X-ray Single-Crystal Analysis, Pharmaco-Toxicological Profile and Enoyl-ACP Reductase-Inhibiting Activity of Leading Sulfonyl Hydrazone Derivatives
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemistry
2.2. Biology
2.2.1. Experimental Animals
2.2.2. Acute Toxicity in Mice
2.2.3. Sub-Acute Toxicity in Mice
2.2.4. Experimental Design
- Group 1—control, untreated group;
- Group 2—mice treated i.p. with isoniazid (INH) 50 mg/kg [16];
- Group 3—mice treated i.p. with 3a at a dose of 45 mg/kg (1/20 LD50);
- Group 4—mice treated i.p. with 3a at a dose of 90 mg/kg (1/10 LD50);
- Group 5—mice treated i.p. with 3b at a dose of 65 mg/kg (1/20 LD50);
- Group 6—mice treated i.p. with 3b at a dose of 130 mg/kg (1/10 LD50).
2.2.5. Histological Evaluation of Tissue Specimens
2.3. Assessment of the Oxidative Stress Biomarkers
2.4. InhA Inhibition Assay
2.5. Single-Crystal X-ray Analysis
3. Results and Discussion
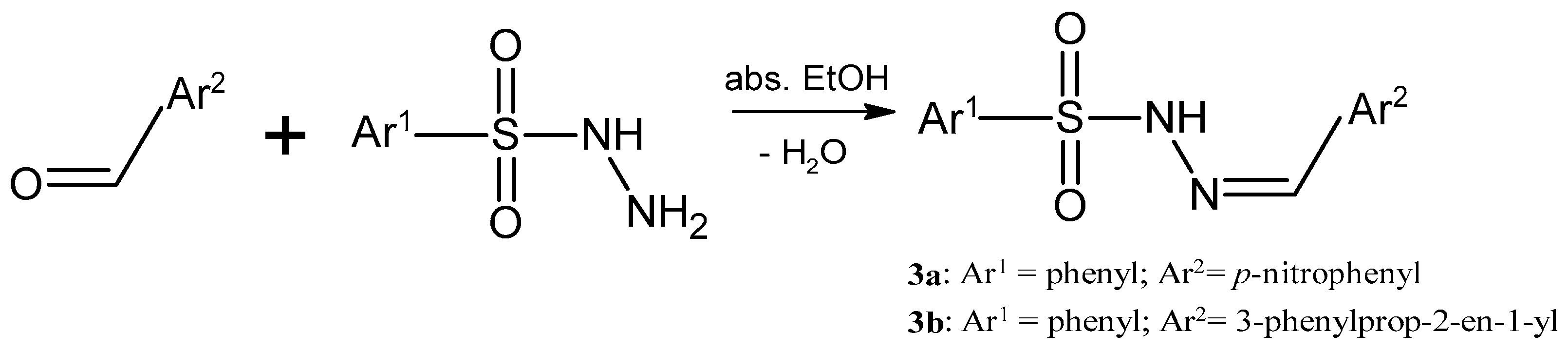
3.1. Synthesis of the Sulfonyl Hydrazone Derivatives
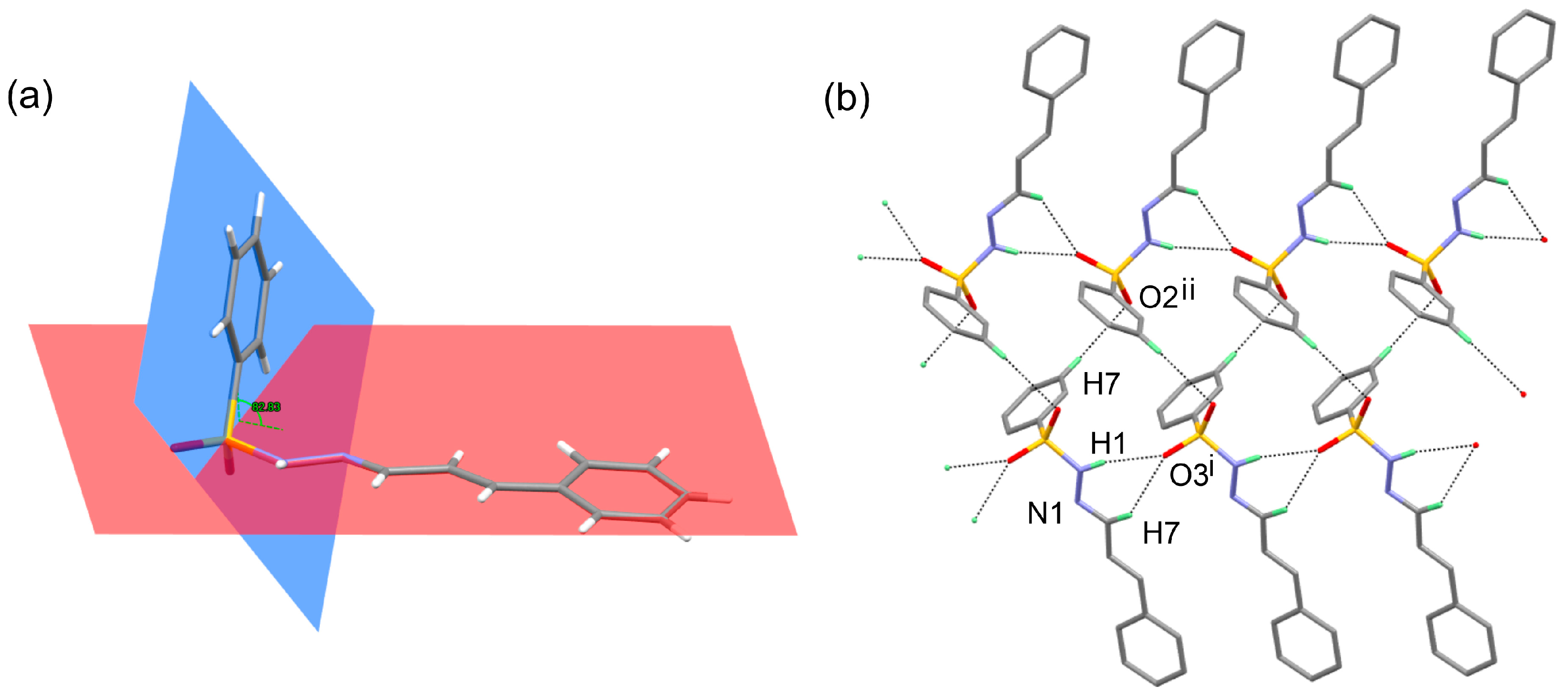
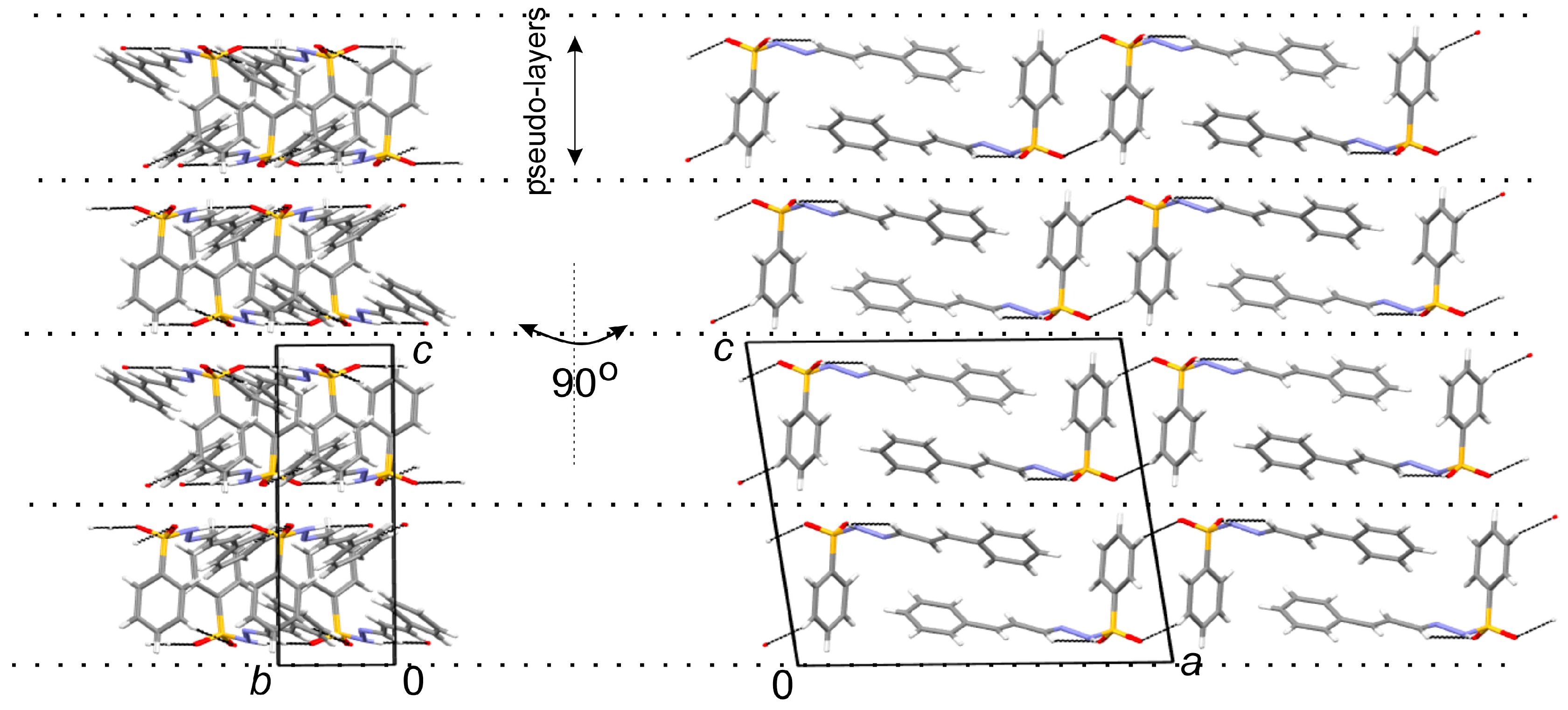
3.2. X-ray Crystallography
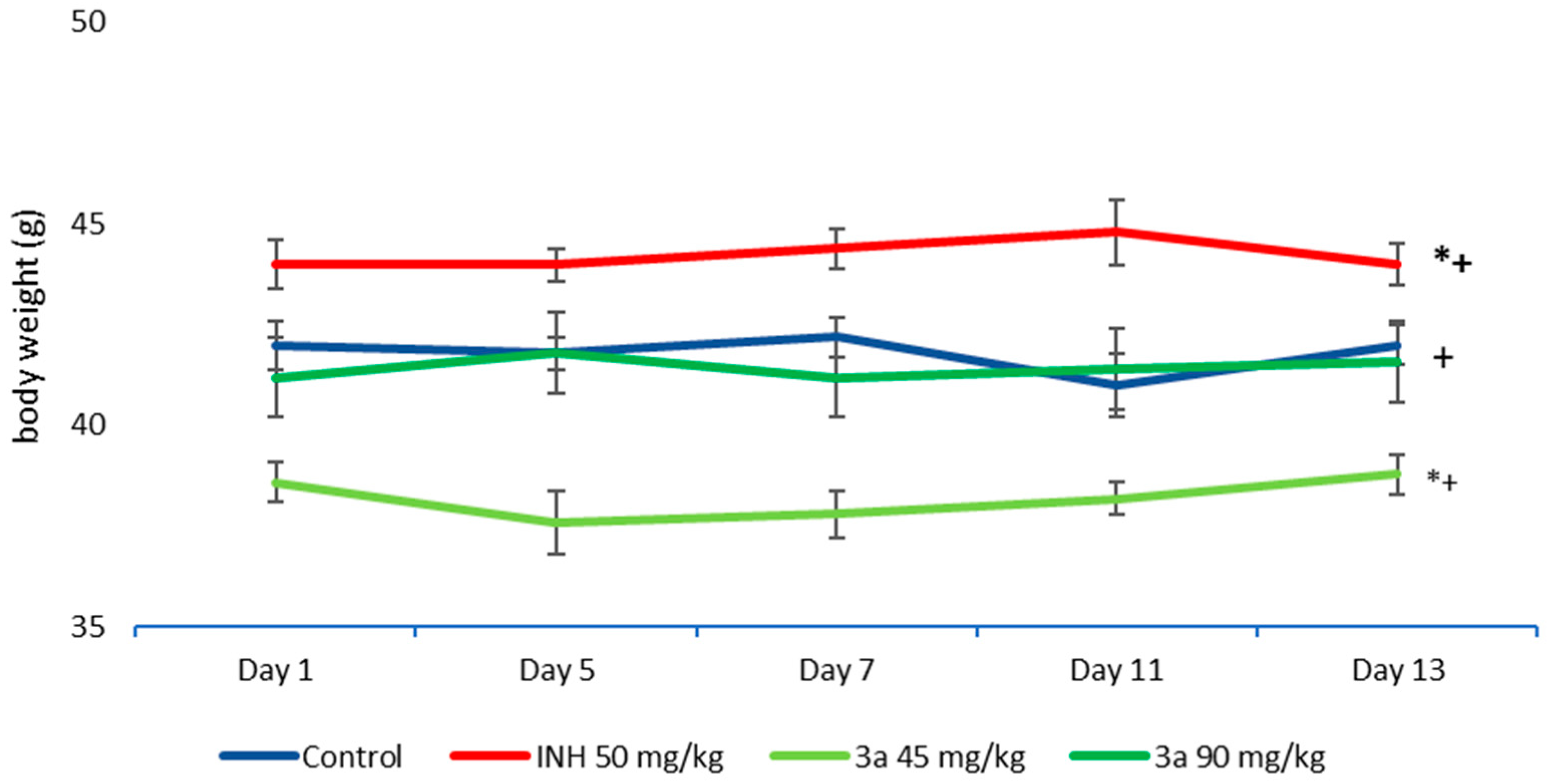
3.3. Acute Toxicity in Mice
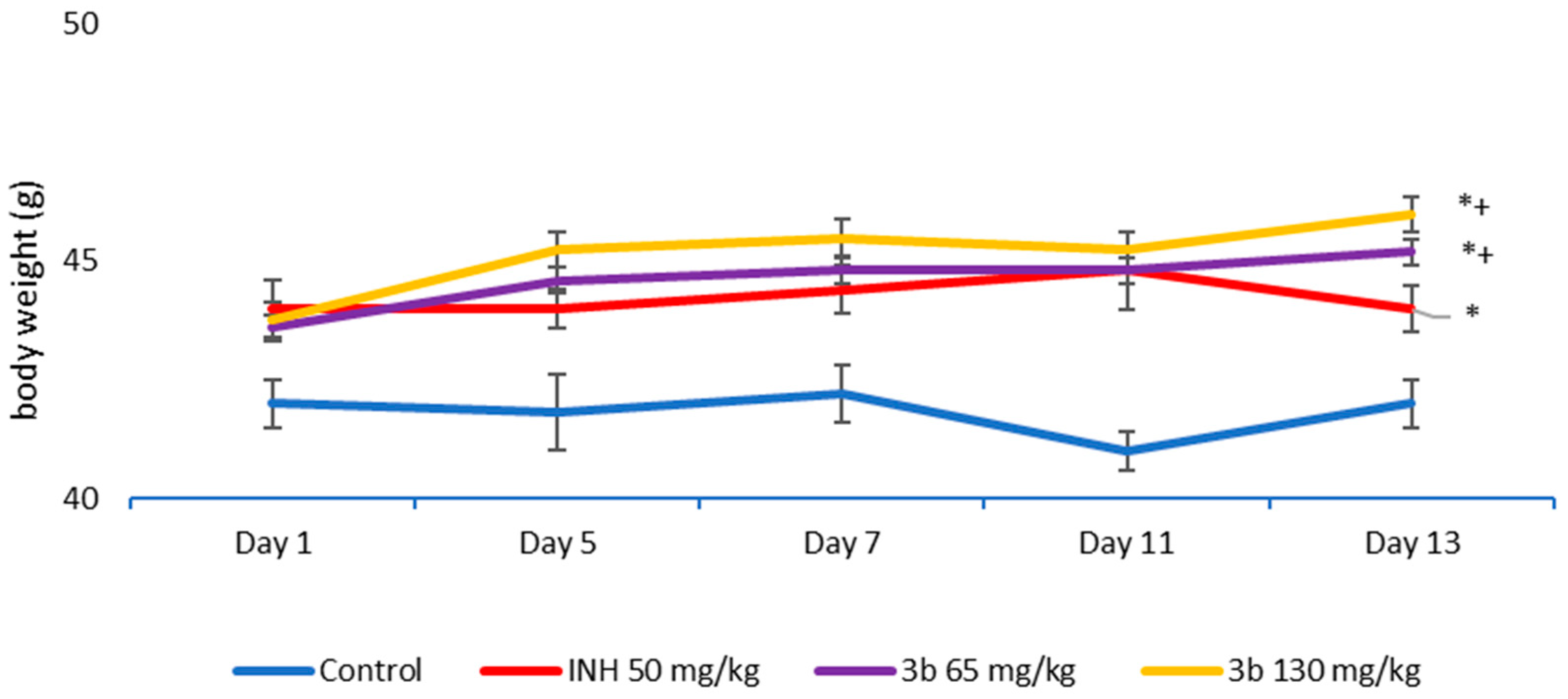
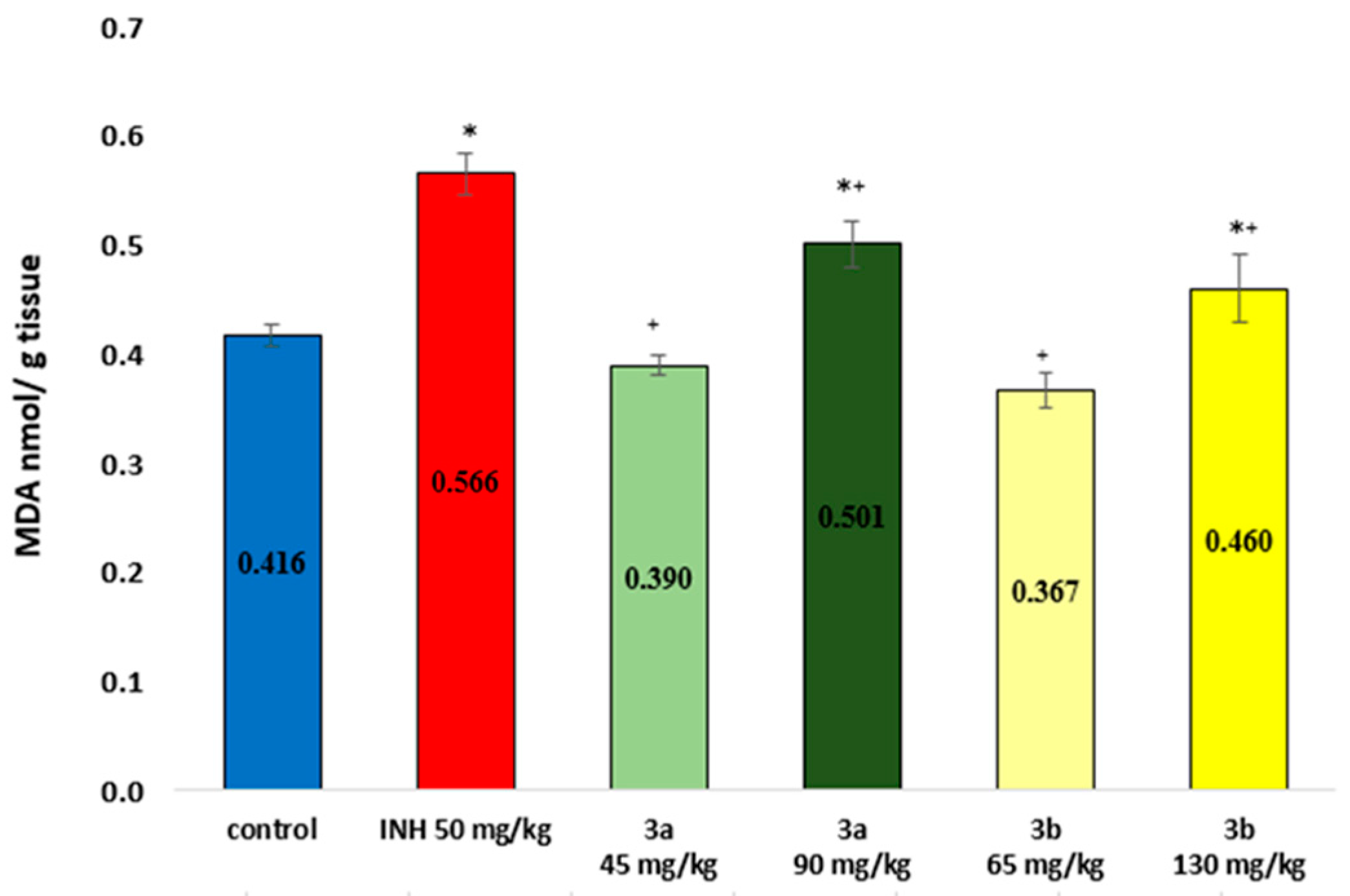
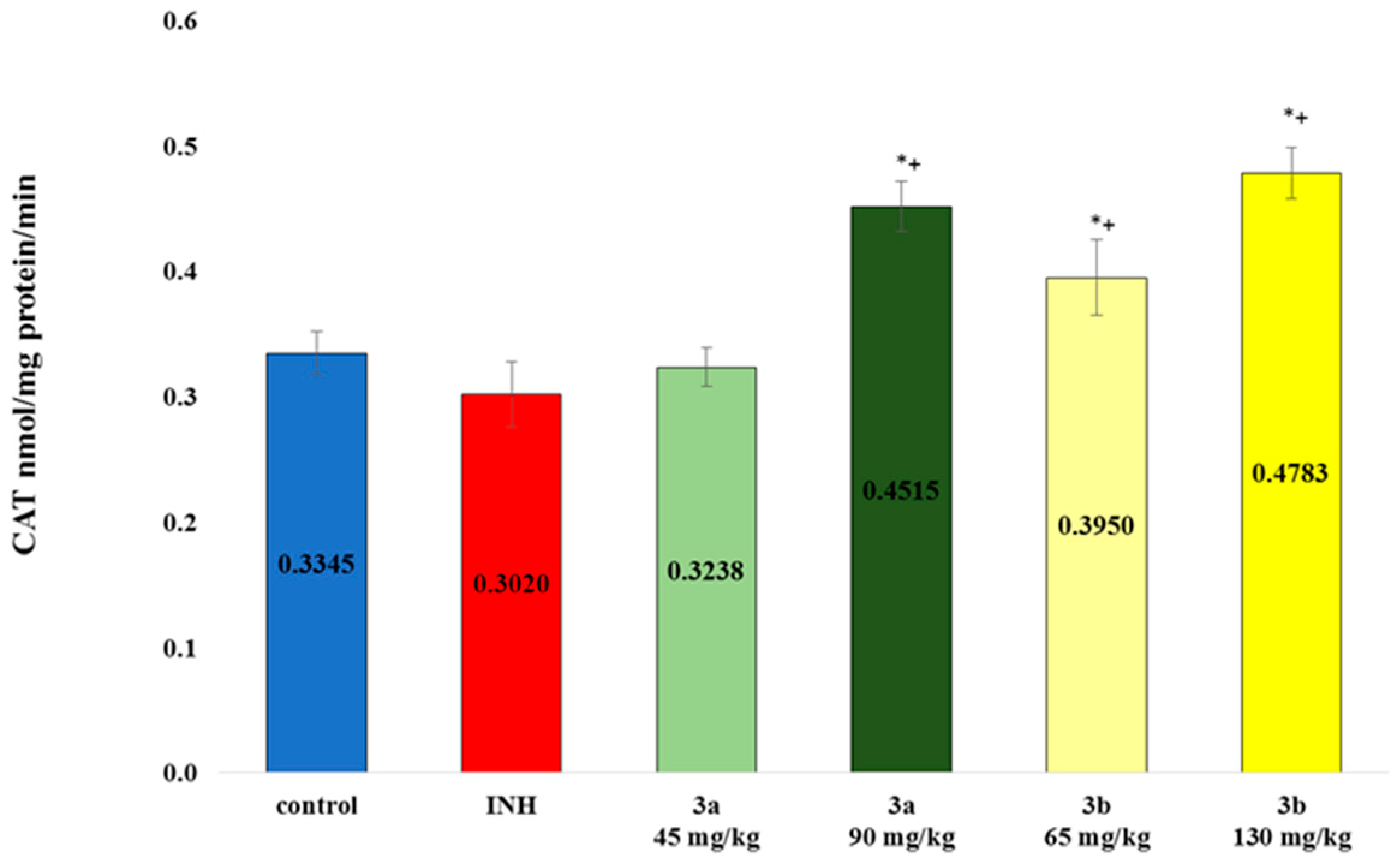
3.4. Sub-Acute Toxicity of Mice, Hematological and Biochemical Parameters, and Markers of Oxidative Stress after the Sub-Acute Toxicity Study
