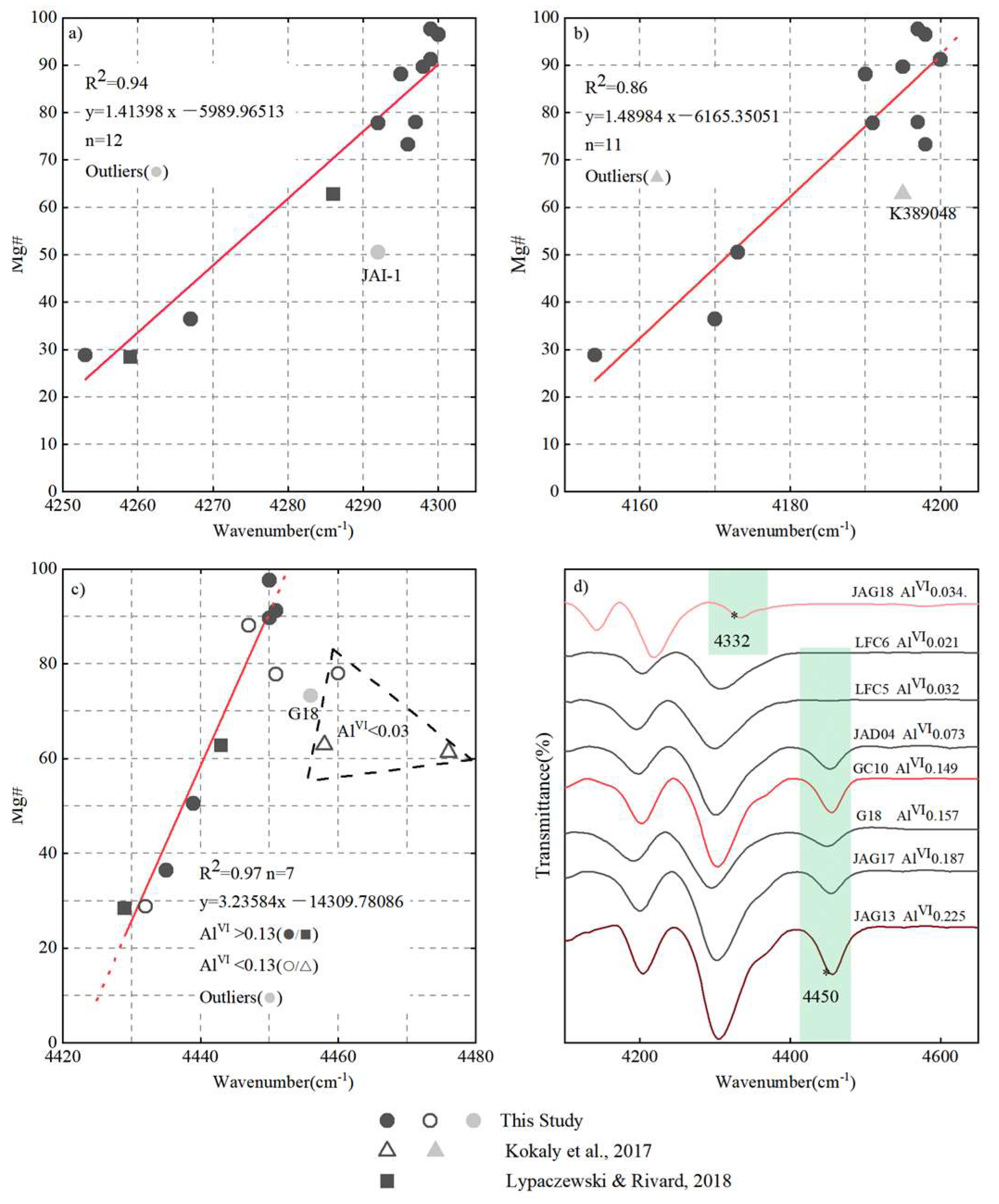
4.1. Assignment of OH Combination Vibration in NIR Spectra
4.2. Assignment of OH First Overtone Vibration in NIR Spectra
4.3. The Relationship between Composition and Characteristic Peaks in NIR Spectra
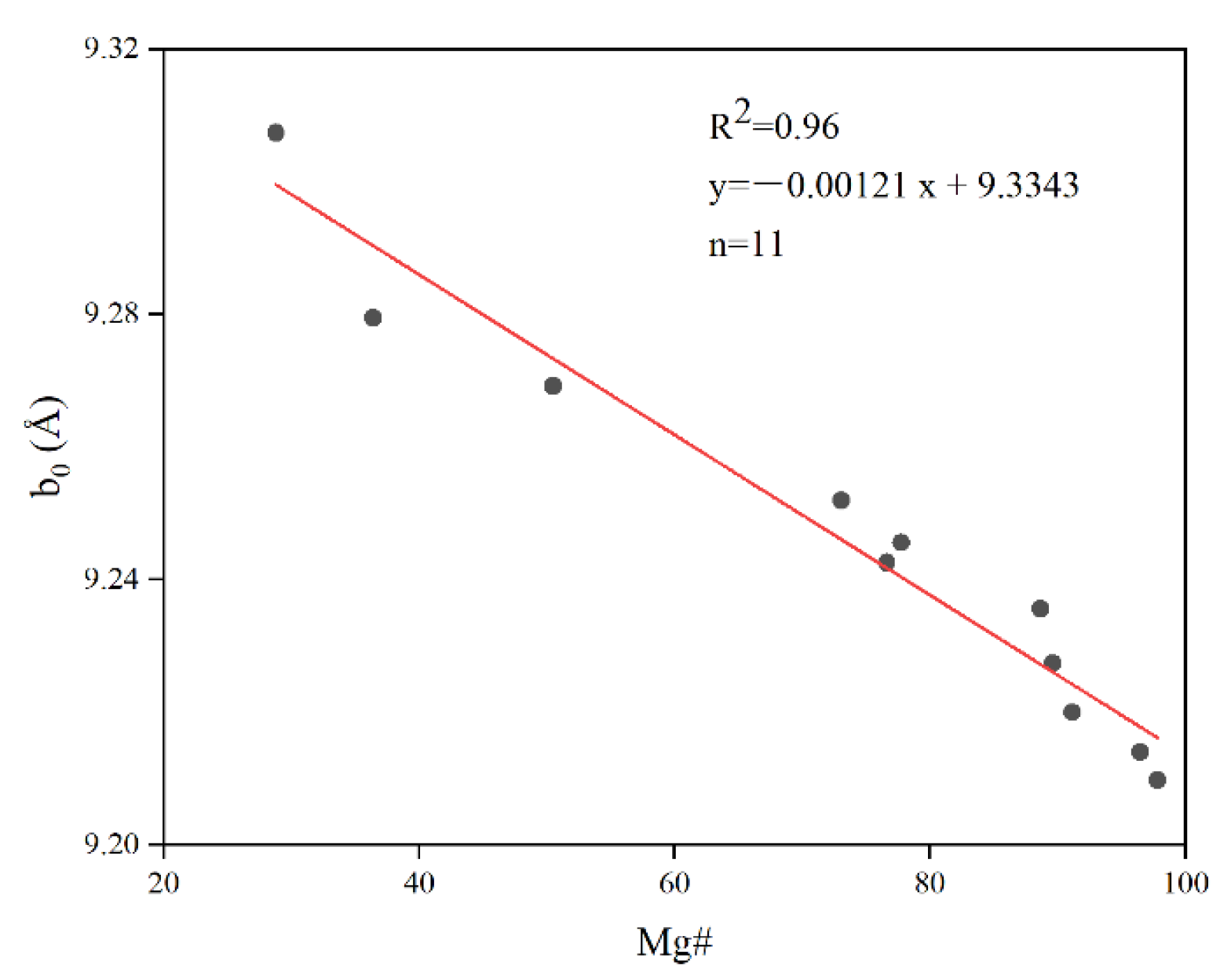
4.4. Correlations between Crystal Chemical Changes and Mg# Content in Biotite–Phlogopite
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beane, R.E. Biotite stability in the porphyry copper environment. Econ. Geol. 1974, 69, 241–256. [Google Scholar] [CrossRef]
- Jacobs, D.C.; Parry, W.T. A comparison of the geochemistry of biotite from some basin and range stocks. Econ. Geol. 1976, 71, 1029–1035. [Google Scholar] [CrossRef]
- Chivas, A.R. Geochemical evidence for magmatic fluids in porphyry copper mineralization: Part I. Mafic silicates from the Koloula igneous complex. Contrib. Mineral. Petrol. 1982, 78, 389–403. [Google Scholar] [CrossRef]
- Selby, D.; Nesbitt, B.E. Chemical composition of biotite from the Casino porphyry Cu–Au–Mo mineralization, Yukon, Canada: Evaluation of magmatic and hydrothermal fluid chemistry. Chem. Geol. 2000, 171, 77–93. [Google Scholar] [CrossRef]
- Ayati, F.; Yavuz, F.; Noghreyan, M.; Haroni, H.A.; Yavuz, R. Chemical characteristics and composition of hydrothermal biotite from the Dalli porphyry copper prospect, Arak, central province of Iran. Mineral. Petrol. 2008, 94, 107–122. [Google Scholar] [CrossRef]
- Afshooni, S.; Mirnejad, H.; Esmaeily, D.; Haroni, H.A. Mineral chemistry of hydrothermal biotite from the Kahang porphyry copper deposit (NE Isfahan), Central Province of Iran. Ore Geol. Rev. 2013, 54, 214–232. [Google Scholar] [CrossRef]
- Parsapoor, A.; Khalili, M.; Tepley, F.; Maghami, M. Mineral chemistry and isotopic composition of magmatic, re-equilibrated and hydrothermal biotites from Darreh-Zar porphyry copper deposit, Kerman (Southeast of Iran). Ore Geol. Rev. 2015, 66, 200–218. [Google Scholar] [CrossRef]
- Ionov, D.A.; Griffin, W.L.; O’Reilly, S.Y. Volatile-bearing minerals and lithophile trace elements in the upper mantle. Chem. Geol. 1997, 141, 153–184. [Google Scholar] [CrossRef]
- Tappert, R.; Foden, J.; Heaman, L.; Tappert, M.C.; Zurevinski, S.E.; Wills, K. The petrology of kimberlites from South Australia: Linking olivine macrocrystic and micaceous kimberlites. J. Volcanol. Geotherm. Res. 2019, 373, 68–96. [Google Scholar] [CrossRef]
- Foster, M.D. Interpretation of the Composition of Trioctahedral Micas; 2330-7102; United States Government Printing Office: Washington, DC, USA, 1960. [Google Scholar]
- David, R.W.; Hans, P.E. Stability of biotite: Experiment, theory, and application. Am. Mineral. J. Earth Planet. Mater. 1965, 50, 1228–1272. [Google Scholar]
- Clemens, J. Granitic magmas with I-type affinities, from mainly metasedimentary sources: The Harcourt batholith of southeastern Australia. Contrib. Mineral. Petrol. 2018, 173, 93. [Google Scholar] [CrossRef]
- Clemens, J.; Stevens, G.; Farina, F. The enigmatic sources of I-type granites: The peritectic connexion. Lithos 2011, 126, 174–181. [Google Scholar] [CrossRef]
- Uchida, E.; Endo, S.; Makino, M. Relationship Between Solidification Depth of Granitic Rocks and Formation of Hydrothermal Ore Deposits. Resour. Geol. 2007, 57, 47–56. [Google Scholar] [CrossRef]
