Indium-Doped SnO2 Based Surface Acoustic Wave Gas Sensor with Ba0.6Sr0.4TiO3 Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
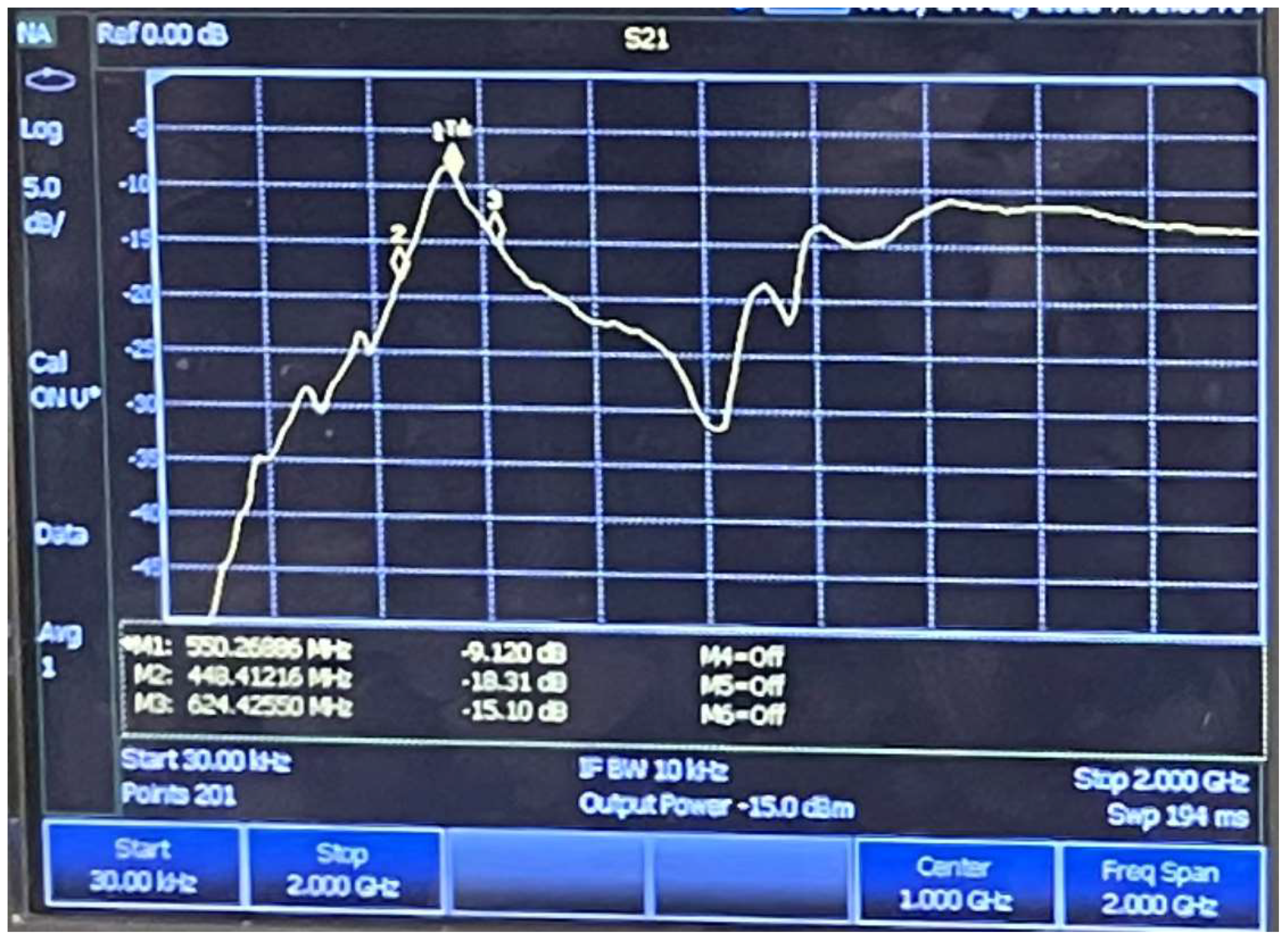
2.2.1. Design and Fabrication of BST-Based SAW Devices
2.2.2. Film Characterization
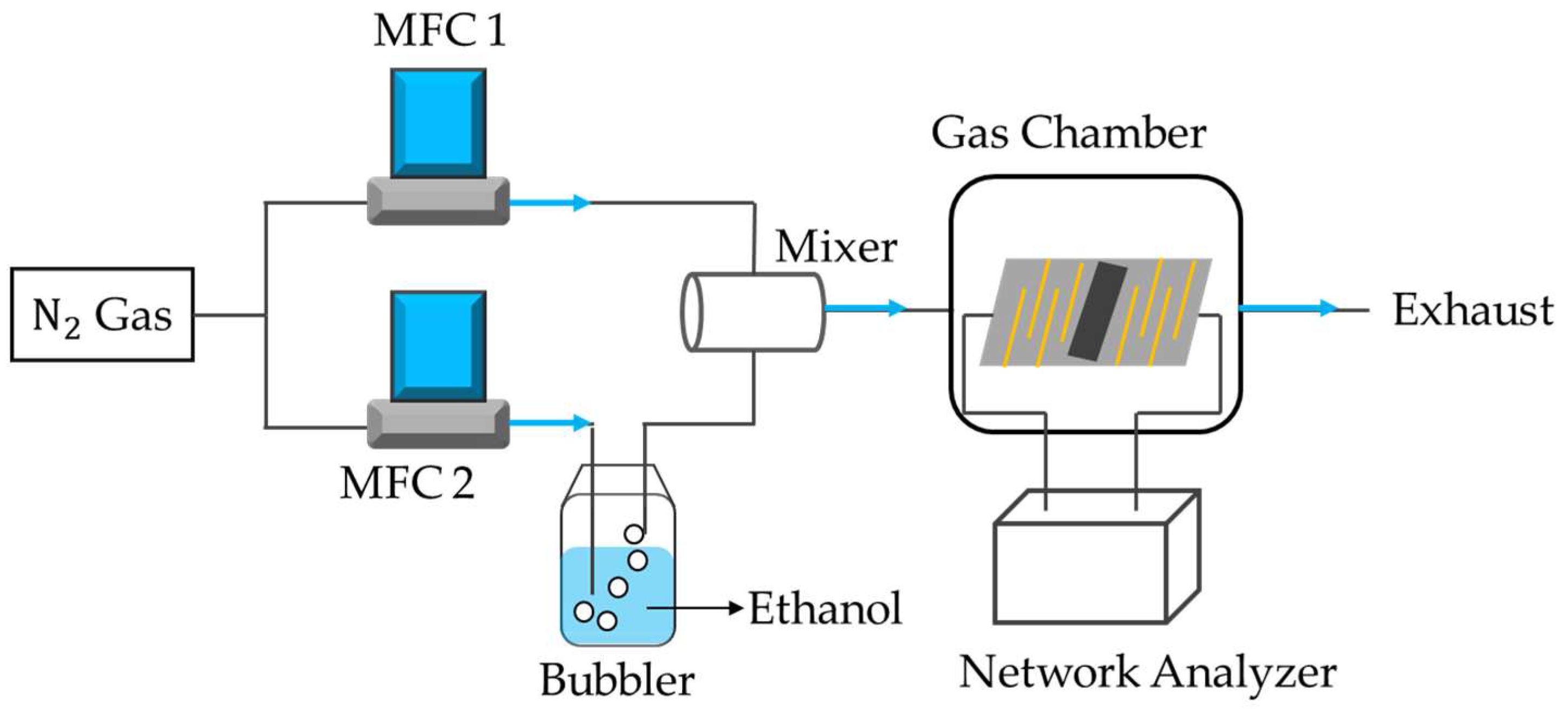
2.2.3. Experimental Setup and Procedures
3. Results and Discussion
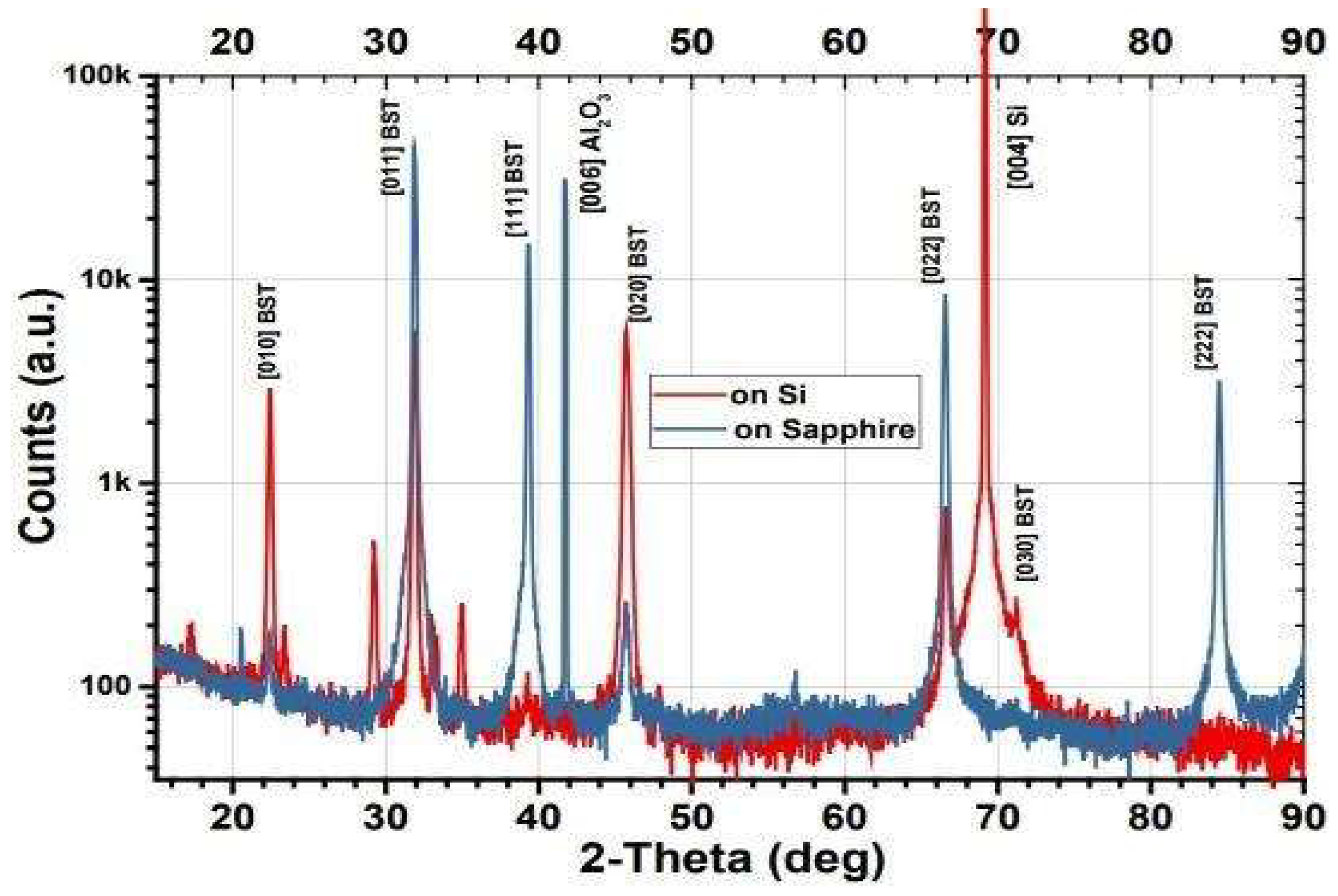
3.1. Film Characterization
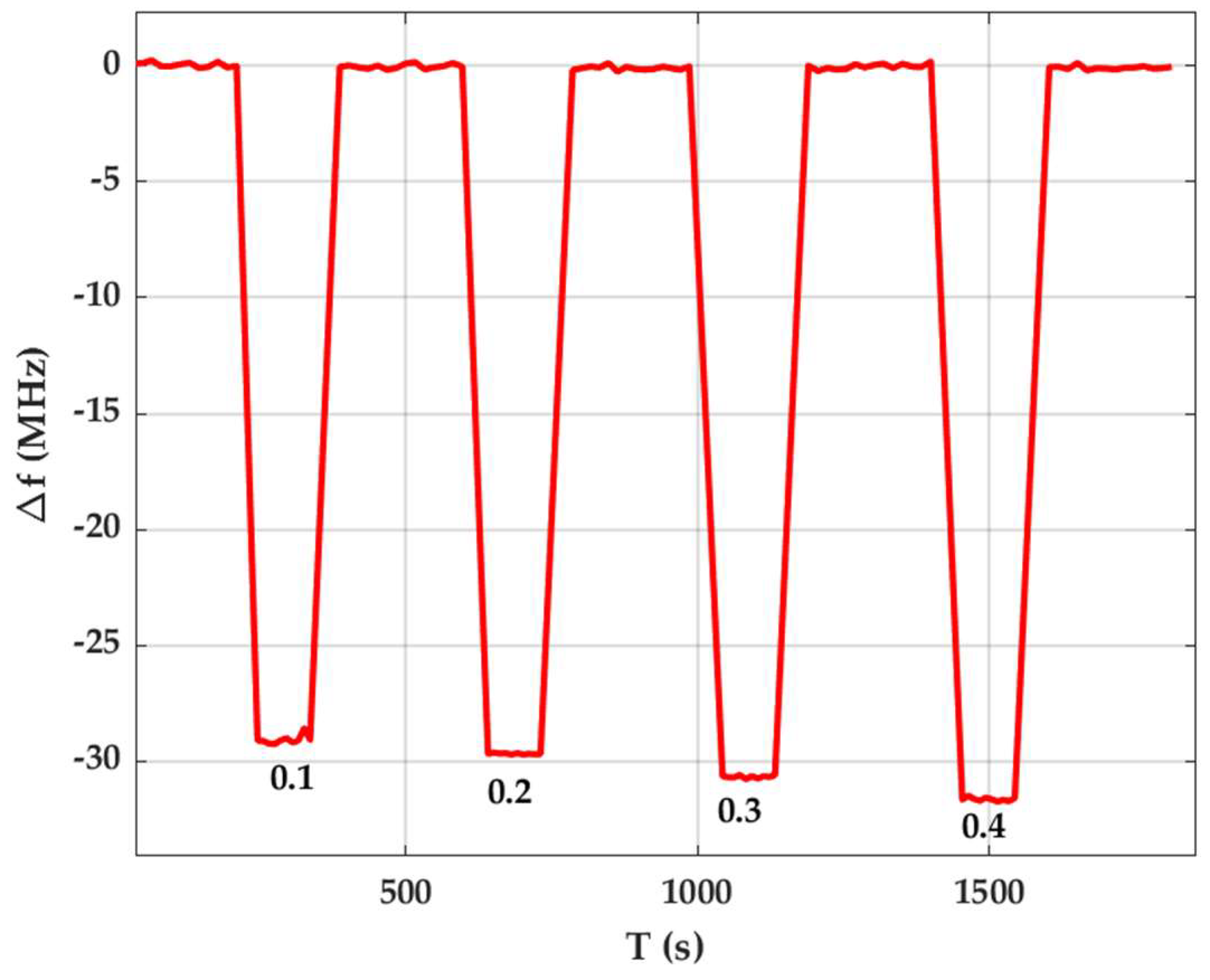
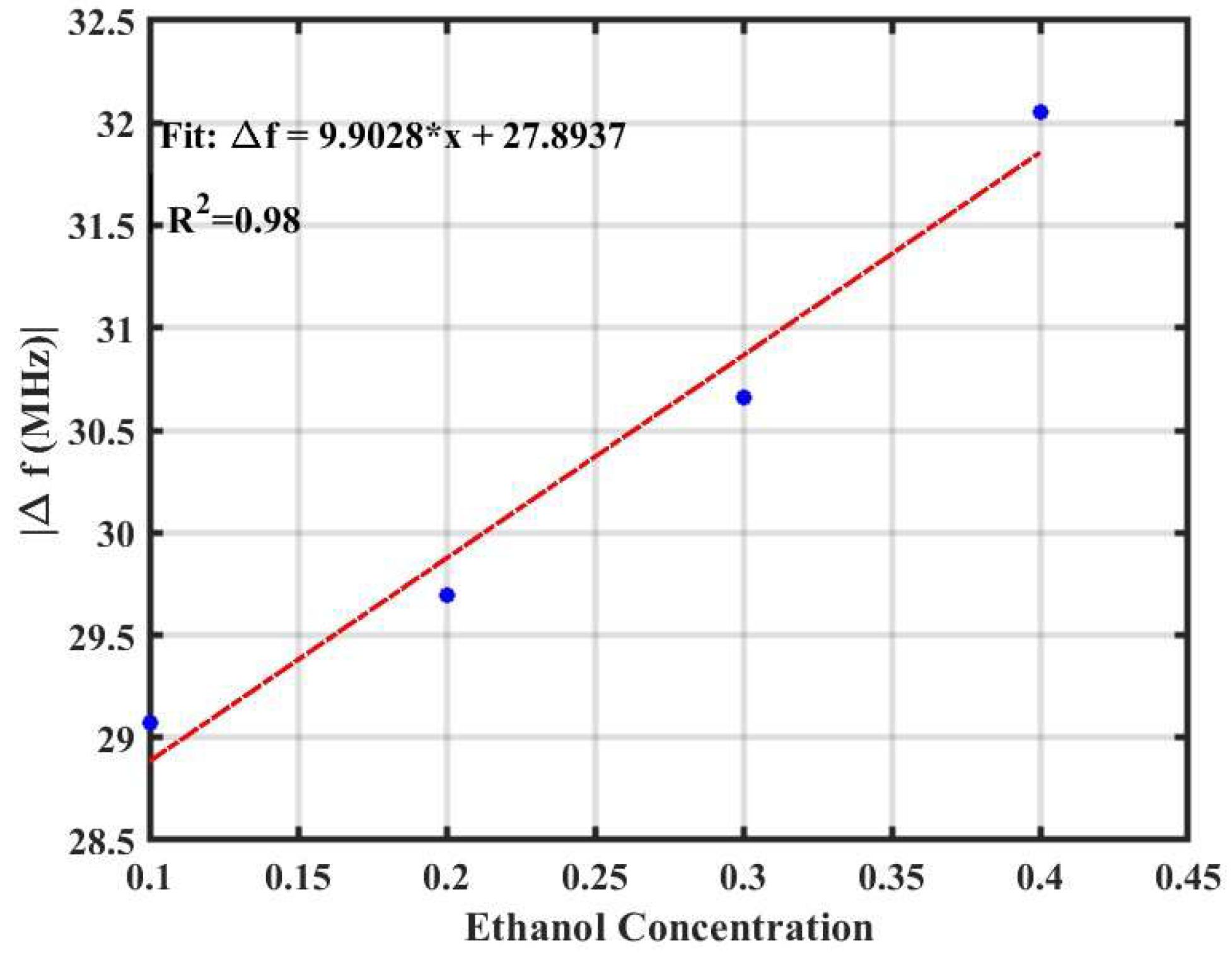
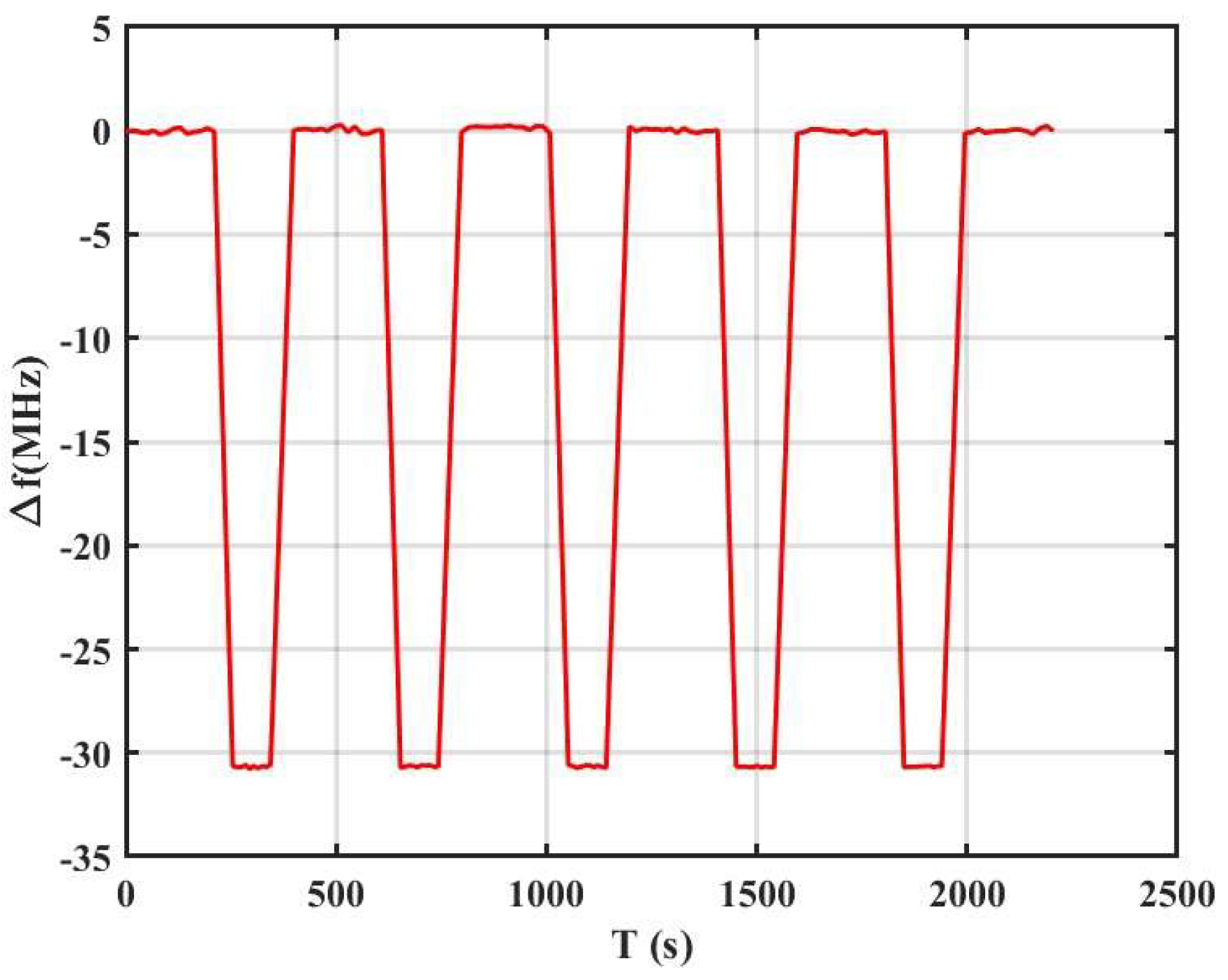
3.2. Gas Sensing Performance
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, M.; Spulber, I.; Gardner, J.W. Surface Acoustic Wave Electronic Tongue for Robust Analysis of Sensory Components. Sens. Actuators B Chem. 2015, 207, 1147–1153. [Google Scholar] [CrossRef]
- Li, D.; Zu, X.; Ao, D.; Tang, Q.; Fu, Y.; Guo, Y.; Bilawal, K.; Faheem, M.B.; Li, L.; Li, S. High Humidity Enhanced Surface Acoustic Wave (SAW) H2S Sensors Based on Sol–Gel CuO Films. Sens. Actuators B Chem. 2019, 294, 55–61. [Google Scholar] [CrossRef]
- Devkota, J.; Greve, D.W.; Hong, T.; Kim, K.-J.; Ohodnicki, P.R. An 860 MHz Wireless Surface Acoustic Wave Sensor with a Metal-Organic Framework Sensing Layer for CO2 and CH4. IEEE Sens. J. 2020, 20, 9740–9747. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, G.; Guo, T.; Liu, X.; Zhang, C.; Yang, J.; Cao, B.; Zhang, C.; Wang, W. Environmental Characteristics of Surface Acoustic Wave Devices for Sensing Organophosphorus Vapor. Sens. Actuators B Chem. 2020, 315, 127986. