Pre-Critical and Giant Post-Freezing and Pre-Melting Effects for Dielectric Properties in a Binary Mixture of Limited Miscibility
Abstract
1. Introduction
2. Materials and Methods
3. Remarks on Continuous and Discontinuous Phase Transitions
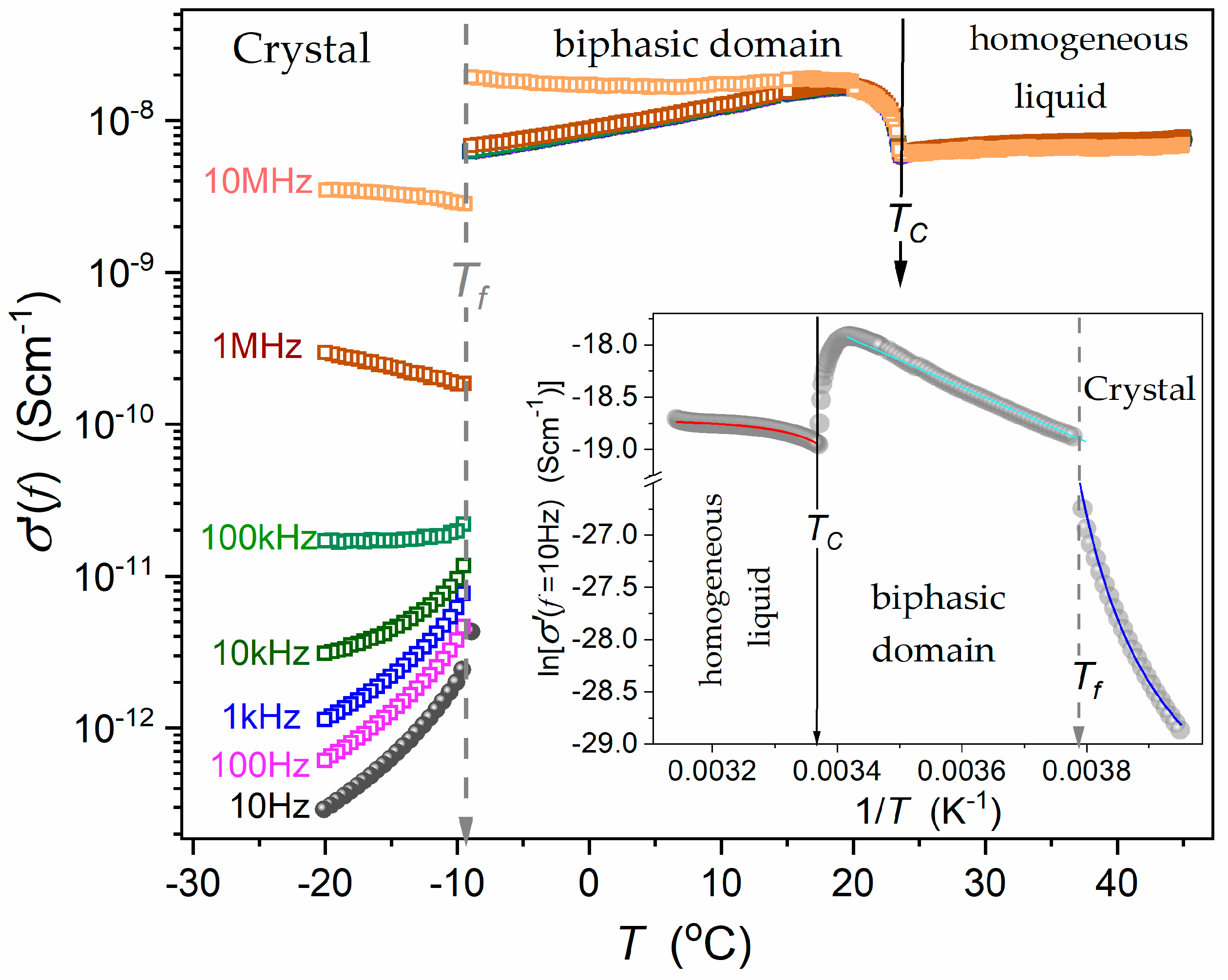
4. Results and Discussion
5. Conclusions
5.1. The Surrounding of the Critical Consolute Point
5.2. The Surrounding of the Liquid–Solid Discontinuous Transition
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanley, H.E. Introduction to Phase Transitions and Critical Phenomena; Oxford University Press: New York, NY, USA, 1971. [Google Scholar]
- Skripov, V.P.; Faizulin, M.Z. Crystal-Liquid-Gas Phase Transitions and Thermodynamic Similarity; Wiley-VCH: Berlin, Germany, 2006. [Google Scholar]
- Atkins, P.; de Paula, J. Atkin’s Physical Chemistry; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Khabibullaev, P.K.; Saidov, A.A. Phase Separation in Soft Matter Physics; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Arkenbout, G.F. Melt Crystallization Technology; Technomic Pub. Co.: Lancaster, UK, 1995. [Google Scholar]
- Rzoska, S.J.; Drozd-Rzoska, A. Criticality-related fundamental bases for new generations of gas–liquid, liquid–liquid, and liquid (LE) extraction technologies. Eur. Phys. J. E 2022, 45, 67. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F. Liquid Phase Extraction; Elsevier Science: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ehrenfest, P. Phasenumwandlungen im ueblichen und erweiterten Sinn, classifiziert nach dem entsprechenden Singularitaeten des thermodynamischen Potentiales. Verhandlingen der Koninklijke Akademie van Wetenschappen (Amsterdam). In Communications from the Physical Laboratory of the University of Leiden; Springer Nature: Berlin/Heidelberg, Germany, 1933; Volume 36, pp. 153–157. [Google Scholar]
- Jaeger, G. The Ehrenfest Classification of Phase Transitions: Introduction and Evolution. Arch. Hist. Exact Sci. 1998, 53, 51–81. [Google Scholar] [CrossRef]
- Wilson, K.G.; The Renormalization Group and Critical Phenomena. Nobel Prize Lecture. Available online: https://www.nobelprize.org/prizes/physics/1982/wilson/lecture/ (accessed on 4 June 2024).
- Anisimov, M.A. Critical Phenomena in Liquid and Liquid Crystals; Gordon and Breach Science Publishers: Philadelphia, PA, USA, 1991. [Google Scholar]
- Honig, J.; Spalek, J. A Primer to the Theory of Critical Phenomena; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Andrews, T. The Bakerian Lecture—On the continuity of the gaseous and liquid states of matter. Phil. Trans. R. Soc. 1869, 159, 575–590. [Google Scholar]
- Gibbs, J.W. Method of Geometrical Representation of the Thermodynamical Properties of Substances by Means of Surfaces. Trans. Conn. Acad. Sci. 1873, 2, 382–404. [Google Scholar]
