Photophysical Properties, Stability and Microstructures of Temperature-Dependent Evolution of Methylammonium Lead Bromide Perovskite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Growth of MAPbBr3 Single Crystals
2.3. In Situ High-Temperature Tests
2.4. Raman Spectroscopy
2.5. X-ray Diffraction (XRD)
2.6. Photoluminescence Spectroscopy
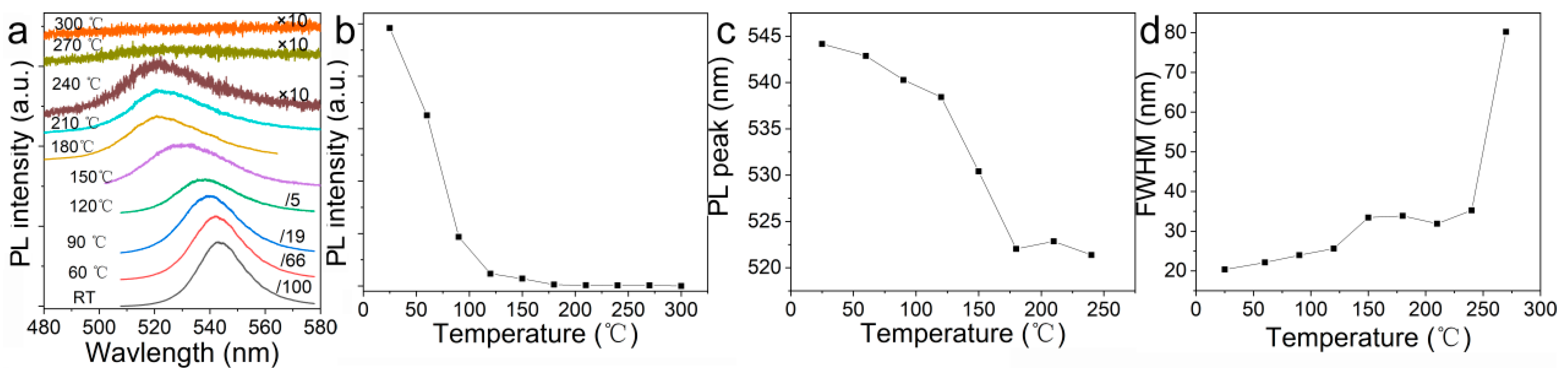
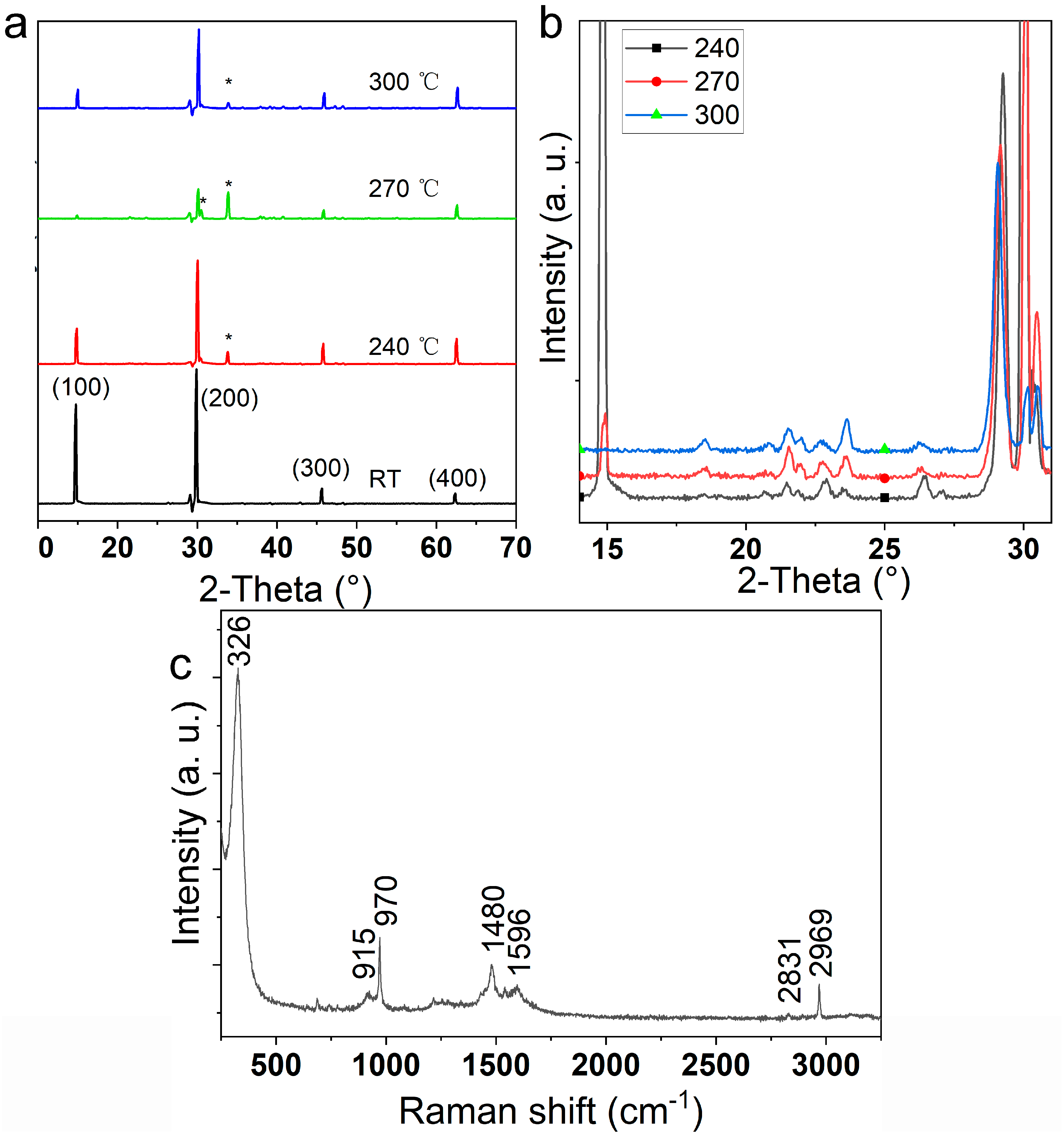
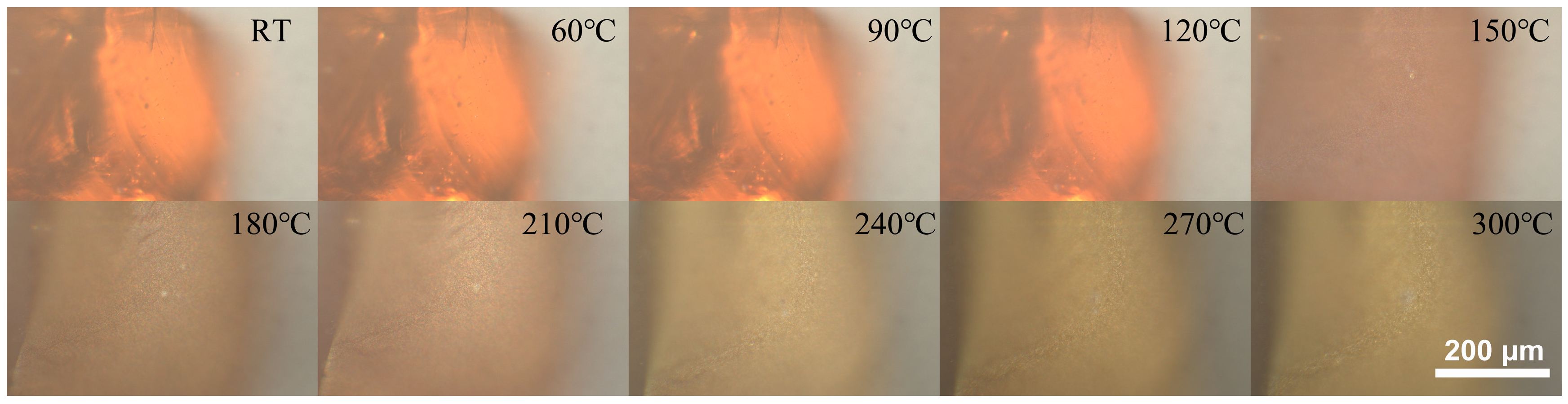
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Ecker, B.R.; Ghosh, M.; Li, M.; Karasiev, V.V.; Hu, S.X.; Huang, J.; Gao, Y. Light-enhanced oxygen degradation of MAPbBr3 single crystal. Phys. Chem. Chem. Phys. 2024, 26, 5027–5037. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.J.; Ono, L.K.; Cho, H.; Kim, K.; Kang, H.; Qi, Y.; Park, J.Y. Pathways of water-induced lead-halide perovskite surface degradation: Insights from in situ atomic-scale analysis. ACS Nano 2023, 17, 25679–25688. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Kumar, S.; Cherf, S.; Aharon, S.; Cahen, D.; Eren, B. Self-healing and -repair of nanomechanical damages in lead halide perovskites. Adv. Funct. Mater. 2023, 33, 2304278. [Google Scholar] [CrossRef]
- Capitani, F.; Marini, C.; Caramazza, S.; Dore, P.; Pisanu, A.; Malavasi, L.; Nataf, L.; Baudelet, F.; Brubach, J.B.; Roy, P.; et al. Locking of methylammonium by pressure-enhanced H-bonding in (CH3NH3)PbBr3 hybrid perovskite. J. Phys. Chem. C 2017, 121, 28125–28131. [Google Scholar] [CrossRef]
- Ma, L.; Li, W.; Yang, K.; Bi, J.; Feng, J.; Zhang, J.; Yan, Z.; Zhou, X.; Liu, C.; Ji, Y.; et al. A- or x-site mixture on mechanical properties of APbX3 perovskite single crystals. APL Mater. 2021, 9, 041112. [Google Scholar] [CrossRef]
- Qin, Z.; Dai, S.; Gajjela, C.C.; Wang, C.; Hadjiev, V.G.; Yang, G.; Li, J.; Zhong, X.; Tang, Z.; Yao, Y.; et al. Spontaneous formation of 2D/3D heterostructures on the edges of 2D Ruddlesden-popper hybrid perovskite crystals. Chem. Mat. 2020, 32, 5009–5015. [Google Scholar] [CrossRef]