- Poulet, F.; Bibring, J.-P.; Mustard, J.; Gendrin, A.; Mangold, N.; Langevin, Y.; Arvidson, R.; Gondet, B.; Gomez, C. Phyllosilicates on Mars and implications for early Martian climate. Nature 2005, 438, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Gendrin, A.; Mangold, N.; Bibring, J.-P.; Langevin, Y.; Gondet, B.; Poulet, F.; Bonello, G.; Quantin, C.; Mustard, J.; Arvidson, R. Sulfates in Martian layered terrains: The OMEGA/Mars Express view. Science 2005, 307, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Milliken, R.E.; Swayze, G.A.; Arvidson, R.E.; Bishop, J.L.; Clark, R.N.; Ehlmann, B.L.; Green, R.O.; Grotzinger, J.P.; Morris, R.; Murchie, S.L. Opaline silica in young deposits on Mars. Geology 2008, 36, 847–850. [Google Scholar] [CrossRef]
- Carter, J.; Poulet, F.; Bibring, J.P.; Mangold, N.; Murchie, S. Hydrous minerals on Mars as seen by the CRISM and OMEGA imaging spectrometers: Updated global view. J. Geophys. Res. Planets 2013, 118, 831–858. [Google Scholar] [CrossRef]
- Cuadros, J.; Mavris, C.; Michalski, J.R. Possible widespread occurrence of vermiculite on Mars. Appl. Clay Sci. 2022, 228, 106643. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Mustard, J.F.; Swayze, G.A.; Clark, R.N.; Bishop, J.L.; Poulet, F.; Des Marais, D.J.; Roach, L.H.; Milliken, R.E.; Wray, J.J. Identification of hydrated silicate minerals on Mars using MRO-CRISM: Geologic context near Nili Fossae and implications for aqueous alteration. J. Geophys. Res. Planets 2009, 114, E00D08. [Google Scholar] [CrossRef]
- Siesler, H.W.; Kawata, S.; Heise, H.M.; Ozaki, Y. Near-Infrared Spectroscopy: Principles, Instruments, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Holze, R. Fundamentals and applications of near infrared spectroscopy in spectroelectrochemistry. J. Solid State Electrochem. 2004, 8, 982–997. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K. Near-Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 1987. [Google Scholar]
- Clark, R.N.; King, T.V.; Klejwa, M.; Swayze, G.A.; Vergo, N. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Res. Solid Earth 1990, 95, 12653–12680. [Google Scholar] [CrossRef]
- Ozaki, Y.; Huck, C.W.; Ishigaki, M.; Ishikawa, D.; Ikehata, A.; Shinzawa, H. Near-infrared spectroscopy in biological molecules and tissues. In Encyclopedia of Biophysics; Roberts, G., Watts, A., European Biophysical Societies, Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Clark, R.N.; Rencz, A.N. Spectroscopy of rocks and minerals, and principles of spectroscopy. Man. Remote Sens. 1999, 3, 3–58. [Google Scholar]
- Duke, E.F. Near infrared spectra of muscovite, Tschermak substitution, and metamorphic reaction progress: Implications for remote sensing. Geology 1994, 22, 621–624. [Google Scholar] [CrossRef]
- Vedder, W. Correlations between infrared spectrum and chemical composition of mica. Am. Mineral. J. Earth Planet. Mater. 1964, 49, 736–768. [Google Scholar]
- Lypaczewski, P.; Rivard, B. Estimating the Mg# and AlVI content of biotite and chlorite from shortwave infrared reflectance spectroscopy: Predictive equations and recommendations for their use. Int. J. Appl. Earth Obs. Geoinf. 2018, 68, 116–126. [Google Scholar]
