Research on the Stability of Different Polar Surfaces in Aluminum Nitride Single Crystals
Abstract
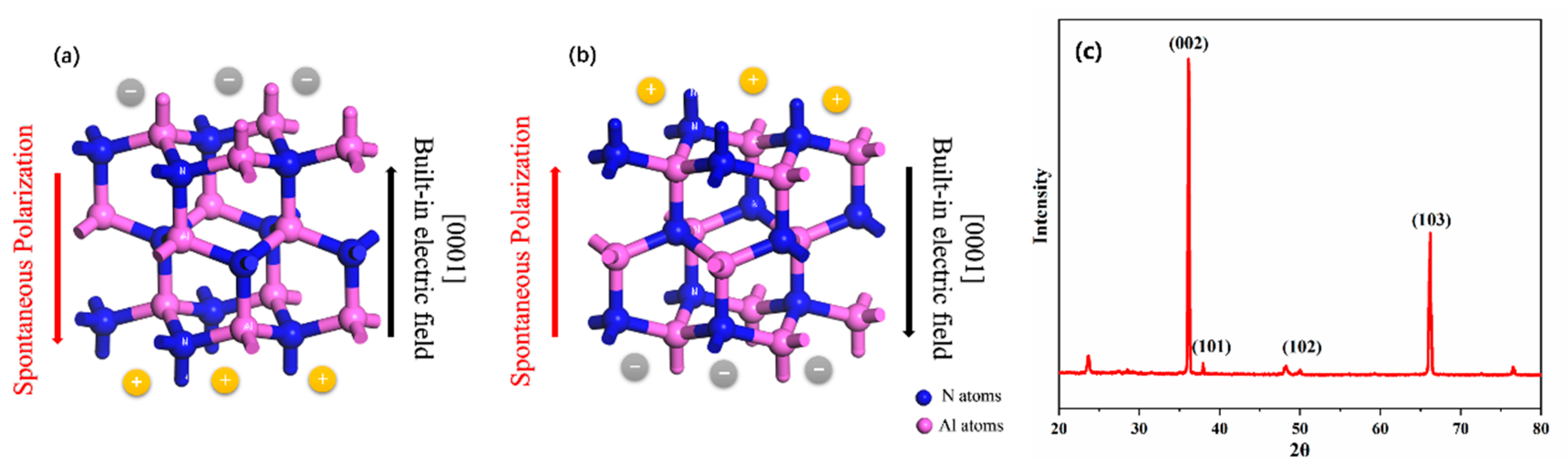
1. Introduction
2. Experiment
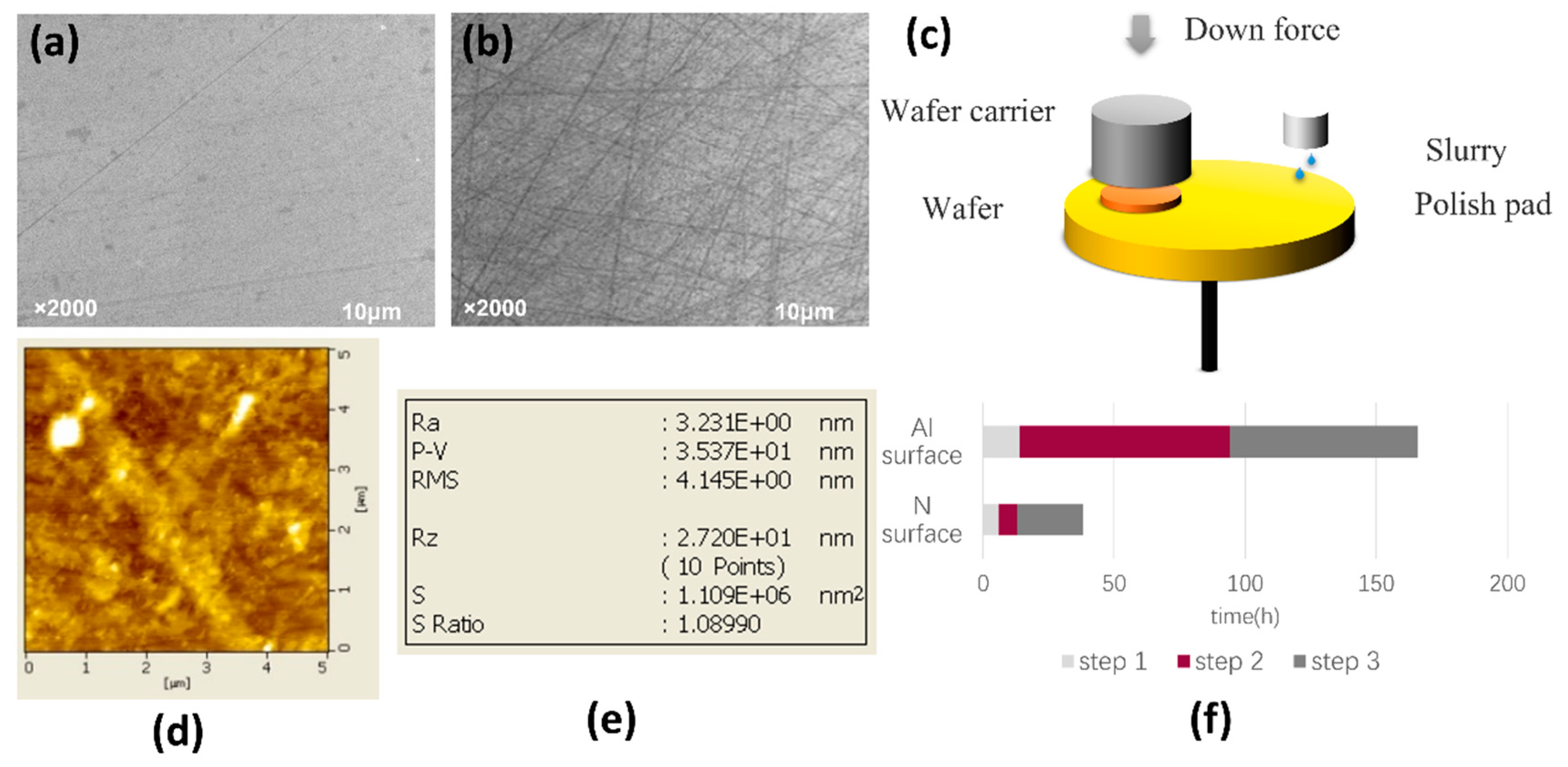
2.1. Polishing of AlN Crystal
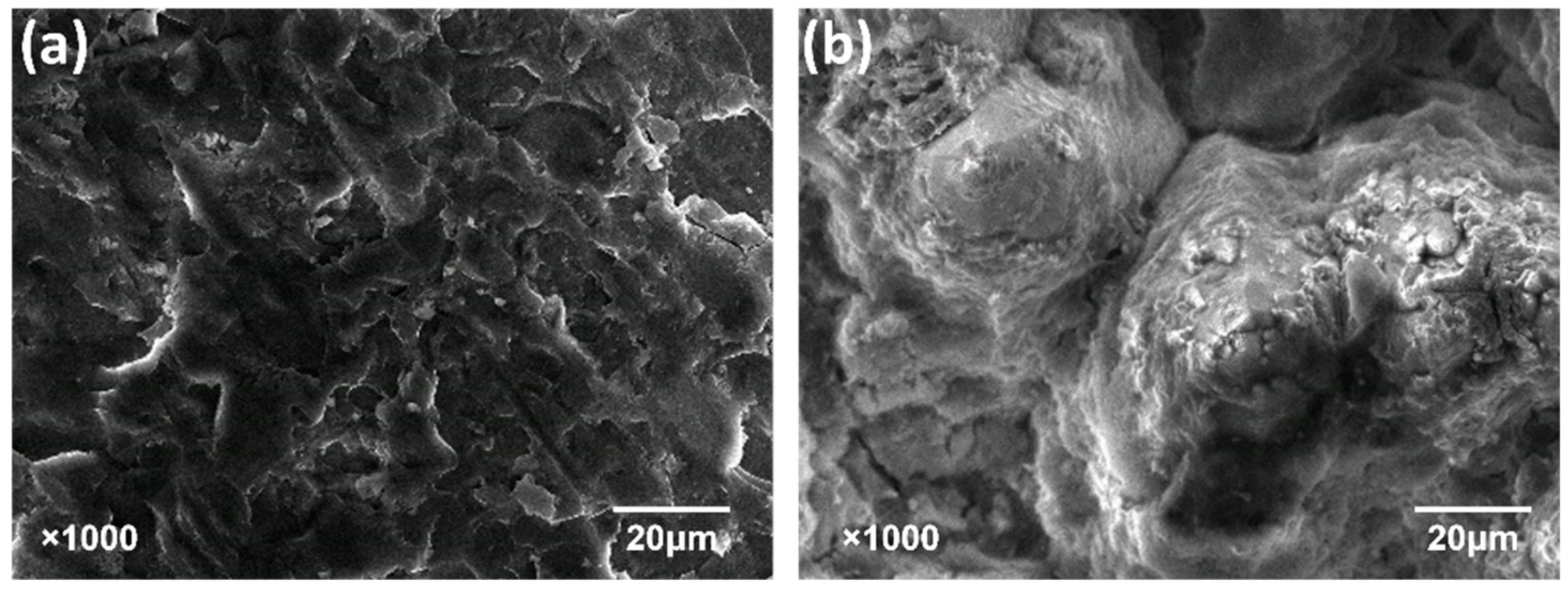
2.2. Corrosion Experiment of AlN Crystal
3. Discussion
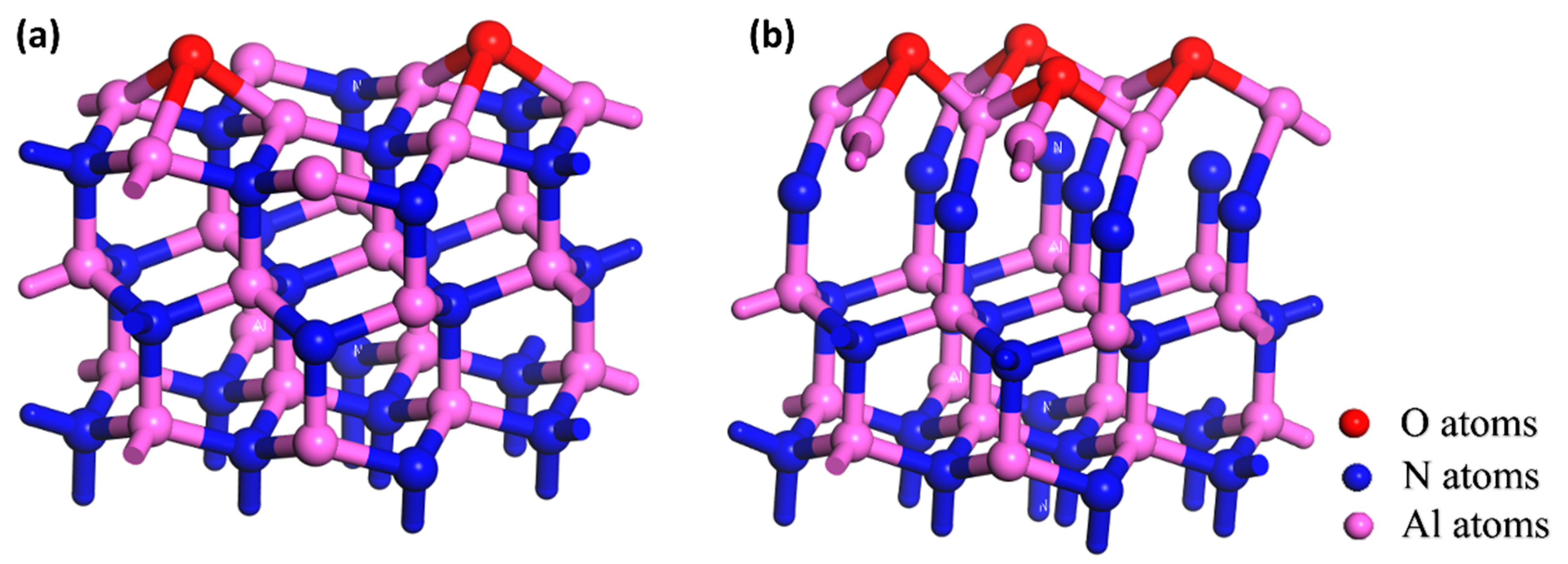
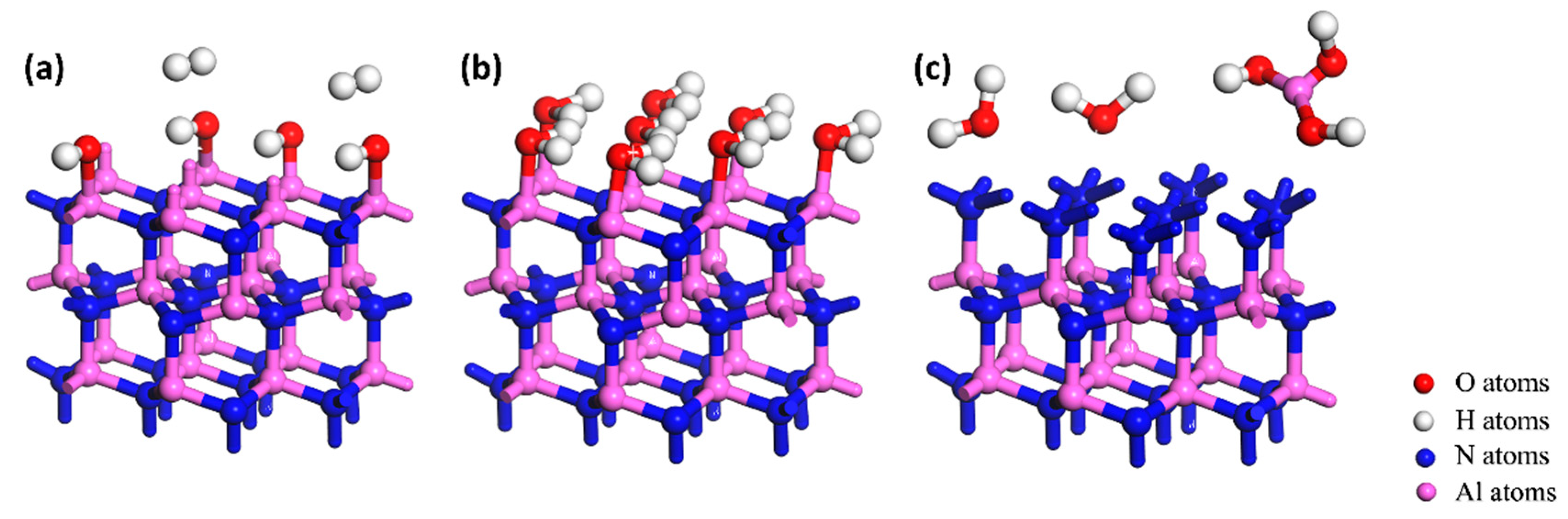
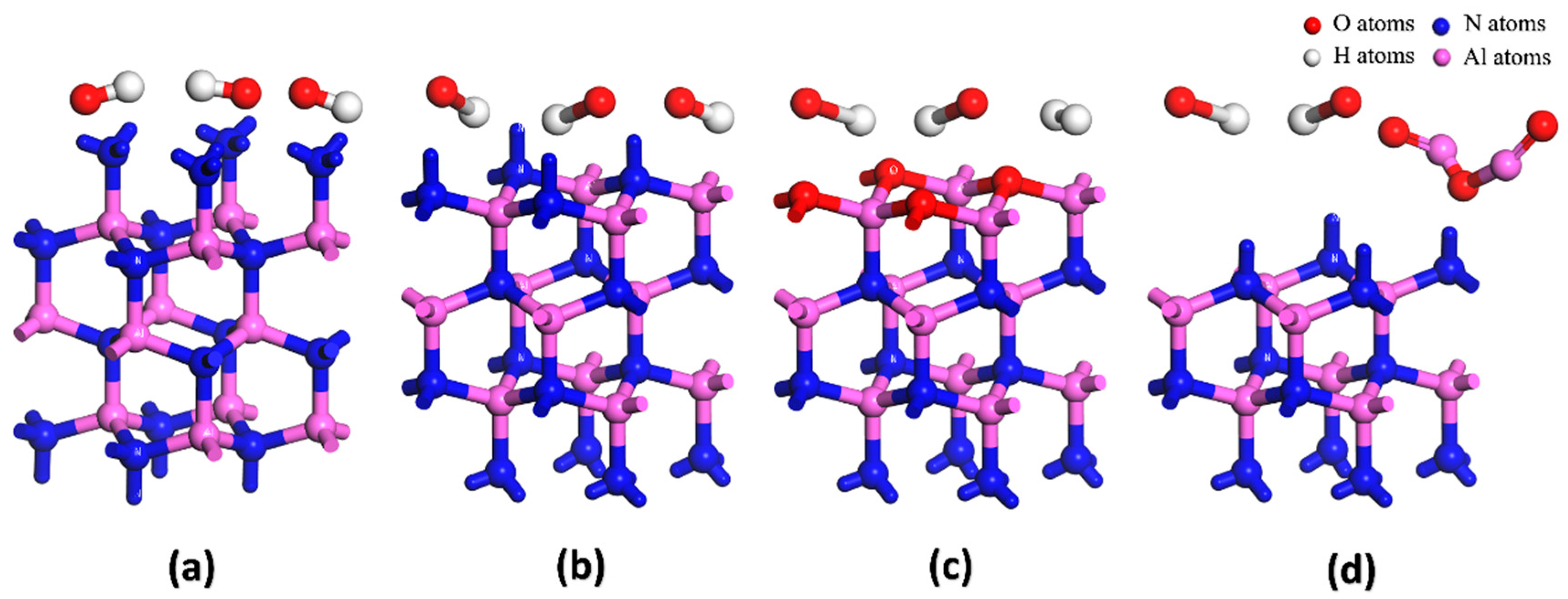
3.1. Differences in the Oxidation of the Polar Surface of AlN Crystal
3.2. Hydrolyzation Differences of the Polar Surfaces of AlN Crystal
3.3. Differences in Corrosion of AlN Crystal Polar Surface in an Alkaline Environment
3.4. Surface Energy Difference of Polar Surfaces in AlN Crystal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Guo, Y.N.; Yan, J.C.; Wu, Q.Q.; Wei, X.C.; Wang, J.X.; Li, J.M. Deep ultraviolet light-emitting diodes with improved performance via nanoporous AlGaN template. Opt. Express 2019, 27, 4917–4926. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yan, C.Y.; Zhou, G.G.; Wen, J.M.; Qin, Z.Y.; Zhou, Q.; Li, B.K.; Zheng, R.S.; Wu, H.L.; Sun, Z.H. Broadband White-Light Emission from Alumina Nitride Bulk Single Crystals. ACS Photonics 2018, 5, 4009–4013. [Google Scholar] [CrossRef]