- Tan, M.; Wang, S.; Rao, F. First-principles study on the modulation of the electronic structure of organic-inorganic hybrid perovskite (MAPbBr3) under pressure. J. Synth. Cryst. 2018, 47, 1589–1595. [Google Scholar]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Degani, M.; An, Q.; Albaladejo-Siguan, M.; Hofstetter, Y.J.; Cho, C.; Paulus, F.; Grancini, G.; Vaynzof, Y. 23.7% efficient inverted perovskite solar cells by dual interfacial modification. Sci. Adv. 2021, 7, eabj7930. [Google Scholar] [CrossRef]
- Shen, Z.; Han, Q.; Luo, X.; Shen, Y.; Wang, Y.; Yuan, Y.; Zhang, Y.; Yang, Y.; Han, L. Efficient and stable perovskite solar cells with regulated depletion region. Nat. Photonics 2024, 18, 450–457. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Graetzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316. [Google Scholar] [CrossRef] [PubMed]
- Ahn, N.; Choi, M. Towards long-term stable perovskite solar cells: Degradation mechanisms and stabilization techniques. Adv. Sci. 2024, 11, e2306110. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; Mcgehee, M.D. Understanding degradation mechanisms and improving stability of perovskite photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, D.; Hu, Y.; Mei, A.; Han, H. Degradation pathways in perovskite solar cells and how to meet international standards. Commun. Mater. 2022, 3, 58. [Google Scholar] [CrossRef]
- Kim, T.; Park, S.; Iyer, V.; Shaheen, B.; Choudhry, U.; Jiang, Q.; Eichman, G.; Gnabasik, R.; Kelley, K.; Lawrie, B.; et al. Mapping the pathways of photo-induced ion migration in organic-inorganic hybrid halide perovskites. Nat. Commun. 2023, 14, 1846. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, N.; Aono, M.; Watanabe, Y. Temperature-dependent time-resolved photoluminescence of (C6H5C2H4NH3)2PBX4 (X=Br and I). Mater. Chem. Phys. 2012, 134, 875–880. [Google Scholar] [CrossRef]