- Post, J.; Crawford, S. Uses of near-infared spectra for the identification of clay minerals. Appl. Clay Sci. 2014, 95, 383–387. [Google Scholar] [CrossRef]
- Wu, S.; He, M.; Yang, M.; Peng, B. Near-Infrared Spectroscopic Study of OH Stretching Modes in Kaolinite and Dickite. Crystals 2022, 12, 907. [Google Scholar] [CrossRef]
- Kokaly, R.; Clark, R.; Swayze, G.; Livo, K.; Hoefen, T.; Pearson, N.; Wise, R.; Benzel, W.; Lowers, H.; Driscoll, R. USGS Spectral Library Version 7 Data: US Geological Survey Data Release; United States Geological Survey (USGS): Reston, VA, USA, 2017; 61p. [Google Scholar]
- Petit, S.; Madejova, J.; Decarreau, A.; Martin, F. Characterization of octahedral substitutions in kaolinites using near infrared spectroscopy. Clays Clay Miner. 1999, 47, 103–108. [Google Scholar] [CrossRef]
- Balan, E.; Saitta, A.M.; Mauri, F.; Calas, G. First-principles modeling of the infrared spectrum of kaolinite. Am. Mineral. 2001, 86, 1321–1330. [Google Scholar] [CrossRef]
- Kaye, W. Near-infrared spectroscopy: I. Spectral identification and analytical applications. Spectrochim. Acta 1954, 6, 257–287. [Google Scholar] [CrossRef]
- Aldega, L.; Cuadros, J.; Laurora, A.; Rossi, A. Weathering of phlogopite to beidellite in a karstic environment. Am. J. Sci. 2009, 309, 689–710. [Google Scholar] [CrossRef]
- Rieppo, L.; Saarakkala, S.; Närhi, T.; Helminen, H.; Jurvelin, J.; Rieppo, J. Application of second derivative spectroscopy for increasing molecular specificity of fourier transform infrared spectroscopic imaging of articular cartilage. Osteoarthr. Cartil. 2012, 20, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Redhammer, G.n.J.; Beran, A.; Schneider, J.; Amthauer, G.; Lottermoser, W. Spectroscopic and structural properties of synthetic micas on the annite-siderophyllite binary: Synthesis, crystal structure refinement, Mössbauer, and infrared spectroscopy. Am. Mineral. 2000, 85, 449–465. [Google Scholar] [CrossRef]
- Yuan, X.; Mayanovic, R.A. An empirical study on Raman peak fitting and its application to Raman quantitative research. Appl. Spectrosc. 2017, 71, 2325–2338. [Google Scholar] [CrossRef]
- Bailey, S. Structures of layer silicates. In Crystal Structures of Clay Minerals and their X-ray Identification; European Mineralogical Union: Strasbourg, France, 1980. [Google Scholar]
- Bailey, S.W. Re-evaluation of ordering and local charge-balance in Ia chlorite. Can. Mineral. 1986, 24, 649–654. [Google Scholar]
- Fleet, M.E.; Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals: Micas; Geological Society: London, UK, 2003. [Google Scholar]
- Nachit, H.; Ibhi, A.; Ohoud, M.B. Discrimination between primary magmatic biotites, reequilibrated biotites and neoformed biotites. Comptes Rendus Geosci. 2005, 337, 1415–1420. [Google Scholar] [CrossRef]
- Stubičan, V.; Roy, R. Isomorphous substitution and infra-red spectra of the layer lattice silicates. Am. Mineral. J. Earth Planet. Mater. 1961, 46, 32–51. [Google Scholar]
- Jenkins, D.M. Empirical study of the infrared lattice vibrations (1100–350 cm−1) of phlogopite. Phys. Chem. Miner. 1989, 16, 408–414. [Google Scholar] [CrossRef]
- Farmer, V.t.; Russell, J. The infra-red spectra of layer silicates. Spectrochim. Acta 1964, 20, 1149–1173. [Google Scholar] [CrossRef]