3.5. Complete Blood Count (CBC) and Biochemistry in the Blood of Mice
3.6. Markers of Oxidative Stress
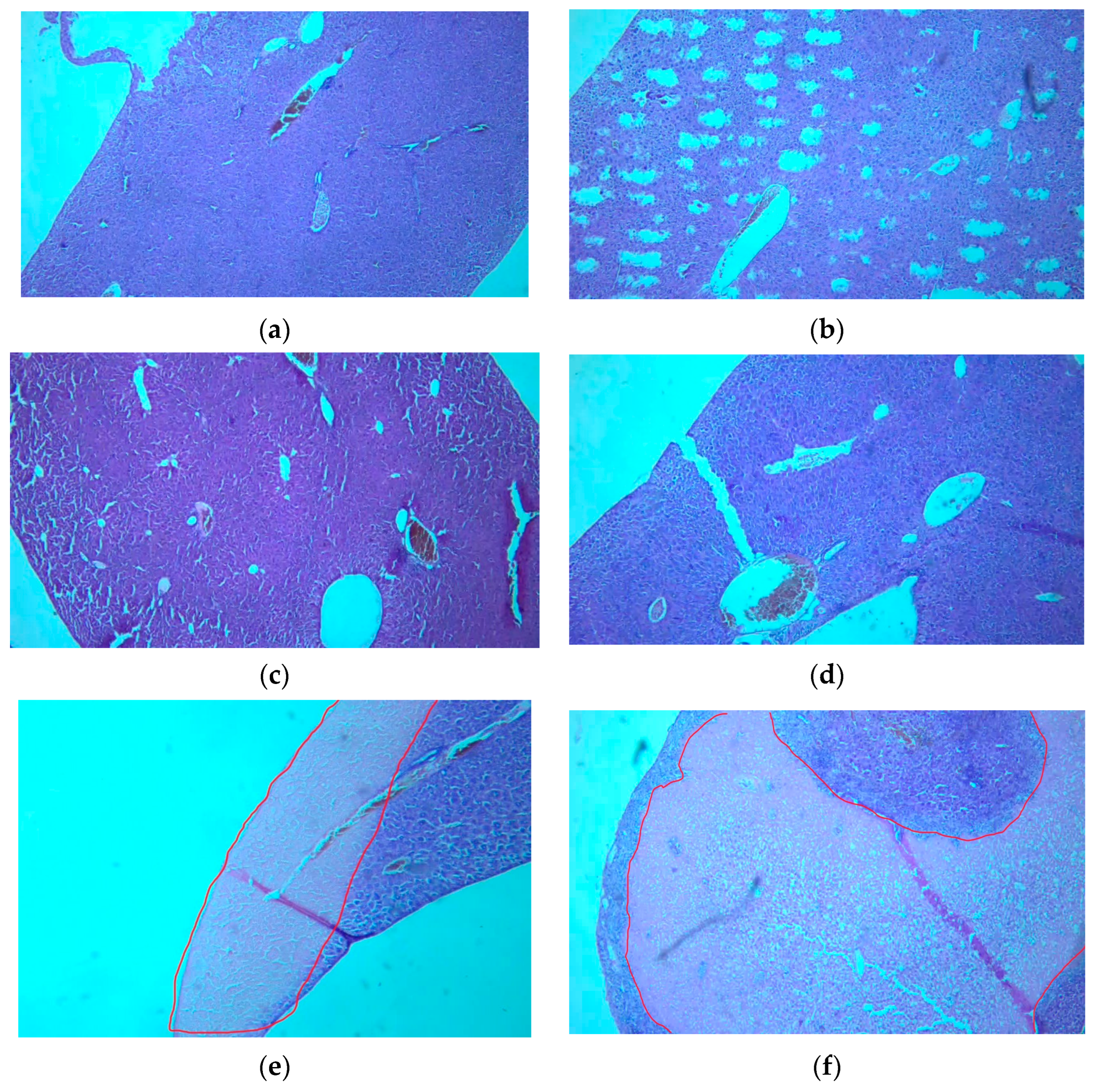
3.7. Histological Examination of Tissue Specimens Post-Mortem
3.7.1. Kidneys
3.7.2. Liver
3.8. In Vitro InhA Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Consolidated Guidelines on Tuberculosis Module 4: Treatment Drug-Susceptible Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Ghiano, D.G.; Recio-Balsells, A.; Bortolotti, A.; Defelipe, L.A.; Turjanski, A.; Morbidoni, H.R.; Labadie, G.R. New one-pot synthesis of anti-tuberculosis compounds inspired on isoniazid. Eur. J. Med. Chem. 2020, 208, 112699. [Google Scholar] [CrossRef] [PubMed]
- Ghiya, S.; Joshi, Y.C. Synthesis and antimicrobial evaluation of hydrazones derived from 4-methylbenzenesulfonohydrazide in aqueous medium. Med. Chem. Res. 2016, 25, 970–976. [Google Scholar] [CrossRef]
- Mascarello, A.; Mori, M.; Chiaradia-Delatorre, L.D.; Menegatti, A.C.O.; Monache, F.D.; Ferrari, F.; Yunes, R.A.; Nunes, R.J.; Terenzi, H.; Botta, B.; et al. Discovery of Mycobacterium tuberculosis Protein Tyrosine Phosphatase B (PtpB) Inhibitors from Natural Products. PLoS ONE 2013, 8, e77081. [Google Scholar] [CrossRef] [PubMed]
