Manufacturing of Sapphire Crystals with Variable Shapes for Cryosurgical Applications
Abstract
:1. Introduction
2. Materials and Methods
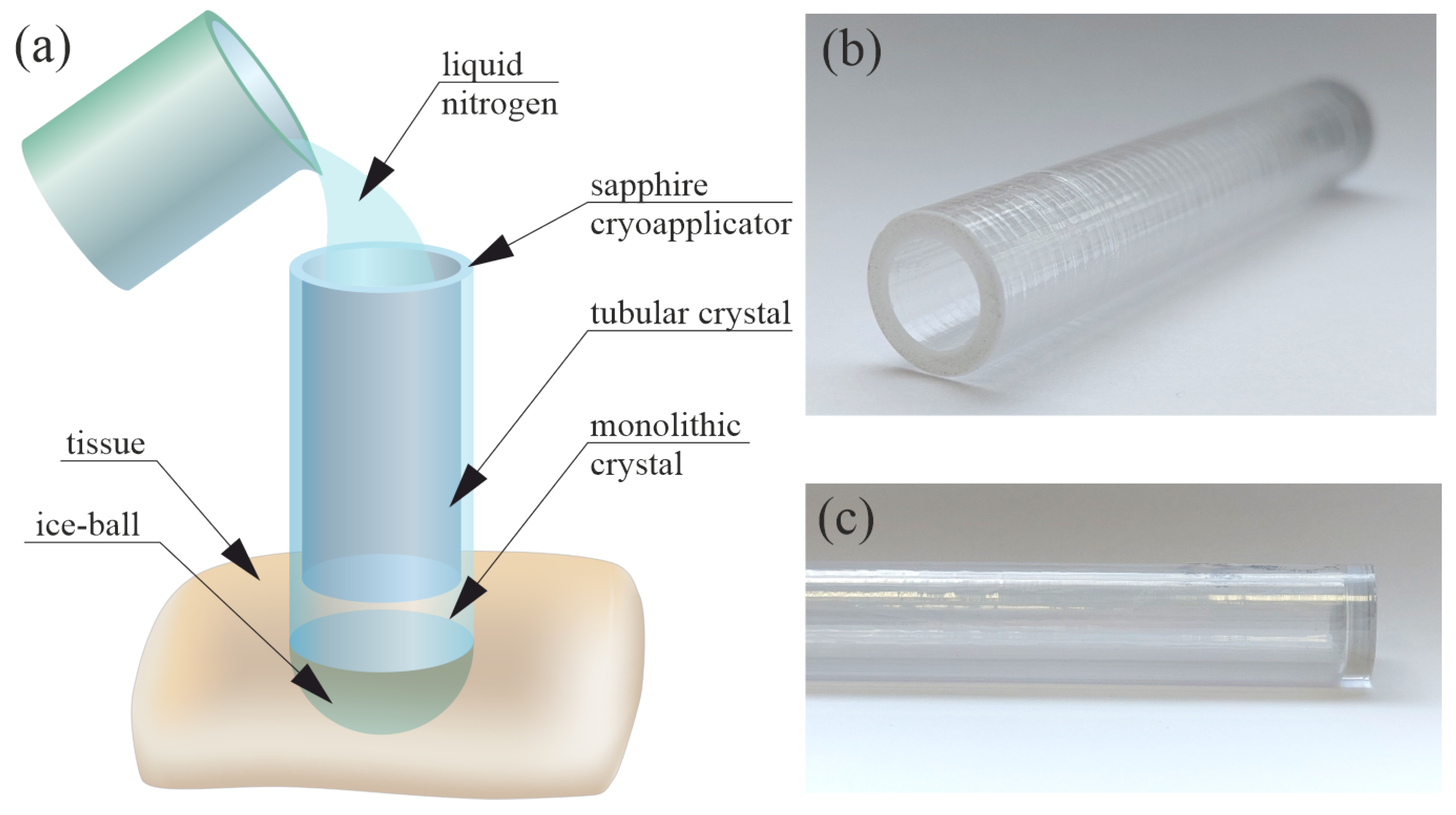
2.1. Manufacture of a Hollow Sapphire Crystal for Cryosurgery
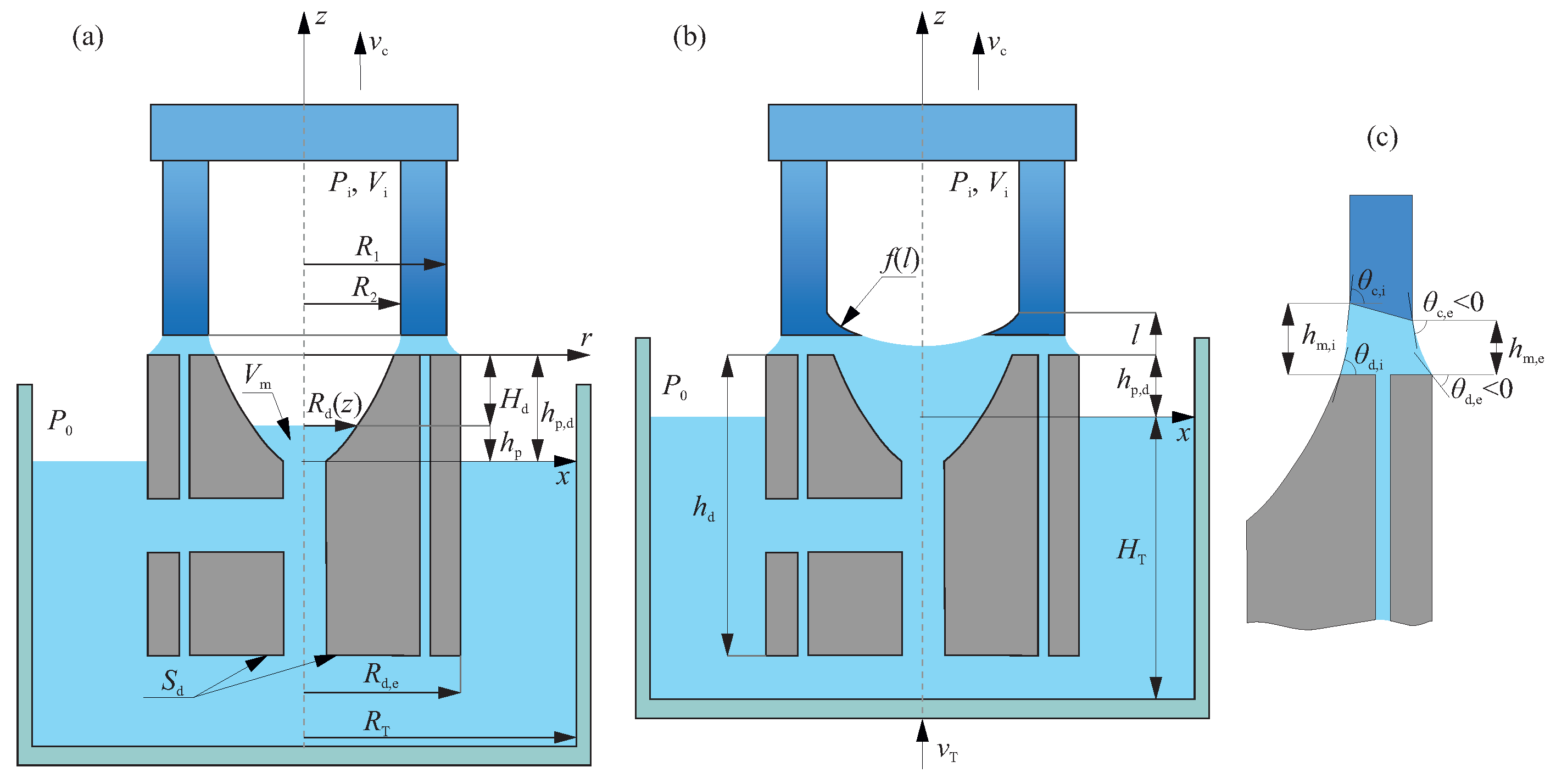
2.2. Mathematical Description of the Weight Signal
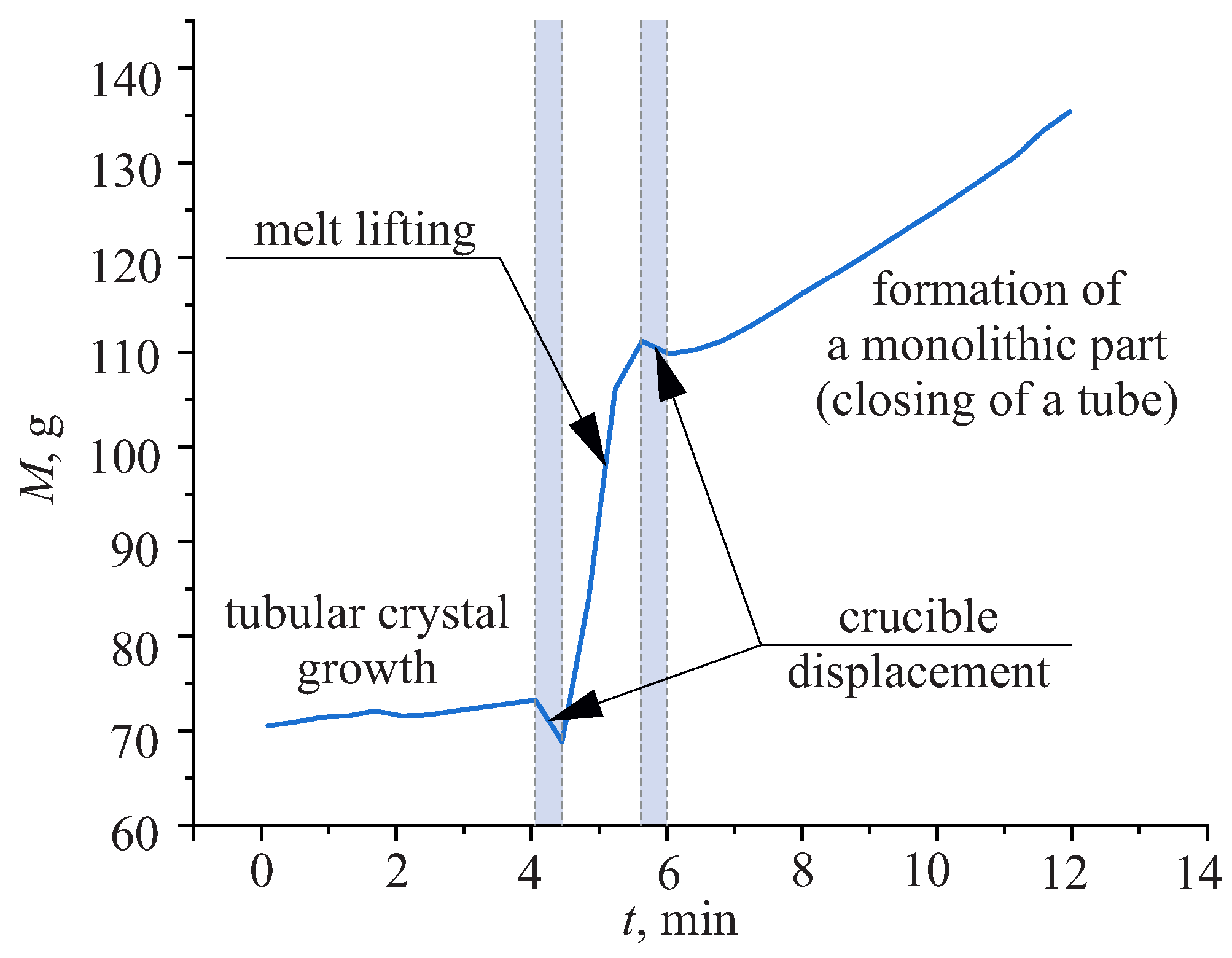
2.2.1. The Stage of a Melt Lifting
2.2.2. The Stage of a Monolithic Part Formation
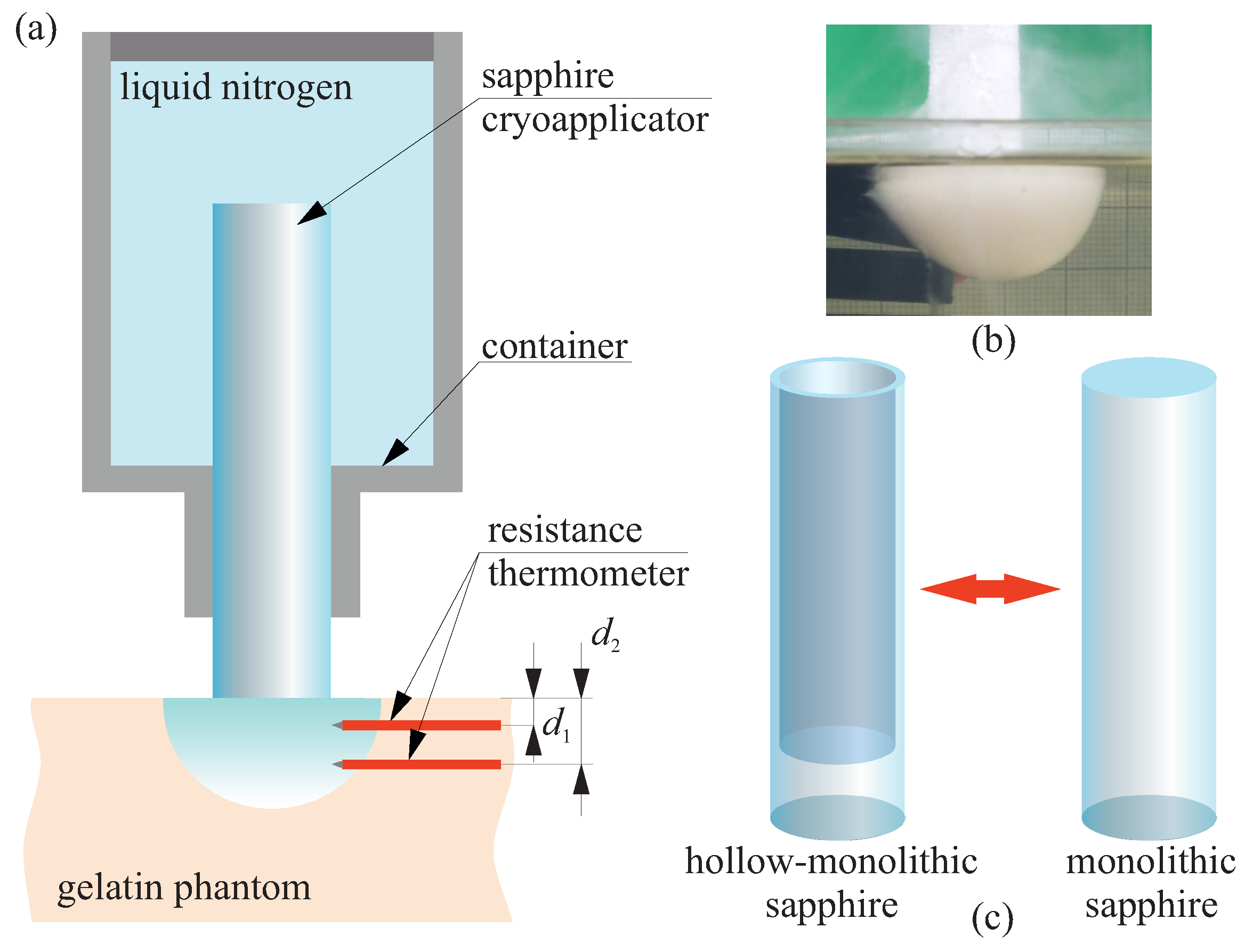
2.3. Study the Advances of Hollow Sapphire Applicator for Cryosurgery
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dobrovinskaya, E.R.; Lytvynov, L.A.; Pishchik, V. Sapphire: Material, Manufacturing, Applications; Springer: Boston, MA, USA, 2009. [Google Scholar] [CrossRef]
- Hutchens, T.C.; Darafsheh, A.; Fardad, A.; Antoszyk, A.N.; Ying, H.S.; Astratov, V.N.; Fried, N.M. Detachable microsphere scalpel tips for potential use in ophthalmic surgery with the erbium:YAG laser. J. Biomed. Opt. 2014, 19, 018003. [Google Scholar] [CrossRef] [PubMed]
- Polletto, T.J.; Ngo, A.K.; Tchapyjnikov, A.; Levin, K.; Tran, D.; Fried, N.M. Comparison of germanium oxide fibers with silica and sapphire fiber tips for transmission of erbium: YAG laser radiation. Lasers Surg. Med. 2006, 38, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Tatla, T.; Nissanka-Jayasuriya, E.; Yuan, A.Y.; Stoyanov, D.; Elson, D.S. Surgical polarimetric endoscopy for the detection of laryngeal cancer. Nat. Biomed. Eng. 2023, 7, 971–985. [Google Scholar] [CrossRef]
- Ramli, R.; Chung, C.C.; Fried, N.M.; Franco, N.; Hayman, M.H. Subsurface tissue lesions created using an Nd:YAG laser and a sapphire contact cooling probe. Lasers Surg. Med. 2004, 35, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bian, H.; Song, X.; Lei, Y.; Sun, M.; Long, W.; Zhong, S.; Jia, L. Interfacial microstructure and mechanical properties of titanium/sapphire joints brazed with AuSn20 filler metal. Crystals 2022, 12, 1687. [Google Scholar] [CrossRef]
