Theoretical Study of the Magnetic Properties of the SmFe12−xMox (x = 1, 2) and SmFe10Mo2H Compounds
Abstract
:1. Introduction
2. Methods
3. Results and Discussions
3.1. Crystal Structures
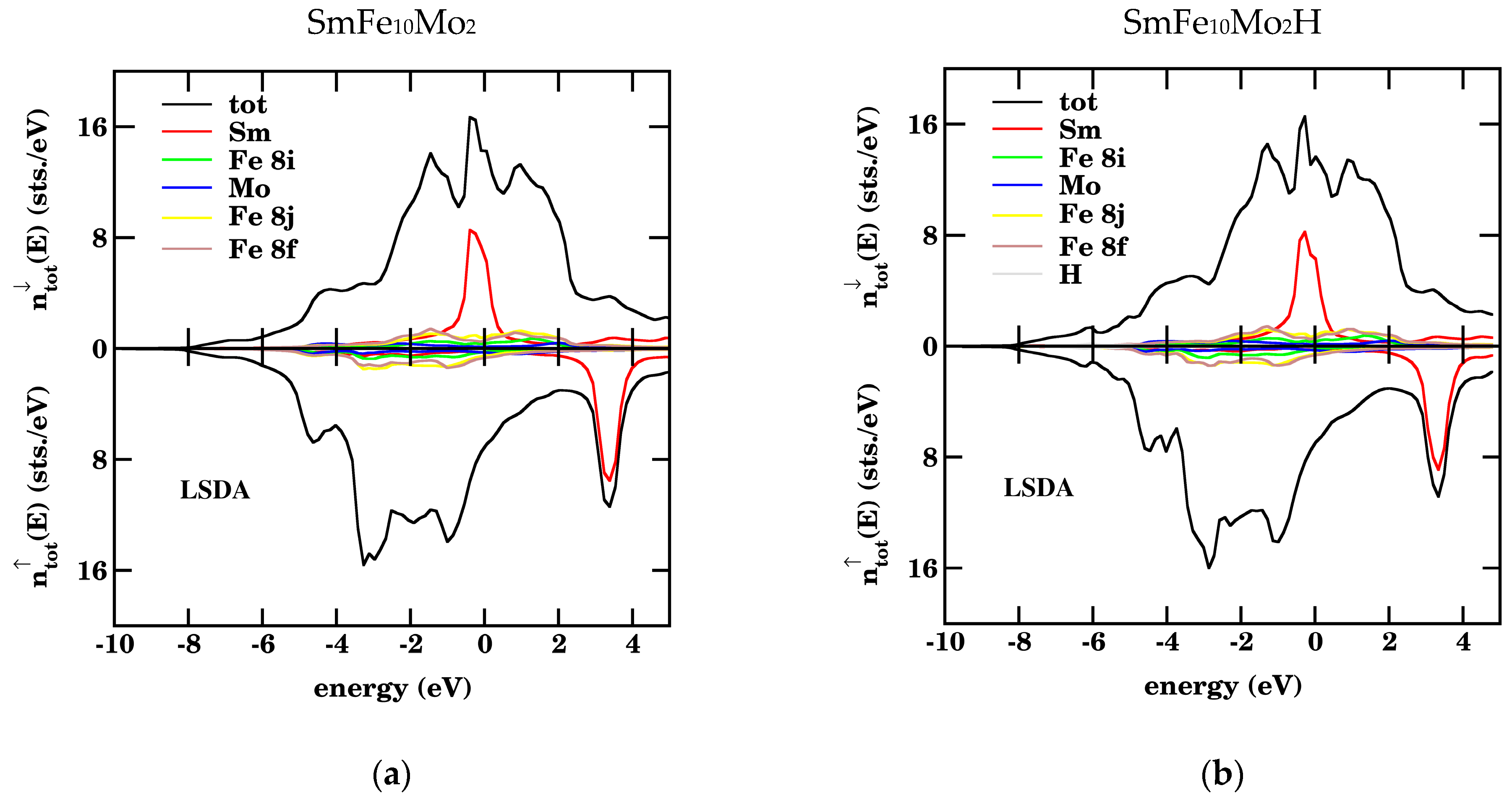