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Long, G.; Tang, Y.L.; Tang, Q.; Zu, X.-T.; Ma, J.; Du, B.; Torun, H.; Fu, Y. Integrated Sensing Layer of Bacterial Cellulose and Polyethyleneimine to Achieve High Sensitivity of ST-Cut Quartz Surface Acoustic Wave Formaldehyde Gas Sensor. J. Hazard. Mater. 2020, 388, 121743. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-L.; Li, Z.-J.; Ma, J.-Y.; Guo, Y.-J.; Fu, Y.-Q.; Zu, X.-T. Ammonia Gas Sensors Based on ZnO/SiO2 Bi-Layer Nanofilms on ST-Cut Quartz Surface Acoustic Wave Devices. Sens. Actuators B Chem. 2014, 201, 114–121. [Google Scholar] [CrossRef]
- Cleland, A.; Pophristic, M.; Ferguson, I. Single-Crystal Aluminum Nitride Nanomechanical Resonators. Appl. Phys. Lett. 2001, 79, 2070–2072. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Luo, J.; Nguyen, N.-T.; Walton, A.; Flewitt, A.J.; Zu, X.-T.; Li, Y.; McHale, G.; Matthews, A.; Iborra, E. Advances in Piezoelectric Thin Films for Acoustic Biosensors, Acoustofluidics and Lab-on-Chip Applications. Prog. Mater. Sci. 2017, 89, 31–91. [Google Scholar] [CrossRef]
- Liang, L.; Kang, X.; Sang, Y.; Liu, H. One-dimensional Ferroelectric Nanostructures: Synthesis, Properties, and Applications. Adv. Sci. 2016, 3, 1500358. [Google Scholar] [CrossRef]
- Liu, M.; Ma, C.; Collins, G.; Liu, J.; Chen, C.; Alemayehu, A.D.; Subramanyam, G.; Ding, Y.; Chen, J.; Dai, C. Ferroelectric BaTiO3/SrTiO3 Multilayered Thin Films for Room-Temperature Tunable Microwave Elements. Nanoscale Res. Lett. 2013, 8, 338. [Google Scholar] [CrossRef]
