Thermal Characterization of [C2Im][NO3] and Multivalent Nitrate Salts Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Section
- (a)
- heating from 25 to 120 °C at 40 °C min−1;
- (b)
- isothermal step at 120 °C for 45 min with the objective of removing impurities, the free water content and to erase the thermal history of the sample [25];
- (c)
- cooling from 120 to −85 °C at 10 °C min−1;
- (d)
- heating from −85 to 100 °C at 10 °C min−1;
- (e)
- cooling from 100 to −85 °C at 5 °C min−1;
- (f)
- heating from −85 to 100 °C at 5 °C min−1.
3. Results
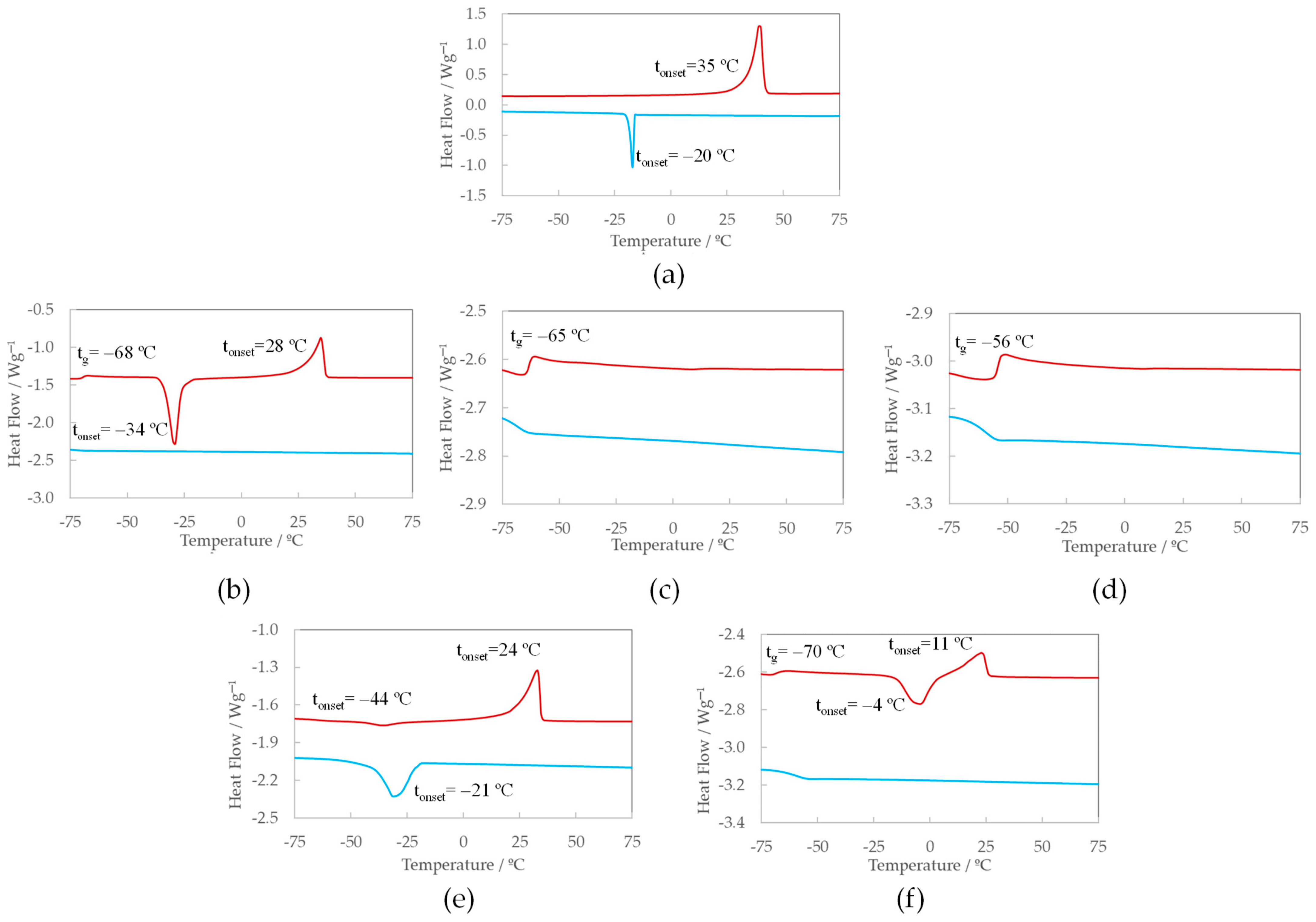
3.1. DSC Results
- The peak of crystallization in the cooling ramps disappears for the blended samples, except for the lowest concentrations of lithium and aluminum mixtures. At intermediate doses of all the mixtures, an exothermic peak upon heating, known as cold crystallization (see further details), emerges; this is followed by the corresponding melting peak.