- Shi, R.; Fang, Q.; Vasenko, A.S.; Long, R.; Fang, W.; Prezhdo, O.V. Structural disorder in higher-temperature phases increases charge carrier lifetimes in metal halide perovskites. J. Am. Chem. Soc. 2022, 144, 19137–19149. [Google Scholar] [CrossRef] [PubMed]
- Nakada, K.; Matsumoto, Y.; Shimoi, Y.; Yamada, K.; Furukawa, Y. Temperature-dependent evolution of raman spectra of methylammonium lead halide perovskites, CH3NH3PbX3 (X = I, Br). Molecules 2019, 24, 626. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Wang, S.; Rao, F.; Yang, S.; Wang, F. Pressures tuning the band gap of organic-inorganic trihalide perovskites (mapbbr3): A first-principles study. J. Electron. Mater. 2018, 47, 7204–7211. [Google Scholar] [CrossRef]
- Park, S.; Seo, Y.; Ahn, C.W.; Woo, W.S.; Kyhm, J.; Lee, S.A.; Kim, I.W.; Hwang, J. Temperature-dependent optical properties of hybrid organic-inorganic perovskite single crystals (CH3NH3PbI3 and CH3NH3PbBr3). J. Phys. D Appl. Phys. 2019, 52, 335302. [Google Scholar] [CrossRef]
- Bermúdez-García, J.M.; Yáñez-Vilar, S.; García-Fernández, A.; Sánchez-Andújar, M.; Castro-García, S.; López-Beceiro, J.; Artiaga, R.; Dilshad, M.; Moya, X.; Señarís-Rodríguez, M.A. Giant barocaloric tunability in [(CH3CH2CH2)4N]Cd[N(CN)2]3 hybrid perovskite. J. Mater. Chem. C 2018, 6, 9867–9874. [Google Scholar] [CrossRef]
- Greenland, C.; Shnier, A.; Rajendran, S.K.; Smith, J.A.; Game, O.S.; Wamwangi, D.; Turnbull, G.A.; Samuel, I.D.W.; Billing, D.G.; Lidzey, D.G. Correlating phase behavior with photophysical properties in mixed-cation mixed-halide perovskite thin films. Adv. Energy Mater. 2020, 10, 1901350. [Google Scholar] [CrossRef]
- Ma, L.; Yan, Z.; Zhou, X.; Pi, Y.; Du, Y.; Huang, J.; Wang, K.; Wu, K.; Zhuang, C.; Han, X. A polymer controlled nucleation route towards the generalized growth of organic-inorganic perovskite single crystals. Nat. Commun. 2021, 12, 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bu, K.; Li, J.; Wang, H.; Xu, Y.; Guo, S.; Luo, H.; Liu, B.; Yang, D.; Gong, Y.; et al. Synthesis of edge-shared octahedral MAPbBr3 via pressure- and temperature-induced multiple-stage processes. Chem. Mat. 2023, 35, 1177–1185. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Shellaiah, M.; Wen Sun, K. Structural and photophysical properties of methylammonium lead tribromide (MAPbBr3) single crystals. Sci. Rep. 2017, 7, 13643. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hunger, J.; Cánovas, E.; Karakus, M.; Mics, Z.; Grechko, M.; Turchinovich, D.; Parekh, S.H.; Bonn, M. Direct observation of mode-specific phonon-band gap coupling in methylammonium lead halide perovskites. Nat. Commun. 2017, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.I.; Jacopin, G.; Meloni, S.; Mattoni, A.; Arora, N.; Boziki, A.; Zakeeruddin, S.M.; Rothlisberger, U.; Grätzel, M. Origin of unusual bandgap shift and dual emission in organic-inorganic lead halide perovskites. Sci. Adv. 2016, 2, e1601156. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Li, C.; Panzer, F.; Narasimhan, K.L.; Graeser, A.; Gujar, T.P.; Köhler, A.; Thelakkat, M.; Huettner, S.; Kabra, D. Effect of thermal and structural disorder on the electronic structure of hybrid perovskite semiconductor CH3NH3PbI3. J. Phys. Chem. Lett. 2016, 7, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Guo, S.; Jin, L.; Mao, Y.; Chen, Y.; Gu, J.; Chen, S.; Huang, X.; Guan, Y.; Li, X.; et al. Two-dimensional lead halide perovskite lateral homojunctions enabled by phase pinning. Nat. Commun. 2024, 15, 3164. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Q.; Zhang, Y.; Cui, C.; Sui, N.; Chi, X.; Zhang, H.; Zhou, Q.; Bao, Y.; Wang, Y. Pressure effects on optoelectronic properties of CsPbBr3 nanocrystals. J. Phys. Chem. C 2020, 124, 11239–11247. [Google Scholar] [CrossRef]
- Wang, C.; Ecker, B.R.; Wei, H.; Huang, J.; Gao, Y. Environmental surface stability of the MAPbBr3 single crystal. J. Phys. Chem. C 2018, 122, 3513–3522. [Google Scholar] [CrossRef]