- Van der Waals, J.D. Over de Continuiteit van den Gasen Vloeistoftoestand (On the Continuity of the Gas and Liquid State). Ph.D. Thesis, University of Leiden, Leiden, The Netherlands, 1873. [Google Scholar]
- Geiger, H.; Scheel, K. Handbuch der Physik Vol. 10; Springer: Berlin/Heidelberg, Germany, 1926. [Google Scholar]
- Mierzwa, M.; Paluch, M.; Rzoska, S.J.; Zioło, J. The liquid−glass and liquid−liquid transitions of TPP at elevated pressure. J. Phys. Chem. B 2008, 112, 10383–10385. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.W.; Largo, J.; Sciortino, F.; Starr, F.W. Hierarchies of networked phases induced by multiple liquid-liquid critical points. Proc. Natl. Acad. Sci. USA 2008, 105, 13711–13715. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H. Liquid–liquid transition and polyamorphism. J. Chem. Phys. 2020, 153, 130901. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, A.; Chakrabarti, D.; Sciortino, F. Topological nature of the liquid–liquid phase transition in tetrahedral liquids. Nat. Phys. 2022, 18, 1248–1253. [Google Scholar] [CrossRef]
- Kumar, A.; Krishnamurthy, H.R.; Gopal, E.S.R. Equilibrium critical phenomena in binary liquid mixtures. Phys. Rep. 1983, 98, 57–143. [Google Scholar] [CrossRef]
- Drozd-Rzoska, A.; Rzoska, S.J.; Kalabiński, J. The impact of pressure on low molecular weight near critical mixtures of limited miscibility. ACS Omega 2020, 5, 20141–20152. [Google Scholar] [CrossRef] [PubMed]
- Rzoska, S.J.; Drozd-Rzoska, A.; Kalabiński, J. Critical concentration in binary mixtures of limited miscibility. Fluid Phase Equilib. 2021, 540, 112979. [Google Scholar] [CrossRef]
- Mei, Q.S.; Lu, K. Melting and superheating of crystalline solids: From bulk to nanocrystals. Prog. Mater. Sci. 2007, 5, 1175–1262. [Google Scholar] [CrossRef]
- Kalabiński, J.; Drozd-Rzoska, A.; Rzoska, S.J. Giant premelting effects for solid-liquid discontinuous transition in nitrobenzene under compression. Crystals 2023, 13, 247. [Google Scholar] [CrossRef]
- Drozd-Rzoska, A.; Rzoska, S.J.; Łoś, J. Supercriticality, glassy dynamics, and the new insight into melting/freezing discontinuous transition in linseed oil. Biophysica 2024, 4, 34–57. [Google Scholar] [CrossRef]
- Kremer, F.; Schonhals, A. Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Landau, L. Zur Theorie der Phasenumwandlungen. Phys. Z. Sowjetunion 1937, 11, 26–47. [Google Scholar]
- Landau, L.; Lifshitz, E. Statistical Physics; Pergamon: London, UK, 1938. [Google Scholar]
- Łoś, J.; Drozd-Rzoska, A.; Rzoska, S.J.; Starzonek, S.; Czupryński, K.; Mukherjee, P. Near-continuous isotropic—Nematic transition in compressed rod-like liquid crystal based nanocolloid. J. Mol. Liq. 2023, 382, 121884. [Google Scholar] [CrossRef]