- Khalifa, A.A.; Bakr, H.M. Updates in biomaterials of bearing surfaces in total hip arthroplasty. Arthroplasty 2021, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Dolganova, I.N.; Zotov, A.K.; Safonova, L.P.; Aleksandrova, P.V.; Reshetov, I.V.; Zaytsev, K.I.; Tuchin, V.V.; Kurlov, V.N. Feasibility test of a sapphire cryoprobe with optical monitoring of tissue freezing. J. Biophotonics 2023, 16, e202200288. [Google Scholar] [CrossRef] [PubMed]
- Dolganova, I.N.; Shikunova, I.A.; Zotov, A.K.; Shchedrina, M.A.; Reshetov, I.V.; Zaytsev, K.I.; Tuchin, V.V.; Kurlov, V.N. Microfocusing sapphire capillary needle for laser surgery and therapy: Fabrication and characterization. J. Biophotonics 2020, 13, e202000164. [Google Scholar] [CrossRef] [PubMed]
- Stock, K.; Stegmayer, T.; Graser, R.; Förster, W.; Hibst, R. Comparison of different focusing fiber tips for improved oral diode laser surgery. Lasers Surg. Med. 2012, 44, 815–823. [Google Scholar] [CrossRef]
- Eguro, T.; Aoki, A.; Maeda, T.; Takasaki, A.A.; Hasegawa, M.; Ogawa, M.; Suzuki, T.; Yonemoto, K.; Ishikawa, I.; Izumi, Y.; et al. Energy output reduction and surface alteration of quartz and sapphire tips following Er:YAG laser contact irradiation for tooth enamel ablation. Lasers Surg. Med. 2009, 41, 595–604. [Google Scholar] [CrossRef]
- Bao, S.H.; Zheng, D.S.; Zhang, S.H.; Yu, G.R.; Lu, H.H.; Gai, B.K. Assessment of Nd:YAG laser via metal cap and sapphire tip delivery system: An experimental and clinical investigation. Chin. Med J. 1993, 106, 61–64. [Google Scholar] [PubMed]
- Ahmad, M.; Ismail, M. Effect of different shapes of recipient site creation micro-blades at varying angles and wound injury. J. Cosmet. Dermatol. 2021, 20, 3610–3615. [Google Scholar] [CrossRef]
- Tatartchenko, V.A. Shaped Crystal Growth. In Springer Handbook of Crystal Growth; Dhanaraj, G., Byrappa, K., Prasad, V., Dudley, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 509–556. [Google Scholar] [CrossRef]