- The glass transition (tg) is present in all saturated mixtures. Particularly in the DSC curves of IL + Mg(NO3)2 mixtures, the glass transition is evident in all studied concentrations, showing a tendency to increase with salt addition, as indicated in Table 2; this is consistent with findings reported in the literature [20,25]. We would like to highlight that this is likely not exclusive to mixtures with Mg, and may also occur in the rest of the salt mixtures. However, the tg is not detected in the rest of the samples since it can appear beyond the lowest temperature range of the equipment used in this work (−80 °C) [27].
- Wider melting peaks shifted to lower temperatures are observed for IL + salt mixtures, which completely disappear at the highest concentrations of the mono and divalent salts mixtures, i.e., the 3 mol·kg−1 IL + LiNO3 mixture [24] and 1 mol·kg−1 and 2 mol·kg−1 IL + Mg(NO3)2 mixtures. Although the DSC curve of the saturated IL + Al(NO3)3 mixture shows clear exothermic and endothermic peaks upon heating (cold crystallization and melting process), the heat associated with these peaks is very small (Table 2). This, together with the appearance of the glass transition temperature at −70 °C, underlines the loss of crystallinity upon salt addition.
3.2. Thermal Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvester, D.S.; Compton, R.G. Electrochemistry in Room Temperature Ionic Liquids: A Review and Some Possible Applications. Z. Phys. Chem. 2006, 220, 1247–1274. [Google Scholar] [CrossRef]
- Semerci, I.; Shikh Mohd Shahrul Nizan, S.Z.; Harirchi, S.; Sar, T.; Awasthi, M.K.; Taherzadeh, M. Applications of Ionic Liquids for the Biochemical Transformation of Lignocellulosic Biomass into Biofuels and Biochemicals: A Critical Review. Biochem. Eng. J. 2023, 193, 108850. [Google Scholar] [CrossRef]
- Tzani, A.; Karadendrou, M.-A.; Kalafateli, S.; Kakokefalou, V.; Detsi, A. Current Trends in Green Solvents: Biocompatible Ionic Liquids. Crystals 2022, 12, 1776. [Google Scholar] [CrossRef]
- Md Moshikur, R.; Chowdhury, M.R.; Moniruzzaman, M.; Goto, M. Biocompatible Ionic Liquids and Their Applications in Pharmaceutics. Green Chem. 2020, 22, 8116–8139. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, J.; Wang, Y.; Hu, J.; Cui, P. Ionic Liquid-Based CO2 Capture in Power Plants for Low Carbon Emissions. Int. J. Greenh. Gas Control 2018, 75, 134–139. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- Pająk, M.; Hubkowska, K.; Czerwiński, A. Nitrate Protic Ionic Liquids as Electrolytes: Towards Hydrogen Sorption in Pd. Electrochim. Acta 2019, 324, 134851. [Google Scholar] [CrossRef]
- Stettner, T.; Balducci, A. Protic Ionic Liquids in Energy Storage Devices: Past, Present and Future Perspective. Energy Storage Mater. 2021, 40, 402–414. [Google Scholar] [CrossRef]
- Matuszek, K.; Piper, S.L.; Brzęczek-Szafran, A.; Roy, B.; Saher, S.; Pringle, J.M.; MacFarlane, D.R. Unexpected Energy Applications of Ionic Liquids. Adv. Mater. 2024; early view. [Google Scholar] [CrossRef]