- Navakoski de Oliveira, K.; Chiaradia, L.D.; Alves Martins, P.G.; Mascarello, A.; Sechini Cordeiro, M.N.; Carvalho Guido, R.V.; Andricopulo, A.D.; Yunes, R.A.; Nunes, R.J.; Vernal, J.; et al. Sulfonyl-hydrazones of cyclic imides derivatives as potent inhibitors of the Mycobacterium tuberculosisprotein tyrosine phosphatase B (PtpB). MedChemComm 2011, 2, 500–504. [Google Scholar] [CrossRef]
- Bhat, M.; Poojary, B.; Kumar, S.M.; Hussain, M.M.; Pai, N.; Revanasiddappa, B.; Kullaiah, B. Structural, crystallographic, Hirshfeld surface, thermal and antimicrobial evaluation of new sulfonyl hydrazones. J. Mol. Struct. 2018, 1159, 55–66. [Google Scholar] [CrossRef]
- Ozmen, U.O.; Olgun, G. Synthesis, characterization and antibacterial activity of new sulfonyl hydrazone derivatives and their nickel(II) complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 70, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł. The bioactivity of benzenesulfonyl hydrazones: A short review. Biomed. Pharmacother. 2021, 141, 111851. [Google Scholar] [CrossRef] [PubMed]
- Siemann, S.; Evanoff, D.P.; Marrone, L.; Clarke, A.J.; Viswanatha, T.; Dmitrienko, G.I. N-arylsulfonyl hydrazones as inhibitors of IMP-1 metallo-beta-lactamase. Antimicrob. Agents Chemother. 2002, 46, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V.T.; Pencheva, T.; Vassilev, N.; K-Yovkova, E.; Mihaylova, R.; Petrov, B.; Valcheva, V. Development of New Antimycobacterial Sulfonyl Hydrazones and 4-Methyl-1,2,3-thiadiazole-Based Hydrazone Derivatives. Antibiotics 2022, 11, 562. [Google Scholar] [CrossRef]