- Sergent, A.P.J.; Robert, J.L. Intersite OH-F distribution in an Al-rich synthetic phlogopite. Eur. J. Miner. 1997, 9, 501–508. [Google Scholar]
- Sijakova-Ivanova, T.; Robeva-Čukovska, L. Mineralogical characteristics of phlogopite from Dupen kamen, Republic of Macedonia. IOSR J. Appl. Geol. Geophys. (IOSR-JAGG) 2016, 4, 72–76. [Google Scholar] [CrossRef][Green Version]
- Robert, J.-L. Etudes Cristallochimiques sur les Micas et les Amphiboles: Applications Petrographiques et Geochimiques. Ph.D. Thesis, Université Paris-Sud, Paris, France, 1981. [Google Scholar]
- Scordari, F.; Ventruti, G.; Sabato, A.; Bellatreccia, F.; Della Ventura, G.; Pedrazzi, G. Ti-rich phlogopite from Mt. Vulture (Potenza, Italy) investigated by a multianalytical approach: Substitutional mechanisms and orientation of the OH dipoles. Eur. J. Mineral. 2006, 18, 379–391. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Y.; Ingrin, J.; Wang, Z.; Xia, Q. Impact of fluorine on the thermal stability of phlogopite. Am. Mineral. 2022, 107, 815–825. [Google Scholar] [CrossRef]
- Farmer, V.; Russell, J.; McHardy, W.; Newman, A.; Ahlrichs, J.; Rimsaite, J. Evidence for loss of protons and octahedral iron from oxidized biotites and vermiculites. Mineral. Mag. 1971, 38, 121–137. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectra of Minerals. Mineral. Soc. Monogr. 1974, 4, 331–363. [Google Scholar]
- Della Ventura, G.; Mottana, A.; Parodi, G.C.; Griffin, W.L. FTIR spectroscopy in the OH-stretching region of monoclinic epidotes from Praborna (St. Marcel, Aosta valley, Italy). Eur. J. Mineral. 1996, 8, 655–665. [Google Scholar] [CrossRef]
- Wang, A.; Freeman, J.J.; Jolliff, B.L. Understanding the Raman spectral features of phyllosilicates. J. Raman Spectrosc. 2015, 46, 829–845. [Google Scholar] [CrossRef]
- McKeown, D.A.; Bell, M.I.; Etz, E.S. Raman spectra and vibrational analysis of the trioctahedral mica phlogopite. Am. Mineral. 1999, 84, 970–976. [Google Scholar] [CrossRef]
- Šontevska, V.; Jovanovski, G.; Makreski, P.; Raškovska, A.; Šoptrajanov, B. Minerals from Macedonia. XXI. Vibrational spectroscopy as identificational tool for some phyllosilicate minerals. Acta Chim. Slov. 2008, 55, 757–766. [Google Scholar]
- Ventruti, G.; Caggianelli, A.; Festa, V.; Langone, A. Crystal chemistry of barian titanian phlogopite from a lamprophyre of the Gargano Promontory (Apulia, Southern Italy). Minerals 2020, 10, 766. [Google Scholar] [CrossRef]
- Tlili, A.; Smith, D.C.; Beny, J.-M.; Boyer, H. A Raman microprobe study of natural micas. Mineral. Mag. 1989, 53, 165–179. [Google Scholar] [CrossRef]
- Langer, K.; Flörke, O.W. Near infrared absorption spectra (4000–9000 cm−1) of opals and the vole of” water” in these SIO2·nH2O minerals. Fortschr. Miner. 1974, 52, 17–51. [Google Scholar]
- Pasquini, C. Near infrared spectroscopy: Fundamentals, practical aspects and analytical applications. J. Braz. Chem. Soc. 2003, 14, 198–219. [Google Scholar] [CrossRef]
- Cheng, H.; Hao, R.; Zhou, Y.; Frost, R.L. Visible and near-infrared spectroscopic comparison of five phyllosilicate mineral samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 180, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Post, J.L.; Noble, P.N. The near-infrared combination band frequencies of dioctahedral smectites, micas, and illites. Clays Clay Miner. 1993, 41, 639–644. [Google Scholar] [CrossRef]