- Varanasi, C.; Leedy, K.; Tomich, D.; Subramanyam, G. Large Area Ba1− xSrxTiO3 Thin Films for Microwave Applications Deposited by Pulsed Laser Ablation. Thin Solid Film. 2009, 517, 2878–2881. [Google Scholar] [CrossRef]
- Annam, K.; Spatz, D.; Shin, E.; Subramanyam, G. Experimental Verification of Microwave Phase Shifters Using Barium Strontium Titanate (BST) Varactors. In Proceedings of the 2019 IEEE National Aerospace and Electronics Conference (NAECON), Dayton, OH, USA, 15–19 July 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 63–66. [Google Scholar]
- Annam, K. Reconfigurable RF/Microwave and Millimeterwave Circuits Using Thin Films of Barium Strontium Titanate and Phase Change Materials; University of Dayton: Dayton, OH, USA, 2021; ISBN 9798780609728. [Google Scholar]
- Goud, J.P.; Kumar, A.; Alkathy, M.S.; Sandeep, K.; Raman, A.; Sahoo, B.; Ryu, J.; Raju, K.J. Thickness Dependence of Microwave Dielectric Tunability in Ba0.5Sr0.5TiO3 Thin Films Deposited by Pulsed Laser Deposition. Ceram. Int. 2023, 49, 1188–1194. [Google Scholar] [CrossRef]
- Gevorgian, S.S.; Tagantsev, A.K.; Vorobiev, A.K. Tuneable Film Bulk Acoustic Wave Resonators; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 1-4471-4944-0. [Google Scholar]
- Sandeep, K.; Pundareekam Goud, J.; James Raju, K. Resonant Spectrum Method for Characterizing Ba0.5Sr0.5TiO3 Based High Overtone Bulk Acoustic Wave Resonators. Appl. Phys. Lett. 2017, 111, 012901. [Google Scholar] [CrossRef]