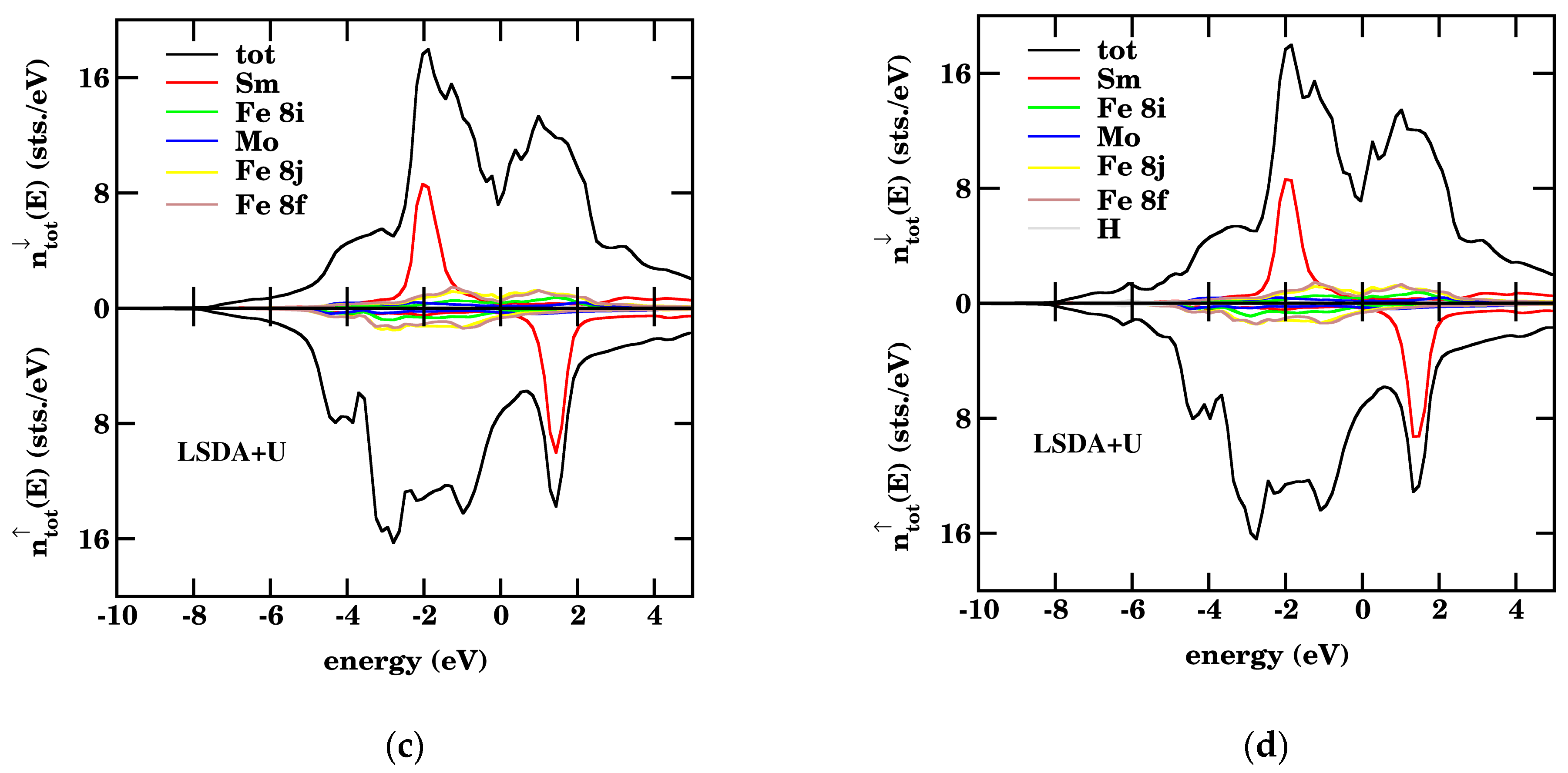
3.2. Density of States
3.3. Magnetic Moments
3.4. Magneto-Crystalline Anisotropy
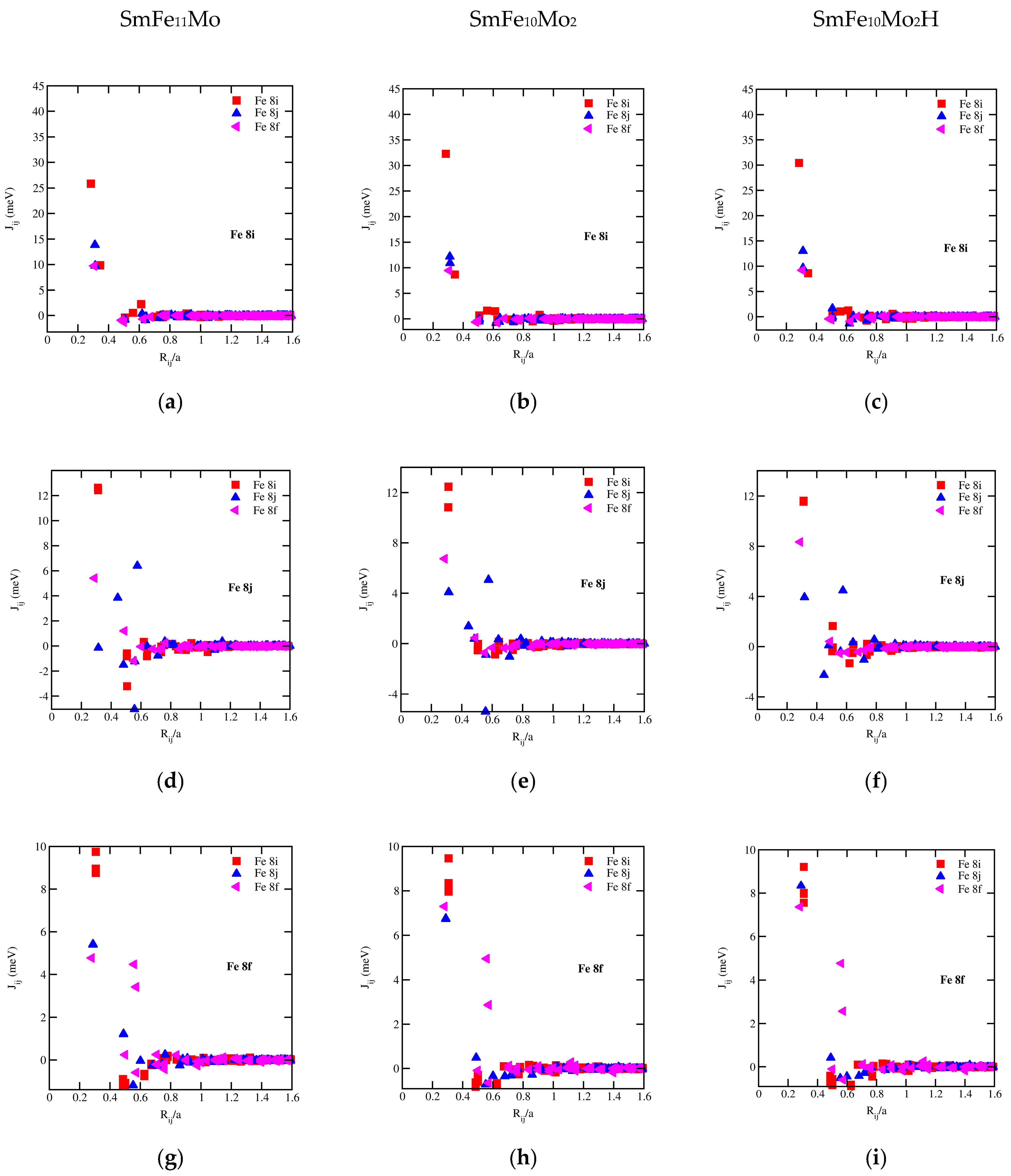
3.5. Exchange-Coupling Parameters and CURIE Temperatures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Report on Critical Raw Materials for the EU 2014. Available online: https://rmis.jrc.ec.europa.eu/uploads/crm-report-on-critical-raw-materials_en.pdf (accessed on 3 June 2024).