- Beran, A. Infrared Spectroscopy of Micas. Rev. Mineral. Geochem. 2002, 46, 351–369. [Google Scholar] [CrossRef]
- Hunt, G.R. Spectral signatures of particulate minerals in the visible and near infrared. Geophysics 1977, 42, 501–513. [Google Scholar] [CrossRef]
- Bishop, J.; Lane, M.; Dyar, M.; Brown, A. Reflectance and emission spectroscopy study of four groups of phyllosilicates: Smectites, kaolinite-serpentines, chlorites and micas. Clay Miner. 2008, 43, 35–54. [Google Scholar] [CrossRef]
- Besson, G.; Drits, V. Refined relationships between chemical composition of dioctahedral fine-grained micaceous minerals and their infrared spectra within the OH stretching region. Part II: The main factors affecting OH vibrations and quantitative analysis. Clays Clay Miner. 1997, 45, 170–183. [Google Scholar] [CrossRef]
- Bishop, J.; Murad, E.; Dyar, M. The influence of octahedral and tetrahedral cation substitution on the structure of smectites and serpentines as observed through infrared spectroscopy. Clay Miner. 2002, 37, 617–628. [Google Scholar] [CrossRef]
- Harris, D.C.; Bertolucci, M.D. Symmetry and Spectroscopy: An Introduction to Vibrational and Electronic Spectroscopy; Courier Corporation: North Chelmsford, MA, USA, 1989. [Google Scholar]
- Valášková, M.; Madejová, J.; Inayat, A.; Matějová, L.; Ritz, M.; Martaus, A.; Leštinský, P. Vermiculites from Brazil and Palabora: Structural changes upon heat treatment and influence on the depolymerization of polystyrene. Appl. Clay Sci. 2020, 192, 105639. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of OH Stretching Frequencies and OH O Hydrogen Bond Lengths in Minerals; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Brigatti, M.F.; Poppi, L. Crystal chemistry of Ba-rich trioctahedral micas-1M. Eur. J. Mineral. 1993, 5, 857–871. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Medici, L.; Poppi, L.; Vaccaro, C. Crystal chemistry of trioctahedral micas-1 M from the Alto Paranaíba Igneous Province, southeastern Brazil. Can. Mineral. 2001, 39, 1333–1345. [Google Scholar] [CrossRef]
- Laurora, A.; Brigatti, M.F.; Mottana, A.; Malferrari, D.; Caprilli, E. Crystal chemistry of trioctahedral micas in alkaline and subalkaline volcanic rocks: A case study from Mt. Sassetto (Tolfa district, Latium, central Italy). Am. Mineral. 2007, 92, 468–480. [Google Scholar] [CrossRef]




| Sample | Mineral | Color | Impurities (a.p.f.u) * |
|---|---|---|---|
| LFC5 | Phlogopite | Golden yellow | Cr, Mn, Ni, Ca, Zn = 0,Na, Ti < 0.07 |
| LFC6 | Phlogopite | Golden yellow | Cr, Mn, Ni, Ca, Zn, Ti = 0, Na < 0.08 |
| GC10 | Phlogopite | Golden yellow | Cr, Mn, Ni, Ca, Zn = 0, Na, Ti < 0.05 |
| JAG13 | Phlogopite | Golden yellow | Cr, Mn, Ni, Ca, Zn = 0,Na, Ti < 0.04 |
| JAG17 | Phlogopite | Brownish yellow | Cr, Mn, Ni, Ca, Zn = 0, Na, Ti < 0.09 |
| JAD04CC | Phlogopite | Brownish yellow | Cr, Mn, Ni, Ca, Zn = 0,Na, Ti < 0.16 |
| JAG18 | Mg–Biotite | Brownish yellow | Cr, Mn, Ni, Ca, Zn, Na < 0.02, Ti < 0.18 |
| G18 | Mg–Biotite | Brownish yellow | Cr, Mn, Ni, Ca, Zn, Na < 0.01, Ti < 0.13 |
| JAI-1 | Mg–Biotite | Sepia | Cr, Mn, Ni, Ca, Zn, Na < 0.02, Ti < 0.12 |