- Debenedetti, P.; Poole, P.; Sastry, S.; Sciortino, F.C. Austen Angell (1933–2021). Nature 2021, 593, 336. [Google Scholar] [CrossRef]
- Vallet, P.; Bouzón-Capelo, S.; Méndez-Morales, T.; Gómez-González, V.; Arosa, Y.; de la Fuente, R.; López-Lago, E.; Rodríguez, J.R.; Gallego, L.J.; Parajó, J.J.; et al. On the Physical Properties of Mixtures of Nitrate Salts and Protic Ionic Liquids. J. Mol. Liq. 2022, 350, 118483. [Google Scholar] [CrossRef]
- Martinelli, A.; Matic, A.; Jacobsson, P.; Börjesson, L.; Fernicola, A.; Scrosati, B. Phase Behavior and Ionic Conductivity in Lithium Bis(Trifluoromethanesulfonyl)Imide-Doped Ionic Liquids of the Pyrrolidinium Cation and Bis(Trifluoromethanesulfonyl)Imide Anion. J. Phys. Chem. B 2009, 113, 11247–11251. [Google Scholar] [CrossRef] [PubMed]
- Parajó, J.J.; Otero-Mato, J.M.; Lobo Ferreira, A.I.M.C.; Varela, L.M.; Santos, L.M.N.B.F. Enthalpy of Solvation of Alkali Metal Salts in a Protic Ionic Liquid: Effect of Cation Charge and Size. J. Mol. Liq. 2022, 360, 119228. [Google Scholar] [CrossRef]
- Lois-Cuns, R.; Otero-Lema, M.; Rivera-Pousa, A.; Vallet, P.; Parajó, J.J.; Cabeza, O.; Montes-Campos, H.; Méndez-Morales, T.; Varela, L.M. Mixtures of Ethylammonium Nitrate and Ethylene Carbonate: Bulk and Interfacial Analysis. J. Mol. Liq. 2023, 385, 122361. [Google Scholar] [CrossRef]
- Chatel, G.; Pereira, J.F.B.; Debbeti, V.; Wang, H.; Rogers, R.D. Mixing Ionic Liquids—“Simple Mixtures” or “Double Salts”? Green Chem. 2014, 16, 2051–2083. [Google Scholar] [CrossRef]
- Neves, C.M.S.S.; Dinis, T.B.V.; Carvalho, P.J.; Schröder, B.; Santos, L.M.N.B.F.; Freire, M.G.; Coutinho, J.A.P. Binary Mixtures of Ionic Liquids in Aqueous Solution: Towards an Understanding of Their Salting-In/Salting-Out Phenomena. J. Solut. Chem. 2019, 48, 983–991. [Google Scholar] [CrossRef]
- Parajó, J.J.; Vallet, P.; Villanueva, M.; Cabeza, O.; Fernández-Carretero, F.; García Luis, A.; Di Pietro, M.E.; Mele, A.; Castiglione, F.; Salgado, J.; et al. Ionogels Based on Protic Ionic Liquid—Lithium Salt Mixtures. J. Mol. Liq. 2024, 397, 124093. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Wang, Y.-H.; Su, S.-G.; Lin, Y.-C.; Kuo, C.-W.; Chang, J.-K.; Sun, I.-W. Influence of LiTFSI Addition on Conductivity, Diffusion Coefficient, Spin–Lattice Relaxation Times, and Chemical Shift of One-Dimensional NMR Spectroscopy in LiTFSI-Doped Dual-Functionalized Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2015, 60, 471–483. [Google Scholar] [CrossRef]
- Girard, G.M.A.; Hilder, M.; Zhu, H.; Nucciarone, D.; Whitbread, K.; Zavorine, S.; Moser, M.; Forsyth, M.; MacFarlane, D.R.; Howlett, P.C. Electrochemical and Physicochemical Properties of Small Phosphonium Cation Ionic Liquid Electrolytes with High Lithium Salt Content. Phys. Chem. Chem. Phys. 2015, 17, 8706–8713. [Google Scholar] [CrossRef]