- de Mooij, D.B.; Buschow, K.H.J. Some novel ternary ThMn12-type compounds. J. Less-Common Met. 1988, 136, 207–215. [Google Scholar] [CrossRef]
- Coey, J.M.D. Perspective and prospects for rare earth permanent magnets. Engineering 2020, 6, 119–131. [Google Scholar] [CrossRef]
- Buschow, K.H.J. Permanent magnet materials based on tetragonal rare earth compounds of the type RFe12−xMx. J. Magn. Magn. Mater. 1991, 100, 79–89. [Google Scholar] [CrossRef]
- Harashima, Y.; Terakura, K.; Kino, H.; Ishibashi, S.; Miyake, T. Nitrogen as the best interstitial dopant among, C, N, O, and F for strong permanent magnet: First-principles study. Phys. Rev. B 2015, 92, 184426. [Google Scholar] [CrossRef]
- Ke, L.; Johnson, D.D. Intrinsic magnetic properties in R(Fe1−xCox)11 Ti Z (R = Y and Ce; Z = H, C, and N). Phys. Rev. B 2016, 94, 024423. [Google Scholar] [CrossRef]
- Tomey, E.; Isnard, O.; Fagan, A.; Desmoulins, C.; Miraglia, S.; Soubeyroux, J.L.; Fruchart, D. Modulation of spin reorientation transitions in the series R(Fe,M)12Xy (R = Y, Nd, Ho; M = Mo, Ti; X = N, H). J. Alloys Compd. 1993, 191, 233–238. [Google Scholar] [CrossRef]
- Isnard, O.; Pop, V. Effect of hydrogen as interstitial element on the magnetic properties of some iron rich intermetallic compounds. J. Alloys Compd. 2011, 509, S549–S554. [Google Scholar] [CrossRef]
- Ohashi, K.; Tawara, Y.; Osugi, R.; Shimao, M. Magnetic properties of Fe-rich rare earth intermetallic compounds with a ThMn12 Structure. J. Appl. Phys. 1998, 64, 5714. [Google Scholar] [CrossRef]
- Müller, A. Magnetic material R,Fe,Mo,(Co) with ThMn12 structure. J. Appl. Phys. 1998, 64, 249–251. [Google Scholar] [CrossRef]
- Schultz, L.; Wecker, J. Coercivity in ThMn12 type magnets. J. Appl. Phys. 1988, 64, 5711. [Google Scholar] [CrossRef]
- Kou, X.C.; Sinnecker, E.H.C.P.; Grössinger, R.; Wiesinger, G.; Zhao, T.; Liu, J.P.; de Boer, F.R. Magnetocrystalline anisotropy of SmFe12−xMox compounds with x = 0.5, 1.0, 1.5, 2.0 or 3.0. J. Magn. Magn. Mater. 1995, 140–144, 1025–1026. [Google Scholar] [CrossRef]
- Kou, X.C.; Grössinger, R.; Wiesinger, G.; Liu, J.P.; de Boer, F.R.; Kleinschroth, I.; Krönmüller, H. Intrinsic magnetic properties of R compounds (R = Y, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, or Tm). Phys. Rev. B 1995, 51, 8254. [Google Scholar] [CrossRef]
- Isnard, O.; Guillot, M. Investigation of the magnetic properties of SmFe10Mo2 and SmFe10Mo2H in high magnetic field. J. Appl. Phys. 1999, 85, 4681–4683. [Google Scholar] [CrossRef]
- Khazzan, S.; Bessais, L.; Van Tendeloo, G.; Mliki, N. Correlation between the nanocrystalline Sm(Fe,Mo)12 and it’s out of equilibrium phase Sm(Fe,Mo)10. J. Magn. Magn. Mater. 2014, 363, 125–132. [Google Scholar] [CrossRef]
- Xu, Y.; Hadjipanayis, G.C. Investigation of rare earth lean Sm-(Fe,Co)-Mo alloys for permanent magnets. J. Magn. Magn. Mater. 2024, 589, 171484. [Google Scholar] [CrossRef]
- Ebert, H. The Munich SPR-KKR Package, Versions 7.7 and 8.6. Available online: http://ebert.cup.uni-muenchen.de/SPRKKR (accessed on 4 June 2024).