- Valcheva, V.; Simeonova, R.; Mileva, M.; Philipov, S.; Petrova, R.; Dimitrov, S.; Georgieva, A.; Tsvetanova, E.; Teneva, Y.; Angelova, V.T. In Vivo Toxicity, Redox-Modulating Capacity and Intestinal Permeability of Novel Aroylhydrazone Derivatives as Anti-Tuberculosis Agents. Pharmaceuticals 2022, 15, 79. [Google Scholar] [CrossRef]
- Teneva, Y.; Simeonova, R.; Valcheva, V.; Angelova, V.T. Recent Advances in Anti-Tuberculosis Drug Discovery Based on Hydrazide-Hydrazone and Thiadiazole Derivatives Targeting InhA. Pharmaceuticals 2023, 16, 484. [Google Scholar] [CrossRef] [PubMed]
- Europe Co. (Ed.) European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (ETS 123); Council of Europe: Strasbourg, France, 1991. [Google Scholar]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
- Chinedu, E.; Arome, D.; Ameh, F.S. A new method for determining acute toxicity in animal models. Toxicol. Int. 2013, 20, 224–226. [Google Scholar] [CrossRef]
- Chen, C.; Wicha, S.G.; de Knegt, G.J.; Ortega, F.; Alameda, L.; Sousa, V.; de Steenwinkel, J.E.M.; Simonsson, U.S.H. Assessing Pharmacodynamic Interactions in Mice Using the Multistate Tuberculosis Pharmacometric and General Pharmacodynamic Interaction Models. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Lillie, R.D. STUDIES ON HISTOCHEMICAL ACYLATION PROCEDURES. I. PHENOLS. J. Histochem. Cytochem. 1964, 12, 522–529. [Google Scholar] [CrossRef]