- Kurlov, V.N.; Rossolenko, S.N.; Abrosimov, N.V.; Lebbou, K. Shaped Crystal Growth. In Crystal Growth Processes Based on Capillarity: Czochralski, Floating Zone, Shaping and Crucible Techniques; Duffar, T., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; Chapter 5; pp. 277–354. [Google Scholar] [CrossRef]
- Akselrod, M.S.; Bruni, F.J. Modern trends in crystal growth and new applications of sapphire. J. Cryst. Growth 2012, 360, 134–145. [Google Scholar] [CrossRef]
- Gong, S.; Zhu, X.; Sun, Y.; Tang, B.; Su, Z. Experimental research on surface characteristics and subsurface damage behavior of monocrystal sapphire induced by helical micro abrasive tools. Ceram. Int. 2022, 48, 21459–21472. [Google Scholar] [CrossRef]
- LaBelle, H. EFG, the invention and application to sapphire growth. J. Cryst. Growth 1980, 50, 8–17. [Google Scholar] [CrossRef]
- Rossolenko, S.N.; Kurlov, V.N.; Asrian, A.A. Analysis of the profile curves of the menisci for the sapphire tubes growth by EFG (Stepanov) technique. Cryst. Res. Technol. 2009, 44, 689–700. [Google Scholar] [CrossRef]
- Kurlov, V. The noncapillary shaping (NCS) method: A new method of crystal growth. J. Cryst. Growth 1997, 179, 168–174. [Google Scholar] [CrossRef]
- Bunoiu, O.M.; Nicoara, I.; Santailler, J.L.; Theodore, F.; Duffar, T. On the void distribution and size in shaped sapphire crystals. Cryst. Res. Technol. 2005, 40, 852–859. [Google Scholar] [CrossRef]
- Borodin, V.A.; Steriopolo, T.A.; Tatarchenko, V.A. The variable shaping technique – a new method of the sapphire growth. Cryst. Res. Technol. 1985, 20, 833–836. [Google Scholar] [CrossRef]
- Borodin, V.A.; Steriopolo, T.A.; Tatarchenko, V.A.; Yalovets, T.N. Control over gas bubble distribution in shaped sapphire crystals. Cryst. Res. Technol. 1985, 20, 301–306. [Google Scholar] [CrossRef]