- Ebert, H.; Ködderitzsch, D.; Minar, J. Calculating condensed matter properties using the KKR-Green’s function method—Recent developments and applications. Rep. Prog. Phys. 2011, 74, 096501. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Herper, H.C.; Skokov, K.P.; Ener, S.; Thunström, P.; Diop, L.V.B.; Gutfleisch, O.; Eriksson, O. Magnetic properties of NdFe11Ti and YFe11Ti, from experiment and theory. Acta Mater. 2023, 242, 118473. [Google Scholar] [CrossRef]
- Monkhorst, H.; Pack, J. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Faulkner, J.S.; Stocks, G.M. Calculating properties with the coherent-potential approximation. Phys. Rev. B 1980, 21, 3222. [Google Scholar] [CrossRef]
- Andersen, O.K.; Skriver, H.L.; Nohl, H.; Johansson, B. Electronic structure of transition metal compounds; ground-state properties of the 3d-monoxides in the atomic sphere approximation. Pure Appl. Chem. 1979, 52, 93. [Google Scholar] [CrossRef]
- Czyżyk, M.T.; Sawatsky, G.A. Local-density functional and on-site correlations: The electronic structure of La2CuO4 and LaCuO3. Phys. Rev. B 1994, 49, 14211. [Google Scholar] [CrossRef] [PubMed]
- Odkhuu, D.; Ochirkhuyag, T.; Hong, S.C. Enhancing Energy Product and Thermal Stability of by Interstitial Doping. Phys. Rev. Appl. 2020, 13, 054076. [Google Scholar] [CrossRef]
- Staunton, J.B.; Szunyogh, L.; Buruzs, A.; Gyorffy, B.L.; Ostanin, S.; Udvardi, L. Temperature dependence of magnetic anisotropy: An ab initio approach. Phys. Rev. B 2006, 74, 144411. [Google Scholar] [CrossRef]
- Mankovsky, S.; Polesya, S.; Minar, J.; Hoffmann, F.; Back, D.H.; Ebert, H. Spin-orbit coupling effect in (Ga,Mn) As films: Anisotropic exchange interactions and magnetocrystalline anisotropy. Phys. Rev. B 2011, 84, 201201. [Google Scholar] [CrossRef]
- Liechtenstein, A.I.; Katsnelson, M.I.; Antropov, V.P.; Gubanov, V.A. Local spin density functional approach to the theory of exchange interactions in ferromagnetic metals and alloys. J. Magn. Magn. Mater. 1987, 67, 65–74. [Google Scholar] [CrossRef]
- Anderson, P.W. Theory of magnetic exchange interactions: Exchange in insulators and semiconductors. Solid State Phys. 1963, 14, 99. [Google Scholar] [CrossRef]