- Rzoska, S.J. Kerr effect and nonlinear dielectric effect on approaching the critical consolute point. Phys. Rev. 1993, 48, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Asta, M.; Laird, B.B. Solid-liquid interfacial premelting. Phys. Rev. Lett. 2013, 110, 096102. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Tuckerman, M.E.; Yu, T.Q.; Ee, W. Microscopic mechanisms of equilibrium melting of a solid. Science 2014, 346, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Pogatscher, S.; Leutenegger, D.; Schawe, J.E.K.; Uggowitzer, P.J.; Löffler, J.F. Solid—Solid phase transitions via melting in metals. Nat. Commun. 2016, 7, 11113. [Google Scholar] [CrossRef] [PubMed]
- Toledano, Ó.; Pancorbo, M.; Alvarellos, J.E.; Gálvez, Ó. Melting in two-dimensional systems: Characterizing continuous and first-order transition. Phys. Rev. B 2021, 103, 094107. [Google Scholar] [CrossRef]
- Kryuchkov, N.P.; Dmitryuk, N.A.; Li, W.; Ovcharov, P.V.; Han, Y.; Sapelkin, A.V.; Yurchenko, S.O. Mean-field model of melting in superheated crystals based on a single experimentally measurable order parameter. Sci. Rep. 2021, 11, 1796. [Google Scholar] [CrossRef] [PubMed]
- Pocheć, M.; Niu, H.; Ren, L.; Bai, S.; Orzechowski, K. Premelting phenomena in n-alcohols from nonanol to dodecanol. J. Phys. Chem. C 2020, 124, 21013–21017. [Google Scholar] [CrossRef]
- Pocheć, M.; Orzechowski, K.; Rutkowski, K. Indicators of premelting in 1-decanol and 1-nonanol studied by FTIR spectroscopy. Surf. Interfaces 2022, 28, 101676. [Google Scholar] [CrossRef]
- Lindemann, F.A. Über die berechning molekularer eigenfrequenzen. Phys. Z 1910, 11, 609–615. [Google Scholar]
- Lawson, A.C. Physics of the Lindemann rule. Phil. Mag. 2009, 89, 1757–1770. [Google Scholar] [CrossRef]
- Lipowsky, R. Critical surface phenomena at first-order bulk transitions. Phys. Rev. Lett. 1982, 49, 1575–1578. [Google Scholar] [CrossRef]
- Lipowsky, R. Surface critical phenomena at first-order phase transitions. Ferroelectrics 1987, 73, 69–81. [Google Scholar] [CrossRef]
- Chełkowski, A. Dielectric Physics; PWN-Elsevier: Warsaw, Poland, 1990. [Google Scholar]
- von Hippel, A. Dielectrics and Waves; Artech House: New York, NY, USA, 1954. [Google Scholar]
- Piekara, A. The Dielectric Constant and Electric Polarization of Mixtures in the Neighborhood of the Critical Point. Phys. Rev. 1932, 42, 448–449. [Google Scholar] [CrossRef]
- Semenchenko, V.K.; Azimov, M. Zh. Fiz. Khim; MAIK Nauka/Interperiodica Publishers: Moscow, Russia, 1955; Volume 80–81, pp. 1343–1345. [Google Scholar]
- Semenchenko, V.K.; Azimov, M. Dielectric constants of binary systems in the critical region. Zh. Fiz. Khim. 1956, 30, 2228–2235. [Google Scholar]
- Semenchenko, V.K.; Azimov, M. Second-order phase transitions and critical phenomena. VII. Dielectric permeability of the nitrobenzene-hexane system in the critical region. Zh. Fiz. Khim. 1956, 30, 1821–1829. (In Russian) [Google Scholar]
- Shakhparov, M.I. The dependence of the dielectric constant on temperature in the critical region. Vestn. Mosk. Univ. Ser. Khim. 1961, I6, 25–30. [Google Scholar]
- Semenchenko, V.K. Thermodynamics of critical and hyper-critical phenomena in solutions. Zh. Fiz. Khim. 1961, 35, 1210–1215. [Google Scholar]