- Polizio, A.H.; Peña, C. Effects of angiotensin II type 1 receptor blockade on the oxidative stress in spontaneously hypertensive rat tissues. Regul. Pept. 2005, 128, 1–5. [Google Scholar] [CrossRef]
- Bump, E.A.; Taylor, Y.C.; Brown, J.M. Role of glutathione in the hypoxic cell cytotoxicity of misonidazole. Cancer Res. 1983, 43, 997–1002. [Google Scholar] [PubMed]
- Aebi, H. [13] Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Chetty, S.; Armstrong, T.; Kharkwal, S.S.; Drewe, W.C.; De Matteis, C.I.; Evangelopoulos, D.; Bhakta, S.; Thomas, N.R. New InhA Inhibitors Based on Expanded Triclosan and Di-Triclosan Analogues to Develop a New Treatment for Tuberculosis. Pharmaceuticals 2021, 14, 361. [Google Scholar] [CrossRef]
- Doğan, H.; Doğan, D.; Gündüz, M.G.; Krishna, V.S.; Lherbet, C.; Sriram, D.; Şahin, O.; Sarıpınar, E. Discovery of hydrazone containing thiadiazoles as Mycobacterium tuberculosis growth and enoyl acyl carrier protein reductase (InhA) inhibitors. Eur. J. Med. Chem. 2020, 188, 112035. [Google Scholar] [CrossRef]
- Doğan, Ş.D.; Gündüz, M.G.; Doğan, H.; Krishna, V.S.; Lherbet, C.; Sriram, D. Design and synthesis of thiourea-based derivatives as Mycobacterium tuberculosis growth and enoyl acyl carrier protein reductase (InhA) inhibitors. Eur. J. Med. Chem. 2020, 199, 112402. [Google Scholar] [CrossRef]
- Bruker, A. APEX 2. Bruker Advanced X-ray Solutions; Bruker: Madison, WI, USA, 2004. [Google Scholar]