- Samanta, D.; Saha, P.; Ghosh, B.; Chaudhary, S.P.; Bhattacharyya, S.; Chatterjee, S.; Mukherjee, G.D. Pressure-induced emergence of visible luminescence in lead free halide perovskite Cs3Bi2Br9: Effect of structural distortion. J. Phys. Chem. C 2021, 125, 3432–3440. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; Zou, B. Pressure-induced structural and optical properties of organometal halide perovskite-based formamidinium lead bromide. J. Phys. Chem. Lett. 2016, 7, 2556–2562. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.R.; Kim, S.H.; Joo, Y.L. Structural dynamics of CH3NH3+ and PbBr3− in tetragonal and cubic phases of CH3NH3PbBr3 hybrid perovskite by nuclear magnetic resonance. Sci. Rep. 2020, 10, 13140. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal degradation of CH3NH3PbI3 perovskite into NH3 and CH3I gases observed by coupled thermogravimetry-mass spectrometry analysis. Energy Environ. Sci. 2016, 9, 3406–3410. [Google Scholar] [CrossRef]
- Li, F.; Lu, J.; Zhang, Q.; Peng, D.; Yang, Z.; Xu, Q.; Pan, C.; Pan, A.; Li, T.; Wang, R. Controlled fabrication, lasing behavior and excitonic recombination dynamics in single crystal CH3NH3PbBr3 perovskite cuboids. Sci. Bull. 2019, 64, 698–704. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.; Ma, L.; Zheng, S.; Li, X.; Cai, S.; Chang, H. Photophysical Properties, Stability and Microstructures of Temperature-Dependent Evolution of Methylammonium Lead Bromide Perovskite. Crystals 2024, 14, 589. https://doi.org/10.3390/cryst14070589
Lai Y, Ma L, Zheng S, Li X, Cai S, Chang H. Photophysical Properties, Stability and Microstructures of Temperature-Dependent Evolution of Methylammonium Lead Bromide Perovskite. Crystals. 2024; 14(7):589. https://doi.org/10.3390/cryst14070589
Chicago/Turabian StyleLai, Yuming, Lin Ma, Shi Zheng, Xiao Li, Shuangyu Cai, and Hai Chang. 2024. "Photophysical Properties, Stability and Microstructures of Temperature-Dependent Evolution of Methylammonium Lead Bromide Perovskite" Crystals 14, no. 7: 589. https://doi.org/10.3390/cryst14070589