- Islam, S.M.; Protasenko, V.; Lee, K.; Rouvimov, S.; Verma, J.; Xing, H.L.; Jena, D. Deep-UV emission at 219 nm from ultrathin MBE GaN/AlN quantum heterostructures. Appl. Phys. Lett. 2017, 111, 091104. [Google Scholar] [CrossRef]
- Xiong, C.; Pernice, W.H.P.; Tang, H.X. Low-loss; silicon integrated, Aluminium nitride photonic circuits and their use for electro-optic signal processing. Nano Lett. 2012, 12, 3562–3568. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Huang, F.; Zheng, R.S.; Wu, H.L. Low-Dimensional Structure Vacuum-Ultraviolet-Sensitive (λ < 200 nm) Photodetector with Fast-Response Speed Based on High-Quality AlN Micro/Nanowire. Adv. Mater. 2015, 27, 3921–3927. [Google Scholar]
- Horng, R.H.; Cho, P.H.; Chang, J.C.; Singh, A.K.; Jhang, S.Y.; Liu, P.L.; Wuu, D.S.; Bairagi, S.; Chen, C.H.; Järrendahl, K.; et al. Growth and Characterization of Sputtered InAlN Nanorods on Sapphire Substrates for Acetone Gas Sensing. Nanomaterials 2024, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Levinshtein, M.E.; Rumyantsev, S.L. Properties of Advanced Semiconductor Materials: GaN, AIN, InN, BN, SiC, SiGe; John Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Grandusky, J.R.; Gibb, S.R.; Mendrick, M.C.; Moe, C.; Wraback, M.; Schowalter, L.J. High output power from 260 nm pseudomorphic ultraviolet light-emitting diodes with improved thermal performance. Appl. Phys. Express 2011, 4, 082101. [Google Scholar] [CrossRef]
- Grandusky, J.R.; Chen, J.F.; Gibb, S.R.; Mendrick, M.C.; Moe, C.G.; Rodak, L.; Garrett, G.A.; Wraback, M.; Schowalter, L.J. 270 nm pseudomorphic ultraviolet light-emitting diodes with over 60 mW continuous wave output power. Appl. Phys. Express 2013, 6, 032101. [Google Scholar] [CrossRef]
- Stutzmann, M.; Ambacher, O.; Eickhoff, M.; Karrer, U.; Pimenta, A.L.; Neuberger, R.; Schalwig, J.; Dimitrov, R.; Schuck, P.J.; Grober, R.D. Playing with polarity. Phys. Status Solidi Basic Res. 2001, 228, 505–512. [Google Scholar] [CrossRef]
- Zuniga-Perez, J.; Consonni, V.; Lymperakis, L.; Kong, X.; Trampert, A.; Fernandez-Garrido, S.; Brandt, O.; Renevier, H.; Keller, S.; Hestroffer, K.; et al. Polarity in GaN and ZnO: Theory, measurement, growth, and devices. Appl. Phys. Rev. 2016, 3, 041303. [Google Scholar] [CrossRef]