- Bruker, A. Saint and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.R.; Tavares, M.T.; Carvalho, C.F.; Silva, N.A.T.; Souza, A.D.F.; Pereira, G.J.V.; Ferreira, F.F.; Parise-Filho, R. Environmentally safe condition for the synthesis of aryl and alkyl sulfonyl hydrazones via one-pot reaction. ACS Sustain. Chem. Eng. 2016, 4, 1899–1905. [Google Scholar] [CrossRef]
- Ozochukwu, I.S.; Okpareke, O.C.; Izuogu, D.C.; Ibezim, A.; Ujam, O.T.; Asegbeloyin, J.N. N’-(Pyridin-3-ylmethylene) benzenesulfonohydrazide: Crystal structure, DFT, Hirshfeld surface and in silico anticancer studies. Eur. J. Chem. 2021, 12, 256–264. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hussain, M.M.; Arshad, M.N.; Awual, M.R.; Asiri, A.M. Arsenic sensor development based on modification with (E)-N′-(2-nitrobenzylidine)-benzenesulfonohydrazide: A real sample analysis. New J. Chem. 2019, 43, 9066–9075. [Google Scholar] [CrossRef]
- Blatova, O.A.; Asiri, A.M.; Al-Amshany, Z.M.; Arshad, M.N.; Blatov, V.A. Molecular packings and specific-bonding patterns in sulfonamides. New J. Chem. 2014, 38, 4099–4106. [Google Scholar] [CrossRef]
- Hodge, H.C.; Sterner, J.H. Tabulation of Toxicity Classes. Am. Ind. Hyg. Assoc. Q. 1949, 10, 93–96. [Google Scholar] [CrossRef]
- Ogawa, K.; Ito, M. Appetite-enhancing Effects of trans-Cinnamaldehyde, Benzylacetone and 1-Phenyl-2-butanone by Inhalation. Planta Med. 2016, 82, 84–88. [Google Scholar] [CrossRef]