- Nieves, P.; Arapan, S.; Maudes-Raedo, J.; Marticorena-Sánchez, R.; Del Brío, N.L.; Kovacs, A.; Echevarria-Bonet, C.; Salazar, D.; Weischenberg, J.; Zhang, H.; et al. Database of novel magnetic materials for high-performance permanent magnet development. Comput. Mat. Sci. 2019, 168, 188–202. [Google Scholar] [CrossRef]
- Bessais, L. Structure and magnetic properties of intermetallic rare-earth-transition-metal compounds: A review. Materials 2022, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Tomey, E.; Bacmann, M.; Fruchart, D.; Soubeyroux, J.L.; Gignoux, D. Influence of hydrogen on the structural and magnetic properties of the RFe10.5Mo1.5 compounds (R = rare earth). J. Alloys Compd. 1995, 231, 195–200. [Google Scholar] [CrossRef]
- Benea, D.; Pop, V.; Minár, J. The effects of V doping on the intrinsic properties of SmFe10Co2 alloys: A theoretical investigation. Comput. Mat. Sci. 2024, 241, 113029. [Google Scholar] [CrossRef]
- Skomski, R.; Coey, J.M.D. Permanent Magnetism; Taylor & Francis Group: Bristol, UK, 1999. [Google Scholar]
- Miyake, T.; Akai, H. Quantum theory of rare-earth magnets. J. Phys. Soc. Jpn. 2018, 87, 041009. [Google Scholar] [CrossRef]
- Benea, D.; Hirian, R.; Gutoiu, S.; Isnard, O.; Pop, V. Intrinsic magnetic properties of the RFe11Ti (R = Y and Gd) alloys by Co, Zr and C doping. Solid State Commun. 2022, 355, 114922. [Google Scholar] [CrossRef]
- Piquer, C.; Grandjean, F.; Long, G.J.; Isnard, O. A magnetic and Mössbauer spectral study of SmFe11Ti, LuFe11Ti, and their respective hydrides. J. Alloys Comp. 2005, 383, 6–14. [Google Scholar] [CrossRef]
- Tereshina, I.S.; Nikitin, S.A.; Ivanova, T.I.; Skokov, K.P. Rare-earth and transition metal sublattice contributions to magnetization and magnetic anisotropy of R(TM,Ti)12 single crystals. J. Alloys Compd. 1998, 275–277, 625–628. [Google Scholar] [CrossRef]
- Schönhöbel, A.M.; Madugundo, R.; Vekilova, O.Y.; Eriksson, O.; Herper, H.C.; Barandiaran, J.M.; Hadjipanayis, G.C. Intrinsic magnetic properties of SmFe12−xVx alloys with reduced V-concentration. J. Alloys Compd. 2019, 786, 969. [Google Scholar] [CrossRef]