- Parajó, J.J.; Vallet, P.; Varela, L.M.; Villanueva, M.; Salgado, J. Ecotoxicity of Binary Mixtures of ILs and Inorganic Salts of Electrochemical Interest. Environ. Sci. Pollut. Res. 2022, 29, 24983–24994. [Google Scholar] [CrossRef]
- Gómez-González, V.; Docampo-Álvarez, B.; Cabeza, O.; Fedorov, M.; Lynden-Bell, R.M.; Gallego, L.J.; Varela, L.M. Molecular Dynamics Simulations of the Structure and Single-Particle Dynamics of Mixtures of Divalent Salts and Ionic Liquids. J. Chem. Phys. 2015, 143, 124507. [Google Scholar] [CrossRef]
- Matveev, V.V.; Ievlev, A.V.; Vovk, M.A.; Cabeza, O.; Salgado-Carballo, J.; Parajó, J.J.; Rodríguez, J.R.; de la Fuente, R.; Lähderanta, E.; Varela, L.M. NMR Investigation of the Structure and Single-Particle Dynamics of Inorganic Salt Solutions in a Protic Ionic Liquid. J. Mol. Liq. 2019, 278, 239–246. [Google Scholar] [CrossRef]
- Vallet, P.; Parajó, J.J.; Santiago-Alonso, A.; Villanueva, M.; Cabeza, Ó.; Varela, L.M.; Salgado, J. Anomalous Behaviour of the Ionic Conductivity of Nanoconfined IL-Lithium Salt Mixtures. J. Mol. Liq. 2024, 401, 124630. [Google Scholar] [CrossRef]
- Salgado, J.; Parajó, J.J.; Villanueva, M.; Rodríguez, J.R.; Cabeza, O.; Varela, L.M. Liquid Range of Ionic Liquid—Metal Salt Mixtures for Electrochemical Applications. J. Chem. Thermodyn. 2019, 134, 164–174. [Google Scholar] [CrossRef]
- Parajó, J.J.; Villanueva, M.; Sánchez, P.B.; Salgado, J. Liquid Window of Some Biologically-Active Ionic Liquids. J. Chem. Thermodyn. 2018, 126, 1–10. [Google Scholar] [CrossRef]
- Crosthwaite, J.M.; Muldoon, M.J.; Dixon, J.K.; Anderson, J.L.; Brennecke, J.F. Phase Transition and Decomposition Temperatures, Heat Capacities and Viscosities of Pyridinium Ionic Liquids. J. Chem. Thermodyn. 2005, 37, 559–568. [Google Scholar] [CrossRef]
- Ishino, K.; Shingai, H.; Hikita, Y.; Yoshikawa, I.; Houjou, H.; Iwase, K. Cold Crystallization and the Molecular Structure of Imidazolium-Based Ionic Liquid Crystals with a p-Nitroazobenzene Moiety. ACS Omega 2021, 6, 32869–32878. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy Applications of Ionic Liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Pitawala, J.; Kim, J.K.; Jacobsson, P.; Koch, V.; Croce, F.; Matic, A. Phase Behaviour, Transport Properties, and Interactions in Li-Salt Doped Ionic Liquids. Faraday Discuss. 2012, 154, 71–80. [Google Scholar] [CrossRef]
- Méndez-Morales, T.; Carrete, J.; Rodríguez, J.R.; Cabeza, Ó.; Gallego, L.J.; Russina, O.; Varela, L.M. Nanostructure of Mixtures of Protic Ionic Liquids and Lithium Salts: Effect of Alkyl Chain Length. Phys. Chem. Chem. Phys. 2015, 17, 5298–5307. [Google Scholar] [CrossRef]
- Ngo, H.P.K.; Planes, E.; Iojoiu, C.; Soudant, P.; Rollet, A.-L.; Judeinstein, P. Transport Properties of Alkali/Alkaline Earth Cations in Ionic-Liquid Based Electrolytes. J. Ion. Liq. 2022, 2, 100044. [Google Scholar] [CrossRef]
- Montanino, M.; Moreno, M.; Carewska, M.; Maresca, G.; Simonetti, E.; Lo Presti, R.; Alessandrini, F.; Appetecchi, G.B. Mixed Organic Compound-Ionic Liquid Electrolytes for Lithium Battery Electrolyte Systems. J. Power Sources 2014, 269, 608–615. [Google Scholar] [CrossRef]