| WS660 [32] | Mg–Biotite | Sepia | Cr, Mn, Ni, Ca, Zn, Na < 0.01, Ti < 0.16 |
| HS28.3B [32] | Mg–Biotite | Sepia | Cr, Ni, Ca, Zn = 0, Mn, Na, Ti < 0.13 |
| K389048 [29] | Mg–Biotite | Sepia | Cr, Mn, Ni, Ca, Zn, Na < 0.02, Ti < 0.1 |
| XL07 | Annite | Sepia | Cr, Mn, Ni, Ca, Zn, Na < 0.04, Ti < 0.23 |
| NB98 | Annite | Sepia | Cr, Mn, Ni, Ca, Zn, Na < 0.01, Ti < 0.13 |
| Bt-8 [29] | Annite | Sepia | Cr, Mn, Ni, Ca, Zn = 0,Na, Ti < 0.12 |
| Sample | XL07 | NB98 | G18 | JAG18 | JAI-1 | LFC5 | LFC6 | JAG13 | JAD04 | GC10 | JAG17 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral (Point) | An (2) | An (3) | Bt (2) | Bt (3) | Bt (1) | Phl (1) | Phl (3) | Phl (2) | Phl (3) | Phl (1) | Phl (2) |
| Si | 2.752 | 2.768 | 2.958 | 2.905 | 2.760 | 3.000 | 3.097 | 2.904 | 2.930 | 2.870 | 2.842 |
| Al | 1.326 | 1.365 | 1.200 | 1.129 | 1.370 | 1.030 | 0.930 | 1.321 | 1.143 | 1.280 | 1.346 |
| Ti | 0.226 | 0.168 | 0.124 | 0.187 | 0.120 | 0.050 | 0.003 | 0.020 | 0.159 | 0.050 | 0.079 |
| Cr | 0.000 | 0.003 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 |
| Fe3+ | 0.191 | 0.165 | 0.124 | 0.099 | 0.170 | 0.080 | 0.028 | 0.014 | 0.063 | 0.050 | 0.054 |
| Fe2+ | 1.535 | 1.408 | 0.548 | 0.508 | 1.130 | 0.550 | 0.101 | 0.045 | 0.231 | 0.200 | 0.218 |
| Mn | 0.046 | 0.018 | 0.004 | 0.006 | 0.020 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.001 |
| Ni | 0.000 | 0.001 | 0.002 | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 |
| Mg | 0.698 | 0.902 | 1.822 | 1.990 | 1.330 | 2.180 | 2.791 | 2.622 | 2.310 | 2.500 | 2.346 |
| Ca | 0.000 | 0.001 | 0.001 | 0.002 | 0.010 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 |
| Na | 0.013 | 0.013 | 0.014 | 0.020 | 0.010 | 0.070 | 0.075 | 0.040 | 0.049 | 0.040 | 0.078 |
| K | 0.966 | 0.976 | 0.933 | 0.900 | 0.810 | 0.940 | 0.864 | 0.928 | 0.883 | 0.930 | 0.899 |
| Zn | 0.003 | 0.003 | 0.002 | 0.003 | 0.010 | 0.000 | 0.001 | 0.000 | 0.003 | 0.000 | 0.005 |
| F | 0.349 | 0.030 | 0.079 | 0.085 | — | 0.304 | 0.545 | 0.160 | 0.066 | 0.066 | 0.062 |
| Cl | 0.017 | 0.010 | 0.029 | 0.028 | — | 0.034 | 0.005 | 0.005 | 0.018 | 0.009 | 0.016 |
| Total | 8.119 | 7.832 | 7.838 | 7.864 | 7.740 | 8.238 | 8.439 | 8.058 | 7.858 | 7.995 | 7.947 |
| Mg# | 28.789 | 36.435 | 73.065 | 76.624 | 50.500 | 77.770 | 96.467 | 97.819 | 88.693 | 91.170 | 89.629 |
| AlIV | 1.249 | 1.232 | 1.043 | 1.095 | 1.240 | 1.000 | 0.903 | 1.096 | 1.070 | 1.130 | 1.159 |
| AlVI | 0.077 | 0.133 | 0.157 | 0.034 | 0.130 | 0.030 | 0.027 | 0.225 | 0.073 | 0.150 | 0.187 |
| FTIR Peak Positions in 400~600 cm−1 Region | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minerals Name | Minerals Name | Assignment [28,44,45,46] | [44] | [45] | Active | ||||||||
| Annite | Mg–Biotite | Phlogopite | |||||||||||
| XL07 | NB98 | G18 | JAG18 | JAD04 | JAG17 | JAG13 | GC10 | LFC5 | LFC6 | ||||
| 432 | 429 | 428 | 428 | 429 | 426 | 430 | 423 | 429 | 425 | — | 410 | — | IF,R |
| 453 | 447 | 438 | 443 | 444 | 443 | 448 | 443 | 441 | 445 | δ(Si–O–Mg) | 450 | — | IF |
| 470 | 465 | 462 | 462 | 465 | 466 | 467 | 465 | 462 | 465 | δ(Si–O–Si) | 470 | 460 | IF |
| 492 | 490 | 492 | 486 | 491 | 494 | 492 | 491 | 488 | 491 | ν(Mg–O) | 500 | 495 | IF |
| 517 | 515 | 521 | 511 | 517 | 521 | 516 | 508 | 513 | 516 | δ(Si–O–Si) | — | 520 | IF |