- Isnard, O.; Fruchart, D. A review on magnetism in Fe based intermetallics, relation between local environment and local magnetic moments. J. Alloys Comp. 1994, 205, 1–15. [Google Scholar] [CrossRef]
- Rodriguez-Crespo, B.; Garcia-Franco, A.; Rosero-Romo, J.J.; Echevarria-Bonet, C.; Porro, J.M.; Saiz, P.G.; Salazar, D. Magnetic Properties of Tetragonal SmFe12−xMox Alloys in Bulk and Melt-Spun Ribbons. Phys. Status Solidi A 2022, 219, 2100725. [Google Scholar] [CrossRef]
- Stevens, K.W.H. Matrix Elements and Operator Equivalents Connected with the Magnetic Properties of Rare Earth Ions. Proc. Phys. Soc. 1952, 65, 209. [Google Scholar] [CrossRef]
- Skomski, R. Simple Models of Magnetism; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Buschow, K.H.J.; de Boer, F.R. Physics of Magnetism and Magnetic Materials; Kluwer Academic Publishers: New York, NY, USA, 2004; ISBN 0-306-47421-2. [Google Scholar]
- Diop, L.V.B.; Kuz’min, M.D.; Skourski, Y.; Skokov, K.P.; Radulov, I.A.; Gutfleisch, O. Determination of the crystal field parameters in SmFe11Ti. Phys. Rev. B 2020, 102, 064423. [Google Scholar] [CrossRef]
- Tereshina, I.S.; Pankratov, N.Y.; Tereshina, E.A.; Verbetski, V.N.; Salamova, A.A.; Mydlarz, T.; Suski, W. Magnetocrystalline anisotropy of hydrogenated and nitrogenated rare-earth intermetallic compound SmFe11Ti. Phys. Met. Metall. 2005, 99, S46. [Google Scholar]
- Sato, K.; Bergqvist, L.; Kudrnovsky, J.; Dederichs, P.H.; Eriksson, O.; Turek, I.; Sanyal, B.; Bouzerar, J.; Katayama-Yoshida, H.; Dinh, V.A.; et al. First-principles theory of dilute magnetic semiconductors. Rev. Mod. Phys. 2010, 82, 1633. [Google Scholar] [CrossRef]
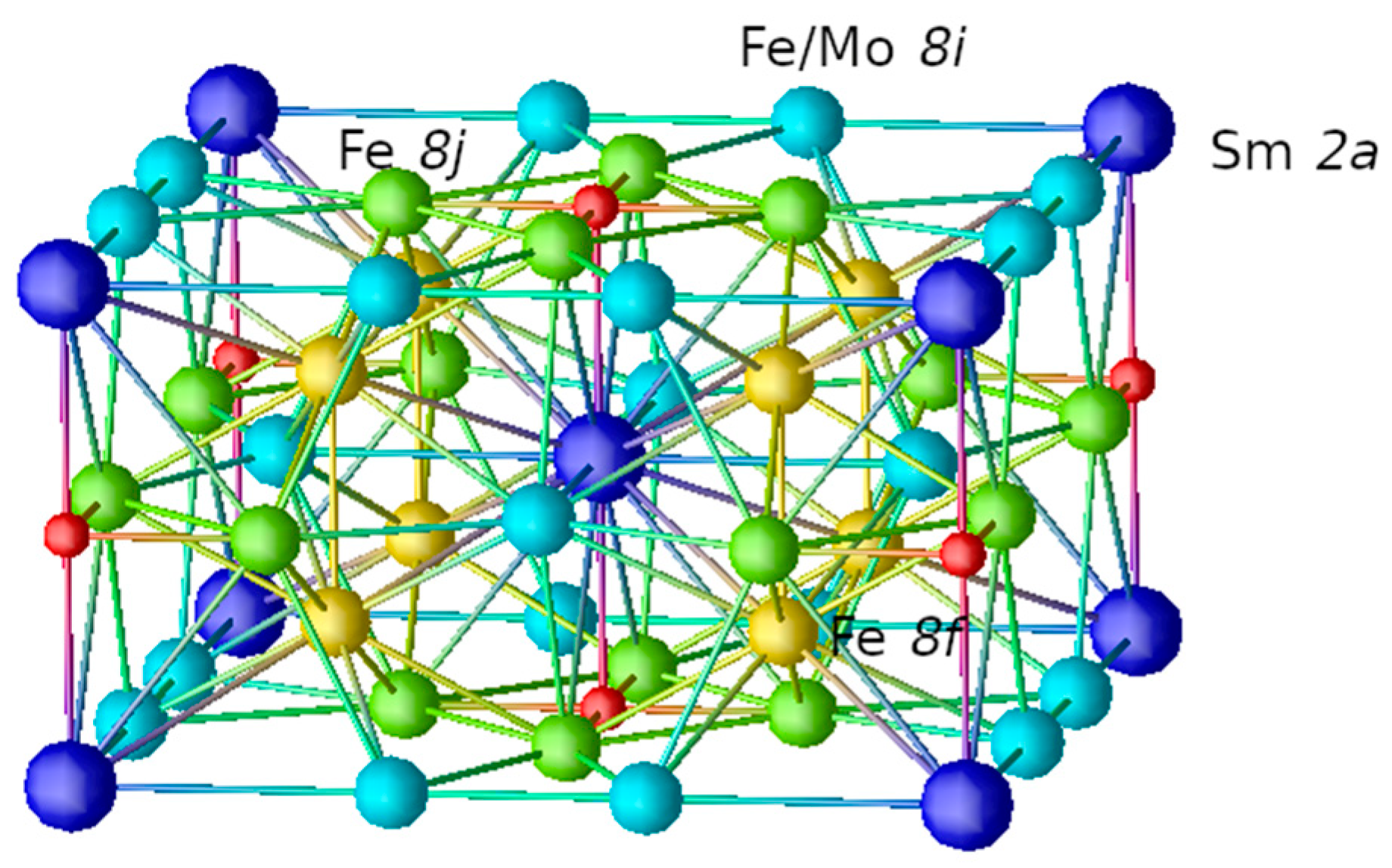
| alat (Å) | clat (Å) | (Å) | (Å) | (Å) | (Å) | (Å) | |
|---|---|---|---|---|---|---|---|
| SmFe11Mo [31] | 8.548 | 4.772 | 1.615 | 1.216 | 1.230 | 1.196 | - |
| SmFe10Mo2 [14] | 8.587 | 4.798 | 1.605 | 1.219 | 1.234 | 1.199 | - |
| SmFe10Mo2H [14] | 8.605 | 4.816 | 1.580 | 1.221 | 1.219 | 1.204 | 0.691 |
| SmFe11Mo | SmFe10Mo2 | SmFe10Mo2H | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LSDA | LSDA+U | LSDA | LSDA+U | LSDA | LSDA+U | |||||||
| ms (µB) | ml (µB) | ms (µB) | ml (µB) | ms (µB) | ml (µB) | ms (µB) | ml (µB) | ms (µB) | ml (µB) | ms (µB) | ml (µB) | |