- Zamkov, V.A. The measurement of dielectric constants in the vicinity of the critical point. Zh. Fiz. Khim. 1962, 36, 1060–1061. [Google Scholar]
- Semenchenko, V.K.; Akhrarov, S. Dielectric constant of binary systems in the critical region. Vopr. Sovrem. Fiz. i. Mat. Akad. Nauk Uz. SSR 1962, 9–14. (In Russian) [Google Scholar]
- Arkhangeiskii, K.V. Fluctuations and thermodynamic stability of systems in the critical and post-critical regions. Fiz. Tverd. Tela. 1964, 27–34. (In Russian) [Google Scholar]
- Arkhangeskii, K.V.; Semenchenko, V.K. Methods and results of measurements of the dielectric parameters of binary liquid mixtures near the critical solution temperature. Zh. Fiz. Khim 1967, 41, 1303–1309. (In Russian) [Google Scholar]
- Givon, M.; Pelah, I.; Efron, U. Behaviour of the dielectric constant of a binary liquid mixture near the critical point. Phys. Lett. A 1974, 48, 121–122. [Google Scholar] [CrossRef]
- Hollecker, M.; Goulon, J.; Thiebaut, J.-M.; Rivail, J.-L. Dielectric behaviour of a molecular liquid mixture in the one-phase precritical region. Chem. Phys. 1975, 11, 99–103. [Google Scholar] [CrossRef]
- Ziejewska, Z.; Piotrowska-Szczepaniak, J.; Zioło, J. Dielectric permittivity of nitrobenzene-hexane solutions in two-phase region. Acta Phys. Pol. A 1979, 56, 347–349. [Google Scholar]
- Hollecker, M.; Goulon, J.; Brondeau, J.; Afsar, M.N.; Chantry, G.W. High frequency dielectric behaviour of critical binary solutions of benzonitrile-isooctane. Chem. Phys. 1977, 26, 267–277. [Google Scholar] [CrossRef]
- Thoen, J.; Kindt, R.; Van Dael, W. Measurements of the temperature and frequency dependence of the dielectric constant near the consolute point of benzonitrile-isooctane. Phys. Lett. A 1980, 76, 445–448. [Google Scholar] [CrossRef]
- Thijsse, B.J. The dielectric constant of SF6 near the critical point. J. Chem. Phys. 1981, 74, 4678–4692. [Google Scholar] [CrossRef]
- Thoen, J.; Kindt, R.; Van Dael, W. The dielectric constant anomaly of nitroethane-cyclohexane near the critical solution point. Phys. Lett. A 1981, 87, 73. [Google Scholar] [CrossRef]
- Jacobs, D.T.; Greer, S.C. Dielectric-constant anomaly near the critical solution point in polystyrene + cyclohexane. Phys. Rev. A 1981, 24, 2075–2083. [Google Scholar] [CrossRef]
- Pestak, M.W.; Chan, M.H.W. Dielectric constant anomaly of CO near its liquid-vapor critical point. Phys. Rev. Lett. 1981, 46, 943–946. [Google Scholar] [CrossRef]
- Kaatze, U.; Woermann, D. Dielectrical relaxation measurements in binary liquid mixtures with an upper critical point. Ber. Bunsen. Phys. Chem. 1982, 86, 81–87. [Google Scholar] [CrossRef]
- Balakrishnan, J.; Gunasekaran, M.K.; Gopal, E.S.R. Low-frequency dielectric constant measurements in the critical polar + non-polar binary liquid system: Methanol + n-heptane. Chem. Phys. Lett. 1982, 88, 305–308. [Google Scholar] [CrossRef]