- Timmermans, J. Plastic Crystals: A Historical Review. J. Phys. Chem. Solids 1961, 18, 1–8. [Google Scholar] [CrossRef]
- Lorenzo, M.; Vilas, M.; Verdía, P.; Villanueva, M.; Salgado, J.; Tojo, E. Long-Term Thermal Stabilities of Ammonium Ionic Liquids Designed as Potential Absorbents of Ammonia. RSC Adv. 2015, 5, 41278–41284. [Google Scholar] [CrossRef]
- Wooster, T.J.; Johanson, K.M.; Fraser, K.J.; MacFarlane, D.R.; Scott, J.L. Thermal Degradation of Cyano Containing Ionic Liquids. Green Chem. 2006, 8, 691–696. [Google Scholar] [CrossRef]

| Name | Molecular Weight (g·mol−1) | Structure | CAS Number | Purity |
|---|---|---|---|---|
| Ethyl Imidazolium Nitrate | 159.14 | 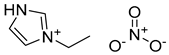 | [C2Im][NO3] 501,693—38—5 | >0.98 a |
| Lithium Nitrate | 68.946 |  | LiNO3 7790—69—4 | >0.999 a |
| Magnesium Nitrate Hexahydrate | 256.41 |  | Mg(NO3)2·6H2O 13,446—18—9 | >0.98 a |
| Aluminum Nitrate Nonahydrate | 375.13 |  | Al(NO3)3·9H2O 7784—27—2 | >0.98 a |
| Compound | tm/°C | tf/°C | tcc/°C | tg (*)/°C | ∆mH/J·g−1 | ∆mH/kJ·mol−1 | ∆mS/J·mol−1·K−1 |
|---|---|---|---|---|---|---|---|
| [C2Im][NO3] [24] | 35 | −20 | 82.7 | 13.2 | 43.0 | ||
| 0.5 m IL + LiNO3 [24] | 32 | 16 | 74.8 | 12.3 | 41.2 | ||
| 1.0 m IL + LiNO3 [24] | 21 | −3 | 51.3 | 8.7 | 28.9 | ||
| 2.0 m IL + LiNO3 [24] | 4 | −15 | −65 | 18.6 | 3.6 | 11.5 | |
| 3.0 m IL + LiNO3 [24] | −69 | ||||||
| 0.5 m IL + Mg(NO3)2 | 28 | −34 | −68 | 51.5 | 8.8 | 29.1 | |
| 1.0 m IL + Mg(NO3)2 | −65 | ||||||
| 2.0 m IL + Mg(NO3)2 | −56 | ||||||
| 0.5 m IL + Al(NO3)3 | 24 | −21 | −44 | 41.2 | 7.3 | 24.7 | |
| 1.0 m IL + Al(NO3)3 | 11 | −4 | −70 | 14.4 | 2.8 | 9.7 |
| Compound | tonset/°C | tpeak/°C | tWooster/°C |
|---|---|---|---|
| [C2Im][NO3] | 224 | 232 | 125 |
| 3 m IL + LiNO3 | 223 | 230 | 127 |
| 2 m IL + Mg(NO3)2 | 222 | 228 | 133 |
| 1 m IL + Al(NO3)3 | 181 | 185 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallet, P.; Parajó, J.J.; Santiago-Alonso, A.; Villanueva, M.; Varela, L.M.; Salgado, J. Thermal Characterization of [C2Im][NO3] and Multivalent Nitrate Salts Mixtures. Crystals 2024, 14, 502. https://doi.org/10.3390/cryst14060502
Vallet P, Parajó JJ, Santiago-Alonso A, Villanueva M, Varela LM, Salgado J. Thermal Characterization of [C2Im][NO3] and Multivalent Nitrate Salts Mixtures. Crystals. 2024; 14(6):502. https://doi.org/10.3390/cryst14060502
Chicago/Turabian StyleVallet, Pablo, Juan José Parajó, Antía Santiago-Alonso, María Villanueva, Luis Miguel Varela, and Josefa Salgado. 2024. "Thermal Characterization of [C2Im][NO3] and Multivalent Nitrate Salts Mixtures" Crystals 14, no. 6: 502. https://doi.org/10.3390/cryst14060502
APA StyleVallet, P., Parajó, J. J., Santiago-Alonso, A., Villanueva, M., Varela, L. M., & Salgado, J. (2024). Thermal Characterization of [C2Im][NO3] and Multivalent Nitrate Salts Mixtures. Crystals, 14(6), 502. https://doi.org/10.3390/cryst14060502