| — | — | 592 | 610 | 608 | 602 | — | 604 | 592 | 611 | OH Libration | 610 | 592 | IF |
| FTIR Peak Positions in 3600~3750 cm−1 Region | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minerals Name | Assignment | Minerals Name | Assignment [51,52,53] | [54] | [50] | Active | ||||||||
| Annite | Mg–Biotite | Phlogopite | ||||||||||||
| XL07 | NB98 | G18 | JAG18 | JAD04 | JAG17 | JAG13 | GC10 | LFC5 | LFC6 | |||||
| 3629 | 3627 | 3627 | 3626 | ν(AlAl□OH) | 3659 | 3649 | 3655 | 3648 | 3654 | 3655 | ν(MgMgFeOH) | 3629 | 3641 | IF,R |
| 3652 | 3648 | 3652 | 3645 | An: [38] ν(Fe2+Fe2+Al3+OH) Mg–Bt: ν(MgMgFeOH) | 3669 | 3662 | — | — | 3669 | 3671 | ν(MgMgFeOH) | 3653 | 3663 | IF,R |
| 3665 | 3669 | 3666 | 3669 | An: [38] ν(FeFeFeOH) Mg–Bt: ν(MgMgFeOH) | 3681 | 3675 | 3671 | 3668 | 3684 | 3679 | ν(MgMgFeOH) | — | — | IF,R |
| 3675 | 3676 | 3673 | 3686 | ν(Mg2FeOH) | — | — | — | — | — | — | — | — | — | |
| 3696 | 3698 | 3698 | 3703 | ν(MgMgMgOH) | — | — | — | — | 3694 | 3692 | ν(MgMgMgOH) | — | — | IF,R |
| 3710 | 3713 | 3706 | 3715 | ν(MgMgMgOH) | 3700 | 3695 | 3691 | 3691 | 3703 | 3707 | ν(MgMgMgOH) | 3668 | 3685 | IF,R |
| — | — | 3717 | — | ν(MgMgMgOH) | 3714 | 3714 | 3714 | 3712 | 3714 | 3719 | ν(MgMgMgOH) | 3703 | 3706 | IF,R |
| Raman Peak Positions in 3600~3750 cm−1 Region | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minerals Name | [58] | [59] | Assignment | Active | |||||||||
| Annite | Mg–Biotite | Phlogopite | |||||||||||
| XL07 | NB98 | G18 | JAG18 | JAD04 | JAG17 | JAG13 | GC10 | LFC5 | LFC6 | ||||
| — | 3627 | — | 3633 | 3630 | 3625 * | 3637 * | 3630 | 3639 | 3632 | — | — | OH Stretching | IF,R |
| — | 3644 | — | 3640 | — | — | 3645 | 3644 | 3648 | 3648 | 3641 | — | R | |
| 3658 | 3657 | — | 3651 | — | — | 3654 | 3655 | — | — | — | — | IF,R | |
| — | — | 3663 | 3663 | — | — | 3661 | — | 3663 | — | — | 3666 | IF,R | |
| 3673 | 3671 | 3677 | 3676 | 3674 | 3673 | 3675 | 3673 | 3678 | 3676 | — | — | IF,R | |
| — | 3686 | 3689 | 3689 | — | — | — | 3680 | 3687 | — | 3682 | — | R | |
| — | 3699 | 3699 | — | — | — | — | — | 3700 | — | — | IF,R | ||
| — | 3714 | 3707 | 3708 | 3716 | 3712 | 3716 | 3714 | 3707 | 3719 | 3698 | 3709 | IF,R | |
| — | — | — | — | — | — | — | 3714 | — | — | — | IF,R | ||
| — | — | — | — | — | — | — | — | 3741 | — | — | — | R | |
| — | — | — | — | 3754 | 3740 | 3736 | — | — | 3751 | — | — | R | |
| — | — | — | — | — | 3749 | 3745 | — | — | — | — | — | R | |
| Assignment | G18 | JAG18 | JAD04 | JAG17 | JAG13 | GC10 | LFC5 | LFC6 |
|---|---|---|---|---|---|---|---|---|
| The first fundamental overtone of ν(OH) | 7153 | 7142 | 7141 | 7141 | 7140 | 7136 | 7139 | 7140 |
| — | 7163 | 7167 | 7164 | 7165 | — | 7164 | 7165 | |
| 7176 | 7182 | 7182 | 7183 | 7181 | 7176 | 7180 | 7181 | |
| — | — | 7206 | — | 7203 | — | 7203 | 7205 | |
| — | — | — | — | — | — | 7233 | 7233 | |
| 7253 | 7241 | 7241 | 7244 | — | — | 7240 | 7240 | |
| 7277 | 7265 | 7258 | 7260 | 7261 | 7262 | 7261 | 7259 | |
| 7301 | — | 7295 | — | — | — | 7292 | 7294 | |
| 7318 | 7309 | 7308 | — | — | — | 7306 | 7308 | |
| — | — | 7329 | — | 7327 | — | 7326 | 7327 | |
| — | 7342 | 7343 | 7343 | 7343 | 7352 | 7342 | 7342 | |
| — | 7378 | 7379 | 7379 | 7379 | 7381 | 7377 | 7378 | |