- Kongbrailatpam, S.S.; Goud, J.P.; Raju, K.J. The Effects of a Coated Material Layer on High-Overtone Bulk Acoustic Resonator and Its Possible Applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 68, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Goud, J.P.; Kumar, A.; Sandeep, K.; Ramakanth, S.; Ghoshal, P.; Raju, K.C.J. Tunable Microwave Device Fabrication on Low-Temperature Crystallized Ba0. 5Sr0. 5TiO3 Thin Films by an Alternating Deposition and Laser Annealing Process. Adv. Electron. Mater. 2021, 7, 2000905. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor. Nanomaterials 2020, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.; Dinan, B.; Akbar, S.A.; Haseeb, A. Gas Sensors Based on One Dimensional Nanostructured Metal-Oxides: A Review. Sensors 2012, 12, 7207–7258. [Google Scholar] [CrossRef]
- Wang, L.; Ma, S.; Li, J.; Wu, A.; Luo, D.; Yang, T.; Cao, P.; Ma, N.; Cai, Y. Mo-Doped SnO2 Nanotubes Sensor with Abundant Oxygen Vacancies for Ethanol Detection. Sens. Actuators B Chem. 2021, 347, 130642. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, L.; Feng, D.; Zhuo, M.; Zhang, M.; Zhang, E.; Xu, Z.; Li, Q.; Wang, T. Superior Ethanol-Sensing Properties Based on Ni-Doped SnO2 p–n Heterojunction Hollow Spheres. Sens. Actuators B Chem. 2012, 166, 61–67. [Google Scholar] [CrossRef]
- Rao, A.; Long, H.; Harley-Trochimczyk, A.; Pham, T.; Zettl, A.; Carraro, C.; Maboudian, R. In Situ Localized Growth of Ordered Metal Oxide Hollow Sphere Array on Microheater Platform for Sensitive, Ultra-Fast Gas Sensing. ACS Appl. Mater. Interfaces 2017, 9, 2634–2641. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wang, L.; Liu, J.; Zhang, B.; Gao, Y.; Sun, P.; Sun, Y.; Zhang, T.; Lu, G. Horseshoe-Shaped SnO2 with Annulus-like Mesoporous for Ethanol Gas Sensing Application. Sens. Actuators B Chem. 2017, 240, 1321–1329. [Google Scholar] [CrossRef]