- Niedernhofer, L.J.; Daniels, J.S.; Rouzer, C.A.; Greene, R.E.; Marnett, L.J. Malondialdehyde, a Product of Lipid Peroxidation, Is Mutagenic in Human Cells. J. Biol. Chem. 2003, 278, 31426–31433. [Google Scholar] [CrossRef]
- Bains, V.K.; Bains, R. The antioxidant master glutathione and periodontal health. Dent. Res. J. 2015, 12, 389–405. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, H.; Thompson, D.C.; Shertzer, H.G.; Nebert, D.W.; Vasiliou, V. Glutathione defense mechanism in liver injury: Insights from animal models. Food Chem. Toxicol. 2013, 60, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Sharma, P.; Tiwary, M.K. Glutathione deficiency in COVID19 illness-does supplementation help? Saudi J. Anaesth. 2021, 15, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]




| Dose mg/kg b.w. | Effect/Lethality | Time of Occurrence | Symptoms |
|---|---|---|---|
| 1500 | 2/3 (66%) | After 24 h | Delayed reflexes, somnolence, lethal outcome |
| 1000 | 1/3 (33%) | After 7 days | Impaired coordination, rapid breathing, lethal outcome |
| 750 | 0/3 | - | - |
| 500 | 0/3 | - | - |
| 250 | 0/3 | - | - |
| Dose mg/kg b.w. | Effect/Lethality | Time of Occurrence | Symptoms |
|---|---|---|---|
| 1500 | 3/3 (100%) | After 24 h | Respiratory failure with long pauses, ataxia, piloerection, seizures, lethal outcome. |
| 1000 | 0/3 | - | - |
| 750 | 0/3 | - | - |