- Feng, S.W.; Liao, P.H.; Leung, B.; Han, J.; Yang, F.W.; Wang, H.C. Efficient carrier relaxation and fast carrier recombination of N -polar InGaN/GaN light emitting diodes. J. Appl. Phys. 2015, 118, 043104. [Google Scholar] [CrossRef]
- Taniyasu, Y.; Kasu, M. Improved emission efficiency of 210-nm deep-ultraviolet Aluminium nitride light-emitting diode. NTT Tech. Rev. 2010, 8, 5. [Google Scholar]
- Weyher, J.L. Defect sensitive etching of nitrides: Appraisal of methods. Cryst. Res. Technol. 2012, 47, 333–340. [Google Scholar] [CrossRef]
- Chen, W.-H.; Qin, Z.-Y.; Tian, X.-Y.; Zhong, X.-H.; Sun, Z.-H.; Li, B.-K.; Zheng, R.-S.; Guo, Y.; Wu, H.-L. The physical vapor transport method for bulk AlN crystal growth. Molecules 2019, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhao, K.; Xu, S.H.; Qin, Z.Y.; Cheng, H.J.; Zhang, L.; Qi, H.T.; Li, J.; Zheng, R.S.; Wu, H.L. Optical property in colorless AlN bulk crystals: Investigation of native defect-induced UV absorption. Scr. Mater. 2021, 190, 91–96. [Google Scholar] [CrossRef]
- Bickermann, M.; Schmidt, S.; Epelbaum, B.M.; Heimann, P.; Nagata, S.; Winnacker, A. Defect-selective etching of aluminum nitride single crystals. Phys. Status Solidi Curr. Top. Solid State Phys. 2007, 4, 2609–2612. [Google Scholar] [CrossRef]