- Shaikh, F.; Chikhale, L.; Nadargi, D.; Mulla, I.; Suryavanshi, S. Structural, Optical and Ethanol Sensing Properties of Dy-Doped SnO2 Nanoparticles. J. Electron. Mater. 2018, 47, 3817–3828. [Google Scholar] [CrossRef]
- Nascimento, E.P.; Firmino, H.C.; Santos, A.M.; Sales, H.B.; Silva, V.D.; Macedo, D.A.; Neves, G.A.; Medeiros, E.S.; Menezes, R.R. Facile Synthesis of Hollow F-doped SnO2 Nanofibers and Their Efficiency in Ethanol Sensing. J. Am. Ceram. Soc. 2021, 104, 1297–1308. [Google Scholar] [CrossRef]
- Jin, W.; Ma, S.; Tie, Z.; Li, W.; Luo, J.; Cheng, L.; Xu, X.; Wang, T.; Jiang, X.; Mao, Y. Synthesis of Hierarchical SnO2 Nanoflowers with Enhanced Acetic Acid Gas Sensing Properties. Appl. Surf. Sci. 2015, 353, 71–78. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of Temperature and Humidity on the Sensing Performance of TiO2 Nanowire-Based Ethanol Vapor Sensors. Nanotechnology 2021, 32, 325501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bai, J.; Huang, B.; Hu, Q.; Cheng, X.; Li, J.; Xie, E.; Wang, Y.; Pan, X. Design of NiCo2O4@ SnO2 Heterostructure Nanofiber and Their Low Temperature Ethanol Sensing Properties. J. Alloys Compd. 2019, 791, 1025–1032. [Google Scholar] [CrossRef]
- Inyawilert, K.; Sukee, A.; Siriwalai, M.; Wisitsoraat, A.; Sukunta, J.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Effect of Er Doping on Flame-Made SnO2 Nanoparticles to Ethylene Oxide Sensing. Sens. Actuators B Chem. 2021, 328, 129022. [Google Scholar] [CrossRef]