| Sm | −5.65 | 2.81 | −5.17 | 4.83 | −5.59 | 2.88 | −5.11 | 4.79 | −5.56 | 2.84 | −5.07 | 4.78 |
| Fe 8i | 2.41 | 0.07 | 2.39 | 0.05 | 2.33 | 0.08 | 2.40 | 0.07 | 2.38 | 0.09 | 2.44 | 0.07 |
| Mo 8i | −0.56 | 0.01 | −0.58 | 0.00 | −0.39 | 0.01 | −0.43 | - | −0.37 | 0.01 | −0.41 | - |
| Fe 8j | 2.19 | 0.08 | 2.17 | 0.06 | 2.05 | 0.08 | 2.09 | 0.06 | 1.97 | 0.08 | 2.02 | 0.06 |
| Fe 8f | 1.76 | 0.05 | 1.79 | 0.04 | 1.64 | 0.06 | 1.70 | 0.05 | 1.78 | 0.07 | 1.82 | 0.05 |
| H | - | - | - | - | - | - | - | - | −0.02 | - | −0.02 | - |
| Total (µB/f.u.) | 16.82 | 3.57 | 17.23 | 5.36 | 13.08 | 3.65 | 13.98 | 5.37 | 13.45 | 3.62 | 14.35 | 5.39 |
| (µB/f.u) | 20.40 | 22.59 | 16.73 | 19.35 | 17.07 | 19.74 | ||||||
| (µB/f.u) | 13.58 [14] a 18.4 [12] a | 14.65 [14] a | ||||||||||
| (106 A/m) | 1.08 | 1.19 | 0.88 | 1.02 | 0.88 | 1.03 | ||||||
| (T) | 1.35 | 1.49 | 1.10 | 1.28 | 1.11 | 1.29 | ||||||
| SmFe11Mo | SmFe10Mo2 | SmFe10Mo2H | ||||
|---|---|---|---|---|---|---|
| LSDA | LSDA+U | LSDA | LSDA+U | LSDA | LSDA+U | |
| Sm 2a | 18.67 | 17.94 | 17.52 | 16.21 | 14.48 | 18.70 |
| Fe/Mo 8i | 0.08 | 0.05 | 0.08 | 0.08 | 0.02 | 0.08 |
| Fe 8j | 0.30 | 0.64 | 0.40 | 0.63 | 0.42 | 0.55 |
| Fe 8f | −0.11 | −0.15 | −0.05 | −0.05 | −0.05 | 0.01 |
| H 2b | - | - | - | - | 0.01 | 0.02 |
| Ku (meV/f.u.) | 19.76 | 21.12 | 19.22 | 18.85 | 16.04 | 21.28 |
| Ku (MJ/m3) | 9.0 | 9.2 | 8.7 | 8.6 | 7.2 | 9.5 |
| MJ/m3 | 2.4 a [14] | 3.2 a [14], 3.7 b [14] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benea, D.; Barna, E.; Pop, V.; Isnard, O. Theoretical Study of the Magnetic Properties of the SmFe12−xMox (x = 1, 2) and SmFe10Mo2H Compounds. Crystals 2024, 14, 598. https://doi.org/10.3390/cryst14070598
Benea D, Barna E, Pop V, Isnard O. Theoretical Study of the Magnetic Properties of the SmFe12−xMox (x = 1, 2) and SmFe10Mo2H Compounds. Crystals. 2024; 14(7):598. https://doi.org/10.3390/cryst14070598
Chicago/Turabian StyleBenea, Diana, Eduard Barna, Viorel Pop, and Olivier Isnard. 2024. "Theoretical Study of the Magnetic Properties of the SmFe12−xMox (x = 1, 2) and SmFe10Mo2H Compounds" Crystals 14, no. 7: 598. https://doi.org/10.3390/cryst14070598