- Shetty, C.; Gunasekaran, M.K.; Vani, V.; Gopal, E.S.R. Electrical resistance and dielectric constant anomaly in the critical liquid mixture methanol + cyclohexane. Pramana-J Phys 1983, 21, 71–78. [Google Scholar] [CrossRef]
- Thoen, J.; Kindt, R.; van Dael, W.; Merabet, M.; Bose, T.K. Low-frequency dielectric dispersion and electric conductivity near the consolute point in some binary liquid mixtures. Phys. A 1989, 156, 92–113. [Google Scholar] [CrossRef]
- Early, M.D. Dielectric constant measurements near the critical point of cyclohexane-aniline. J. Chem. Phys. 1992, 96, 641–647. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Lahiri, D.L.; Ghosh, R. Electrical conductivity anomaly in binary liquid mixtures near the critical point. Jpn. J. Appl. Phys. 1992, 31, 2151–2156. [Google Scholar] [CrossRef]
- Orzechowski, K. Electrical properties of an ethanol–dodecane mixture near the upper critical solution point. J. Chem. Soc. Faraday Trans. 1994, 90, 2757–2763. [Google Scholar] [CrossRef]
- Hamelin, J.; Bose, T.K.; Thoen, J. Critical behavior of the dielectric constant in the triethylamine-water binary liquid mixture: Evidence of an intrinsic effect. Phys. Rev. E 1996, 53, 779–784. [Google Scholar] [CrossRef]
- Orzechowski, K. Dielectric properties of methanol+hexane critical mixtures without and with ionic additives. J. Mol. Liq. 1997, 73–74, 291. [Google Scholar] [CrossRef]
- Rzoska, S.J.; Urbanowicz, P.; Drozd-Rzoska, A.; Paluch, M.; Habdas, P. Pressure behaviour of dielectric permittivity on approaching the critical consolute point. Europhys. Lett. 1999, 45, 334–340. [Google Scholar] [CrossRef]
- Rzoska, S.J.; Drozd-Rzoska, A.; Zioło, J.; Habdas, P.; Jadżyn, J. Critical anomaly of dielectric permittivity for the temperature and pressure paths on approaching the critical consolute point. Phys. Rev. E 2001, 64, 061104. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Rzoska, S.J.; Drozd-Rzoska, A.; Jadzyn, J. Dielectric permittivity and electric conductivity studies in the one and in the two-phase region of nitrobenzene– dodecane critical point. J. Chem. Phys. 2003, 118, 9357–9363. [Google Scholar] [CrossRef]
- Leys, J.; Losada-Pérez, P.; Cordoyiannis, G.; Cerdeiriña, C.A.; Glorieux, C.; Thoen, J. Temperature, concentration, and frequency dependence of the dielectric constant near the critical point of the binary liquid mixture nitrobenzene-tetradecane. J. Chem. Phys. 2010, 132, 014508. [Google Scholar] [CrossRef] [PubMed]
- Losada-Perez, P.; Perez-Sanchez, G.; Cerdeirina, C.A.; Thoen, J. Dielectric constant of fluids and fluid mixtures at criticality. Phys. Rev. E 2010, 81, 041121. [Google Scholar] [CrossRef] [PubMed]
- Orzechowski, K.; Kosmowska, M.; Adamczyk, M. Electric Permittivity Anomaly Close to the Critical Consolute Point of a Nitrobenzene + Octane Liquid Mixture. J. Phys. Chem. B 2012, 116, 2492–2497. [Google Scholar] [CrossRef] [PubMed]