- Varanasi, C.; Leedy, K.D.; Tomich, D.H.; Subramanyam, G.; Look, D.C. Improved Photoluminescence of Vertically Aligned ZnO Nanorods Grown on BaSrTiO3 by Pulsed Laser Deposition. Nanotechnology 2009, 20, 385706. [Google Scholar] [CrossRef]
- Alemayehu, B.; Shin, E.; Vasilyev, V.; Subramanyam, G. Synthesis and Characterization of Indium-Doped SnO2-Based Impedance Spectroscopy Sensor for Real-Time Humidity Sensing Applications. Crystals 2024, 14, 82. [Google Scholar] [CrossRef]
- Thomson, G.W. The Antoine Equation for Vapor-Pressure Data. Chem. Rev. 1946, 38, 1–39. [Google Scholar] [CrossRef]
- Shaikh, F.; Chikhale, L.; Mulla, I.; Suryavanshi, S. Facile Co-Precipitation Synthesis and Ethanol Sensing Performance of Pd Loaded Sr Doped SnO2 Nanoparticles. Powder Technol. 2018, 326, 479–487. [Google Scholar] [CrossRef]
- He, Y.; Tang, P.; Li, J.; Zhang, J.; Fan, F.; Li, D. Ultrafast Response and Recovery Ethanol Sensor Based on SnO2 Quantum Dots. Mater. Lett. 2016, 165, 50–54. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.; Kang, H.; Shen, X.; Wang, T.; Jiang, X.; Chen, Q. Preparation of Ag-Doped ZnO-SnO2 Hollow Nanofibers with an Enhanced Ethanol Sensing Performance by Electrospinning. Mater. Lett. 2017, 209, 188–192. [Google Scholar] [CrossRef]
- Kotchasak, N.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Yordsri, V.; Liewhiran, C. Highly Sensitive and Selective Detection of Ethanol Vapor Using Flame-Spray-Made CeOx-Doped SnO2 Nanoparticulate Thick Films. Sens. Actuators B Chem. 2018, 255, 8–21. [Google Scholar] [CrossRef]