| 500 | 0/3 | - | - |
| 250 | 0/3 | - | - |
| Compound | Concentration (µM) | Initial Velocity of Reaction | % Inhibition of InhA |
|---|---|---|---|
| 3a | 1 | 0.0009 | 35.2 |
| 10 | 0.0006 | 54.1 | |
| 25 | 0.0006 | 52.9 | |
| 50 | 0.0005 | 64.7 | |
| 100 | 0.0002 | 82.3 | |
| 3b | 1 | 0.0009 | 29.3 |
| 10 | 0.0006 | 54.1 | |
| 25 | 0.0006 | 58.8 | |
| 50 | 0.0005 | 64.7 | |
| 100 | 0.0006 | 58.8 | |
| Triclosan | 100 | 0.0002 | 82.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teneva, Y.; Simeonova, R.; Besarboliev, O.; Sbirkova-Dimitrova, H.; Angelova, V.T. X-ray Single-Crystal Analysis, Pharmaco-Toxicological Profile and Enoyl-ACP Reductase-Inhibiting Activity of Leading Sulfonyl Hydrazone Derivatives. Crystals 2024, 14, 560. https://doi.org/10.3390/cryst14060560
Teneva Y, Simeonova R, Besarboliev O, Sbirkova-Dimitrova H, Angelova VT. X-ray Single-Crystal Analysis, Pharmaco-Toxicological Profile and Enoyl-ACP Reductase-Inhibiting Activity of Leading Sulfonyl Hydrazone Derivatives. Crystals. 2024; 14(6):560. https://doi.org/10.3390/cryst14060560
Chicago/Turabian StyleTeneva, Yoanna, Rumyana Simeonova, Orlin Besarboliev, Hristina Sbirkova-Dimitrova, and Violina T. Angelova. 2024. "X-ray Single-Crystal Analysis, Pharmaco-Toxicological Profile and Enoyl-ACP Reductase-Inhibiting Activity of Leading Sulfonyl Hydrazone Derivatives" Crystals 14, no. 6: 560. https://doi.org/10.3390/cryst14060560