- Drozd-Rzoska, A.; Rzoska, S.J. The super- and subcritical effects for dielectric constant in diethyl ether. J. Chem. Phys. 2016, 144, 224506. [Google Scholar] [CrossRef] [PubMed]
- Orzechowski, K.; Kosmowska, M. Dielectric Properties of Critical Conducting Mixtures. In Nonlinear Dielectric Phenomena in Complex Liquids. NATO Science Series II: Mathematics, Physics and Chemistry; Rzoska, S.J., Zhelezny, V.P., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 157. [Google Scholar]
- Losada-Pérez, P. Liquid-liquid criticality in the dielectric constant and refractive index: A perspective. Eur. Phys. J. E 2019, 42, 110. [Google Scholar] [CrossRef] [PubMed]
- Drozd-Rzoska, A.; Rzoska, S.J. High-pressure behavior of dielectric constant in a binary critical mixture. Phys. Rev. E 2020, 102, 042610. [Google Scholar] [CrossRef]
- Fisher, H.J. Faraday’s Experimental Researches in Electricity: Guide to a First Reading; Green Lion Press: London, UK, 2001. [Google Scholar]
- Goulon, S.; Greffe, J.-L.; Oxtoby, D.W. Droplet model for the analysis of the dielectric properties of critical binary mixtures. J. Chem. Phys. 1979, 70, 4742–4750. [Google Scholar] [CrossRef]
- Sengers, J.V.; Bedeaux, D.; Mazur, P.; Greer, S.C. Behavior of the dielectric constant of fluids near a critical point. Phys. A 1980, 104, 573–594. [Google Scholar] [CrossRef]
- Aharony, A.; Fisher, M.E. Nonlinear scaling fields and corrections to scaling near criticality. Phys. Rev. B 1983, 27, 4394–4400. [Google Scholar] [CrossRef]
- Mistura, L. Behaviour of the dielectric constant near a critical point in fluid systems. J. Chem. Phys. 1974, 59, 4563. [Google Scholar] [CrossRef]
- Cailletet, L.; Mathias, E. Recherches sur les densités de gaz liquéfies et de leurs vapeurs saturées. Compt. Acad. Sci. 1886, 102, 1202–1207. [Google Scholar]
- Reif-Acherman, S. The history of the rectilinear diameter law. Quim. Nova 2010, 33, 2003–2010. [Google Scholar] [CrossRef][Green Version]
- Rowlinson, J.S. Physics of liquds: Are diameters rectilinear? Nature 1986, 319, 362. [Google Scholar] [CrossRef]
- Anisimov, M.A.; Wang, J. The nature of asymmetry in fluid criticality. Phys. Rev. Lett. 2006, 97, 025703. [Google Scholar] [CrossRef]
- Jüngst, S.; Knuth, B.; Hensel, F. Observation of singular diameters in the coexistence curves of metals. Phys. Rev. Lett. 1985, 55, 2160–2163. [Google Scholar] [CrossRef] [PubMed]
- Drozd-Rzoska, A.; Łoś, J.; Rzoska, S.J. The dominance of pretransitional effects in the liquid crystal based nanocolloids: Nematogenic MBBA with the transverse permanent dipole moment and BaTiO3 nanoparticles. Nanomaterials 2024, 14, 655. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Chirra, S.K.; Iqbal, A. Correction of the Electrode Polarization and Ionic Conductance Effects in the Measurements of Permittivity and Loss of the Dielectric Material. Available online: https://ssrn.com/abstract=4861077 (accessed on 24 June 2024).