- Pasquali, P.; Mickeviciute, G. Cryosurgery. In European Handbook of Dermatological Treatments; Katsambas, A.D., Lotti, T.M., Dessinioti, C., D’Erme, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1217–1228. [Google Scholar] [CrossRef]
- Gage, A.A.; Baust, J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998, 37, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Korpan, N.N. (Ed.) Basics of Cryosurgery; Springer: Vienna, Austria, 2001. [Google Scholar]
- Deng, W.; Chen, L.; Wang, Y.; Liu, X.; Wang, G.; Liu, W.; Zhang, C.; Zhou, X.; Li, Y.; Fu, B. Cryoablation versus partial nephrectomy for clinical stage T1 renal masses: A systematic review and meta-analysis. J. Cancer 2019, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
- Backman, E.; Polesie, S.; Berglund, S.; Gillstedt, M.; Sjöholm, A.; Modin, M.; Paoli, J. Curettage vs. cryosurgery for superficial basal cell carcinoma: A prospective, randomised and controlled trial. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Pustinsky, I.; Dvornikov, A.; Kiva, E.; Chulkova, S.; Egorova, A.; Gladilina, I.; Peterson, S.; Lepkova, N.; Grishchenko, N.; Galaeva, Z.; et al. Cryosurgery for basal cell skin cancer of the head: 15 years of experience. Life 2023, 13, 2231. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.P.; Chang, A.; Sze, C.; Polascik, T.J. Oncological and Functional Outcomes of Patients Undergoing Individualized Partial Gland Cryoablation of the Prostate: A Single-Institution Experience. J. Endourol. 2021, 35, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.K.; Miller, R.J.; Ahmed, S.; Lotz, M.J.; Baust, J. Ten-Year Biochemical Disease Control for Patients with Prostate Cancer Treated with Cryosurgery as Primary Therapy. Urology 2008, 71, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, K.; Kodresko, A.; Ghazal, H.; Mokbel, R.; Trembley, J.; Jouhara, H. The Evolving Role of Cryosurgery in Breast Cancer Management: A Comprehensive Review. Cancers 2023, 15, 4272. [Google Scholar] [CrossRef]