- Slack, G.A.; McNelly, T.F. Growth of high purity AlN crystals. J. Cryst. Growth 1976, 34, 263–279. [Google Scholar] [CrossRef]
- Ansart, F.; Bernard, J.; Traverse, J.P. Oxidation of aluminium nitride at high temperatures. Evaluation of the progression rate of the oxidation front. Adv. Mater. 1994, 93, 285–289. [Google Scholar]
- Bellosi, A.; Landi, E.; Tampieri, A. Oxidation behavior of Aluminium nitride. J. Mater. Res. 1993, 8, 565–572. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Tuan, W.-H. Oxidation mechanism of Aluminium nitride revisited. J. Adv. Ceram. 2017, 6, 27–32. [Google Scholar] [CrossRef]
- Ye, H.; Chen, G.; Zhu, Y.; Wei, S.H. Asymmetry of adsorption of oxygen at wurtzite AlN (0001) and (000-1) surfaces: First-principles calculations. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 3–6. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.; Nam, S.J.; Hun, Y.; Seo, J.; Lee, H.; Park, K.S. Inductively coupled plasma etching of III-N layers by using a Cl2/N2 plasma. J. Korean Phys. Society 2002, 41, L184–L187. [Google Scholar]
- Fukumoto, S.; Hookabe, T.; Tsubakino, H. Hydrolysis behavior of Aluminium nitride in various solutions. J. Mater. Sci. 2000, 35, 2743–2748. [Google Scholar] [CrossRef]
- Kocjan, A.; Dakskobler, A.; Krnel, K.; Kosmač, T. The course of the hydrolysis and the reaction kinetics of AlN powder in diluted aqueous suspensions. J. Eur. Ceram. Soc. 2011, 31, 815–823. [Google Scholar] [CrossRef]
- Hu, C.L.; Chen, Y.L.; Li, J.Q. First-principles Calculations of H2O Adsorption Reaction on the GaN(0001) Surface. Chin. J. Struct. Chem. 2009, 28, 240–244. [Google Scholar]
- Chen, Y.; Hou, X.; Fang, Z.; Wang, E.; Chen, J.; Bei, G. Adsorption and Reaction of Water on the AlN(0001) Surface from First Principles. J. Phys. Chem. C 2019, 123, 5460–5468. [Google Scholar] [CrossRef]
- Northrup, J.E.; Di Felice, R.; Neugebauer, J. Atomic structure and stability of AlN(0001) and (000̱1) surfaces. Phys. Rev. B Condens. Matter Mater. Phys. 1997, 55, 13878–13883. [Google Scholar] [CrossRef]
- Holec, D.; Mayrhofer, P.H. Surface energies of AlN allotropes from first principles. Scr. Mater. 2012, 67, 760–762. [Google Scholar] [CrossRef]