- Qin, S.; Tang, P.; Feng, Y.; Li, D. Novel Ultrathin Mesoporous ZnO-SnO2 Nn Heterojunction Nanosheets with High Sensitivity to Ethanol. Sens. Actuators B Chem. 2020, 309, 127801. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Z.; Ma, J.; Wang, L.; Yang, J.; Du, B.; Yu, Q.; Zu, X. Highly Sensitive Surface Acoustic Wave (SAW) Humidity Sensors Based on Sol–Gel SiO2 Films: Investigations on the Sensing Property and Mechanism. Sens. Actuators B Chem. 2015, 215, 283–291. [Google Scholar] [CrossRef]
- Kirschner, J. Surface Acoustic Wave Sensors (SAWS). Micromech. Syst. 2010, 6, 1–11. [Google Scholar]
- Tang, Y.; Xu, X.; Han, S.; Cai, C.; Du, H.; Zhu, H.; Zu, X.; Fu, Y. ZnO-Al2O3 Nanocomposite as a Sensitive Layer for High Performance Surface Acoustic Wave H2S Gas Sensor with Enhanced Elastic Loading Effect. Sens. Actuators B Chem. 2020, 304, 127395. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, W.; Wang, B.; Dai, X.; Xie, W.; Yang, Y.; Zhang, R.; Shi, X.; Zhu, H.; Luo, J. H2S Gas Sensing Performance and Mechanisms Using CuO-Al2O3 Composite Films Based on Both Surface Acoustic Wave and Chemiresistor Techniques. Sens. Actuators B Chem. 2020, 325, 128742. [Google Scholar] [CrossRef]
- Tang, Y.; Ao, D.; Li, W.; Zu, X.; Li, S.; Fu, Y.Q. NH3 Sensing Property and Mechanisms of Quartz Surface Acoustic Wave Sensors Deposited with SiO2, TiO2, and SiO2-TiO2 Composite Films. Sens. Actuators B Chem. 2018, 254, 1165–1173. [Google Scholar] [CrossRef]