- Kwong, A.; Co, M.; Fukuma, E. Prospective Clinical Trial on Expanding Indications for Cryosurgery for Early Breast Cancers. Clin. Breast Cancer 2023, 23, 363–368. [Google Scholar] [CrossRef]
- Baust, J.; Gage, A.; Bjerklund Johansen, T.; Baust, J. Mechanisms of cryoablation: Clinical consequences on malignant tumors. Cryobiology 2014, 68, 1–11. [Google Scholar] [CrossRef]
- Surtees, B.; Young, S.; Hu, Y.; Wang, G.; McChesney, E.; Kuroki, G.; Acree, P.; Thomas, S.; Blair, T.; Rastogi, S.; et al. Validation of a low-cost, carbon dioxide-based cryoablation system for percutaneous tumor ablation. PLoS ONE 2019, 14, e0207107. [Google Scholar] [CrossRef]
- Ward, R.C.; Lourenco, A.P.; Mainiero, M.B. Ultrasound-Guided Breast Cancer Cryoablation. Am. J. Roentgenol. 2019, 213, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Velez, A.; DeMaio, A.; Sterman, D. Cryoablation and immunity in non-small cell lung cancer: A new era of cryo-immunotherapy. Front. Immunol. 2023, 14, 1203539. [Google Scholar] [CrossRef] [PubMed]
- Ranjbartehrani, P.; Etheridge, M.; Ramadhyani, S.; Natesan, H.; Bischof, J.; Shao, Q. Characterization of Miniature Probes for Cryosurgery, Thermal Ablation, and Irreversible Electroporation on Small Animals. Adv. Ther. 2022, 5, 2100212. [Google Scholar] [CrossRef]
- Goette, J.; Weimar, T.; Vosseler, M.; Raab, M.; Walle, U.; Czesla, M.; Doll, N. Freezing Equals Freezing? Performance of Two Cryoablation Devices in Concomitant Mitral Valve Repair. Thorac. Cardiovasc. Surg. 2016, 64, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Steblyuk, A.N.; Gunter, V.E.; Khodorenko, V.N.; Bykova, E.V.; Avakimyan, R.A.; Geiko, I.A.; Dmitrieva, A.L. Results of chalazion cryosurgery with an increased risk of complications. Acta Biomed. Sci. 2021, 6, 181–189. (In Russian) [Google Scholar] [CrossRef]