- Dreyer, C.E.; Janotti, A.; Van De Walle, C.G. Absolute surface energies of polar and nonpolar planes of GaN. Phys. Rev. B Condens. Matter Mater. Phys. 2014, 89, 081305. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Ren, Y.; Tan, X.; Xu, P. First principle calculations study of AlN surface terminal structure evolution under different conditions. Surf. Interface Anal. 2020, 52, 167–173. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, W.; Qin, Z.; Jin, L.; Sun, Z.; Wu, H. Research on the Stability of Different Polar Surfaces in Aluminum Nitride Single Crystals. Crystals 2024, 14, 337. https://doi.org/10.3390/cryst14040337
Liu Z, Li W, Qin Z, Jin L, Sun Z, Wu H. Research on the Stability of Different Polar Surfaces in Aluminum Nitride Single Crystals. Crystals. 2024; 14(4):337. https://doi.org/10.3390/cryst14040337
Chicago/Turabian StyleLiu, Zhao, Wenliang Li, Zuoyan Qin, Lei Jin, Zhenhua Sun, and Honglei Wu. 2024. "Research on the Stability of Different Polar Surfaces in Aluminum Nitride Single Crystals" Crystals 14, no. 4: 337. https://doi.org/10.3390/cryst14040337
APA StyleLiu, Z., Li, W., Qin, Z., Jin, L., Sun, Z., & Wu, H. (2024). Research on the Stability of Different Polar Surfaces in Aluminum Nitride Single Crystals. Crystals, 14(4), 337. https://doi.org/10.3390/cryst14040337