| — | 7420 | 7424 | 7425 | 7422 | 7424 | 7418 | 7420 |
| Sample | Measured Peaks (cm−1) | Fundamental Peaks (cm−1) | Factor | Sample | Measured Peaks (cm−1) | Fundamental Peaks (cm−1) | Factor |
|---|---|---|---|---|---|---|---|
| LFC5 | Average: | 1.955 | G18 | Average: | 1.955 | ||
| 1 | 7259 | 3714 | 1.955 | 1 | 7258 | 3717 | 1.953 |
| 2 | 7240 | 3703 | 1.955 | 2 | 7237 | 3706 | 1.953 |
| 3 | 7233 | 3694 | 1.958 | 3 | 7222 | 3698 | 1.953 |
| 4 | 7205 | 3684 | 1.956 | 4 | 7195 | 3673 | 1.959 |
| 5 | 7181 | 3669 | 1.957 | 5 | 7176 | 3666 | 1.958 |
| 6 | 7165 | (3669 + 3654)/2 | 1.957 | 6 | 7153 | (3666 + 3652)/2 | 1.955 |
| 7 | 7140 | 3654 | 1.954 | 7 | 7130 | 3652 | 1.952 |
| 8 | 7118 | 3648 * | 1.951 | 8 | 7107 2nd | — | — |
| LFC6 | Average: | 1.955 | JAG18 | Average: | 1.956 | ||
| 1 | 7261 | 3719 | 1.952 | 1 | 7263 | 3715 | 1.955 |
| 2 | 7240 | 3707 | 1.953 | 2 | 7241 | 3703 | 1.955 |
| 3 | 7233 | 3692 | 1.959 | 3 | — | — | — |
| 4 | 7203 | 3679 | 1.958 | 4 | 7219 2nd | 3686 | 1.958 |
| 5 | 7180 | 3671 | 1.956 | 5 | 7182 | 3669 | 1.958 |
| 6 | 7164 | (3671 + 3655)/2 | 1.956 | 6 | 7163 | (3669 + 3645)/2 | 1.959 |
| 7 | 7139 | 3655 | 1.953 | 7 | 7142 | 3645 | 1.959 |
| 8 | 7117 | 3648 * | 1.951 | 8 | 7119 2nd | 3640 * | 1.956 |
| JAG13 | Average: | 1.954 | JAD04 | Average: | 1.956 | ||
| 1 | 7261 | 3714 | 1.955 | 1 | 7258 | 3714 | 1.954 |
| 2 | 7242 2nd | — | — | 2 | 7241 | 3700 | 1.957 |
| 3 | — | — | — | 3 | — | — | — |
| 4 | 7203 | 3691 | 1.952 | 4 | 7206 | 3681 | 1.958 |
| 5 | 7181 | 3671 | 1.956 | 5 | 7182 | 3669 | 1.957 |
| 6 | 7165 | (3671 + 3655)/2 | 1.956 | 6 | 7167 | (3669 + 3659)/2 | 1.956 |
| 7 | 7140 | 3655 | 1.953 | 7 | 7141 | 3659 | 1.952 |
| 8 | 7119 | 3645 * | 1.953 | 8 | 7119 | — | — |
| GC10 | Average: | 1.954 | JAG17 | Average: | 1.956 | ||
| 1 | 7262 | 3712 | 1.956 | 1 | 7260 | 3714 | 1.955 |
| 2 | 7240 | — | — | 2 | 7240 | 3695 | 1.959 |
| 3 | — | — | — | 3 | — | — | — |
| 4 | 7208 2nd | 3691 | 1.953 | 4 | — | — | — |
| 5 | 7176 | 3668 | 1.956 | 5 | 71792nd | 3675 | 1.953 |
| 6 | 7160 2nd | (3668 + 3648)/2 | 1.957 | 6 | 7164 | 3662 | 1.956 |
| 7 | 7136 | 3648 | 1.956 | 7 | 7141 | 3649 | 1.957 |
| 8 | 7090 2nd | 3644 * | 1.946 | 8 | 71162nd | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; He, M.; Wu, S.; Yang, M.; Peng, B. Near-Infrared Spectroscopic Study of Biotite–Phlogopite (Mg# = 30~99): OH-Stretching Modes and Mg# Content Prediction Equation. Crystals 2024, 14, 336. https://doi.org/10.3390/cryst14040336
Yang Z, He M, Wu S, Yang M, Peng B. Near-Infrared Spectroscopic Study of Biotite–Phlogopite (Mg# = 30~99): OH-Stretching Modes and Mg# Content Prediction Equation. Crystals. 2024; 14(4):336. https://doi.org/10.3390/cryst14040336
Chicago/Turabian StyleYang, Zhentao, Mingyue He, Shaokun Wu, Mei Yang, and Bijie Peng. 2024. "Near-Infrared Spectroscopic Study of Biotite–Phlogopite (Mg# = 30~99): OH-Stretching Modes and Mg# Content Prediction Equation" Crystals 14, no. 4: 336. https://doi.org/10.3390/cryst14040336
APA StyleYang, Z., He, M., Wu, S., Yang, M., & Peng, B. (2024). Near-Infrared Spectroscopic Study of Biotite–Phlogopite (Mg# = 30~99): OH-Stretching Modes and Mg# Content Prediction Equation. Crystals, 14(4), 336. https://doi.org/10.3390/cryst14040336