- Gunjal, A.; Srivastava, A.; Atrey, M. Performance evaluation of liquid nitrogen-cooled cryoprobes using a combined numerical and experimental approach. Cryogenics 2023, 129, 103627. [Google Scholar] [CrossRef]
- Pushkarev, A.V.; Ryabikin, S.S.; Tsiganov, D.I.; Zotov, A.K.; Kurlov, V.N.; Dolganova, I.N. Comparison of Probe Materials for Tissue Cryoablation: Operational Properties of Metal and Sapphire Cryoprobes. J. Biomed. Photonics Eng. 2022, 8, 040501. [Google Scholar] [CrossRef]
- de Jager, N.S.; van Oostenbrugge, T.J.; Pätz, T.; Jenniskens, S.F.M.; Fütterer, J.J.; Langenhuijsen, J.F.; Overduin, C.G. Intraoperative MRI-derived volumetric ablation margins and initial correlation with local outcome after MRI-guided cryoablation of renal tumors. Cancer Imaging 2023, 23, 31. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, I.; Poulsen, F.R.; Pedersen, C.B.; Kristensen, B.W.; Schytte, T.; Andersen, T.L.; Langhorn, L.; Graumann, O.; Krone, W.; Høilund-Carlsen, P.F.; et al. Preclinical cerebral cryoablation in non-tumor bearing pigs. Sci. Rep. 2022, 12, 1977. [Google Scholar] [CrossRef]
- Abrosimov, N.V.; Kurlov, V.N.; Rossolenko, S.N. Automated control of Czochralski and shaped crystal growth processes using weighing techniques. Prog. Cryst. Growth Charact. Mater. 2003, 46, 1–57. [Google Scholar] [CrossRef]
- Bruni, F.J. Control methodology for EFG sapphire crystals. J. Cryst. Growth 2022, 579, 126447. [Google Scholar] [CrossRef]
- Kurlov, V.; Rossolenko, S. Growth of shaped sapphire crystals using automated weight control. J. Cryst. Growth 1997, 173, 417–426. [Google Scholar] [CrossRef]
- Rossolenko, S. Menisci masses and weights in Stepanov (EFG) technique: Ribbon, rod, tube. J. Cryst. Growth 2001, 231, 306–315. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Jordan, M.; Gomboc, T.; Kamenik, B.; Vihar, B.; Kokol, V.; Zadravec, M. Solidification of Gelatine Hydrogels by Using a Cryoplatform and Its Validation through CFD Approaches. Gels 2022, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Kong, J.Y.; Miyawaki, O.; Nakamura, K. The “Itrinsic” Thermal Conductivity of Wet Soy Protein and Its Use in Predicting the Effective Thermal Conductivity of Soybean Curd. J. Food Sci. 2006, 46, 1357–1361. [Google Scholar] [CrossRef]