- Assali, A.; Kanouni, F.; Laidoudi, F.; Arab, F.; Bouslama, M. Structural and Electromechanical Properties of Sr-Substituted Barium Titanate (BST) as Potential Material for High Performance Electroacoustic Devices. Mater. Today Commun. 2020, 25, 101643. [Google Scholar]
- Raj, V.B.; Singh, H.; Nimal, A.; Sharma, M.; Tomar, M.; Gupta, V. Distinct Detection of Liquor Ammonia by ZnO/SAW Sensor: Study of Complete Sensing Mechanism. Sens. Actuators B Chem. 2017, 238, 83–90. [Google Scholar] [CrossRef]



| Sensing Materials | T [°C] | Response Time | Recovery Time | Refs. |
|---|---|---|---|---|
| Pd loaded Sr doped SnO2 | 270 | 1 | 5 | [34] |
| SnO2 Quantum dots | 190 | 1 | 1 | [35] |
| Ag-doped ZnO-SnO2 | 200 | 5 | 5 | [36] |
| Ce-doped SnO2 | 350 | 1 | 120 | [37] |
| ZnO-SnO2 heterostructures | 240 | 7 | 24 | [38] |
| In-doped SnO2 | RT | 39 | 50 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alemayehu, B.; Annam, K.; Shin, E.; Subramanyam, G. Indium-Doped SnO2 Based Surface Acoustic Wave Gas Sensor with Ba0.6Sr0.4TiO3 Film. Crystals 2024, 14, 366. https://doi.org/10.3390/cryst14040366
Alemayehu B, Annam K, Shin E, Subramanyam G. Indium-Doped SnO2 Based Surface Acoustic Wave Gas Sensor with Ba0.6Sr0.4TiO3 Film. Crystals. 2024; 14(4):366. https://doi.org/10.3390/cryst14040366
Chicago/Turabian StyleAlemayehu, Birhanu, Kaushik Annam, Eunsung Shin, and Guru Subramanyam. 2024. "Indium-Doped SnO2 Based Surface Acoustic Wave Gas Sensor with Ba0.6Sr0.4TiO3 Film" Crystals 14, no. 4: 366. https://doi.org/10.3390/cryst14040366