- Popovic, M.; Minceva, M. Thermodynamic properties of human tissues. Therm. Sci. 2020, 24, 151. [Google Scholar] [CrossRef]
- Giering, K.; Minet, O.; Lamprecht, I.; Müller, G. Review of Thermal Properties of Biological Tissues. Proc. SPIE 1995, 25, 45–65. [Google Scholar]
- Mcintosh, R.L.; Anderson, V. A comprehensive tissue properties database provided for the thermal assessment of a human at rest. Biophys. Rev. Lett. 2010, 5, 129–151. [Google Scholar] [CrossRef]
- Xu, X.; Rioux, T.; Castellani, M. The specific heat of the human body is lower than previously believed: The Journal Temperature toolbox. Temperature 2022, 10, 1–5. [Google Scholar] [CrossRef]
- Patel, S.A.; Erickson, L.E. Estimation of heats of combustion of biomass from elemental analysis using available electron concepts. Biotechnol. Bioeng. 1981, 23, 2051–2067. [Google Scholar] [CrossRef]
- Agafonkina, I.V.; Belozerov, A.G.; Berezovsky, Y.M.; Korolev, I.A.; Pushkarev, A.V.; Tsiganov, D.I.; Shakurov, A.V.; Zherdev, A.A. Thermal Properties of Biological Tissue Gel-Phantoms in a Wide Low-Temperature Range. J. Eng. Phys. Thermophys. 2021, 94, 790–803. [Google Scholar] [CrossRef]
- Stewart, G.; Preketes, A.; Horton, M.; Ross, W.; Morris, D. Hepatic Cryotherapy: Double-Freeze Cycles Achieve Greater Hepatocellular Injury in Man. Cryobiology 1995, 32, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.; Yu, B.; Lewandowski, R.J.; Kim, D.H. Recent progress in cryoablation cancer therapy and nanoparticles mediated cryoablation. Theranostics 2022, 12, 2175–2204. [Google Scholar] [CrossRef] [PubMed]
- Berth-Jones, J.; Bourke, J.; Eglitis, H.; Harper, C.; Kirk, P.; Pavord, S.; Rajapakse, R.; Weston, P.; Wiggins, T.; Hutchinson, P. Value of a second freeze-thaw cycle in cryotherapy of common warts. Br. J. Dermatol. 1994, 131, 883–886. [Google Scholar] [CrossRef]
- Baust, J.G.; Santucci, K.L.; Snyder, K.K.; Robilotto, A.; Baust, J.M. Mechanisms of Tissue Injury in Cryosurgery. In The Application of Heat in Oncology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; Chapter 4; pp. 45–71. [Google Scholar] [CrossRef]
- Sumari, S.N.; Mat Zin, N.A.; Wan Ismail, W.F.; Islam, M.A. Global Prevalence and Risk of Local Recurrence Following Cryosurgery of Giant Cell Tumour of Bone: A Meta-Analysis. Cancers 2022, 14, 3338. [Google Scholar] [CrossRef]



| Tissue | , | , | , , | , | References |
|---|---|---|---|---|---|
| Gelatin aqueous solution 10% | 3.80 | 0.511 | 1.34 | 0.998 | [49,50] |
| Liver | 3.54 | 0.566 | 1.50 | 1.050 | [51,52,53,54,55] |
| Brain | 3.62 | 0.528 | 1.32 | 1.050 | [51,52,53,54,55] |
| Kidney | 3.76 | 0.545 | 1.32 | 1.050 | [51,52,53,54,55] |
| Heart | 3.69 | 0.587 | 1.48 | 1.060 | [51,52,53,54,55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolganova, I.N.; Zotov, A.K.; Rossolenko, S.N.; Shikunova, I.A.; Shikunov, S.L.; Dolganov, K.B.; Zaytsev, K.I.; Kurlov, V.N. Manufacturing of Sapphire Crystals with Variable Shapes for Cryosurgical Applications. Crystals 2024, 14, 346. https://doi.org/10.3390/cryst14040346
Dolganova IN, Zotov AK, Rossolenko SN, Shikunova IA, Shikunov SL, Dolganov KB, Zaytsev KI, Kurlov VN. Manufacturing of Sapphire Crystals with Variable Shapes for Cryosurgical Applications. Crystals. 2024; 14(4):346. https://doi.org/10.3390/cryst14040346
Chicago/Turabian StyleDolganova, Irina N., Arsen K. Zotov, Sergey N. Rossolenko, Irina A. Shikunova, Sergey L. Shikunov, Kirill B. Dolganov, Kirill I. Zaytsev, and Vladimir N. Kurlov. 2024. "Manufacturing of Sapphire Crystals with Variable Shapes for Cryosurgical Applications" Crystals 14, no. 4: 346. https://doi.org/10.3390/cryst14040346