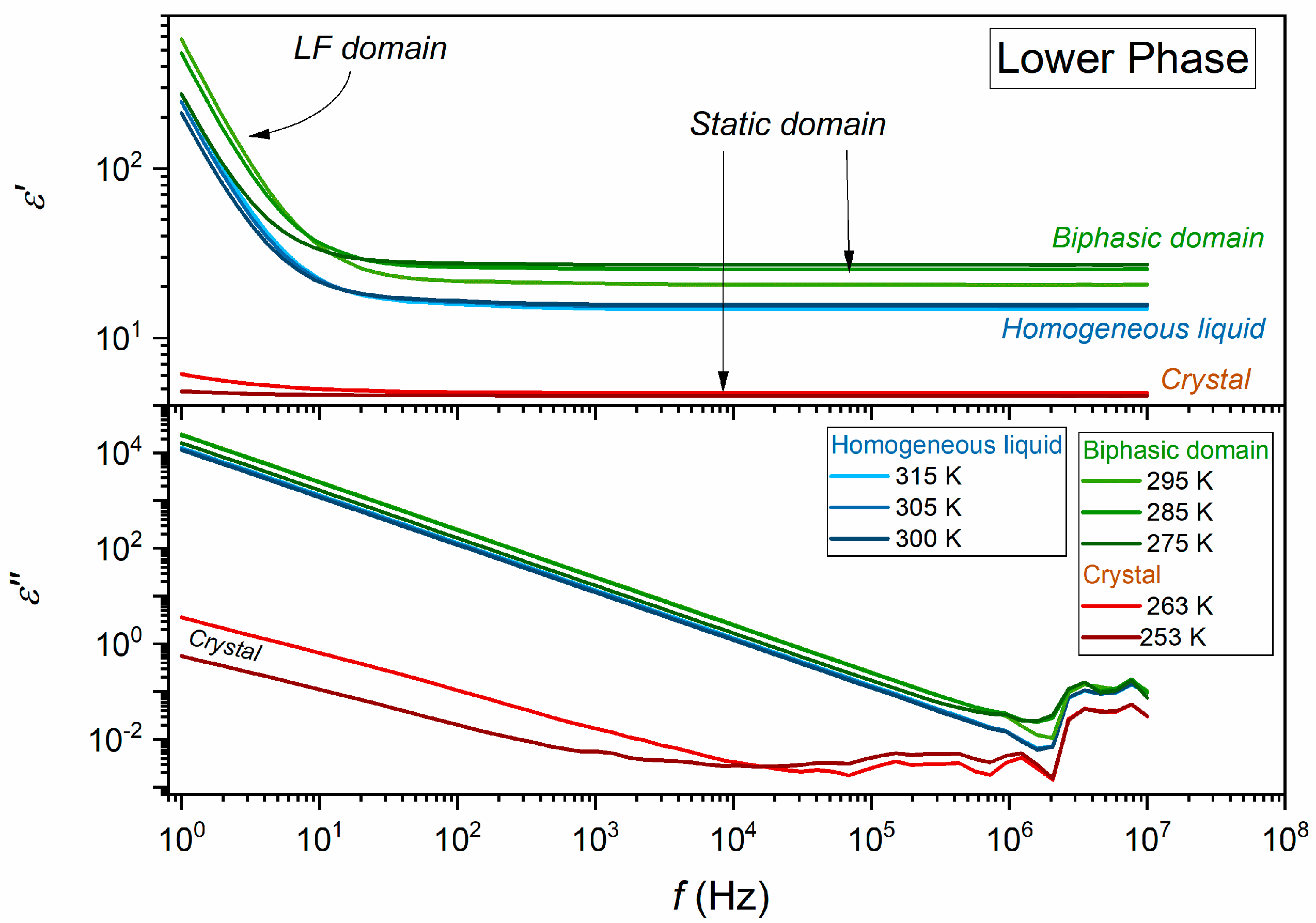
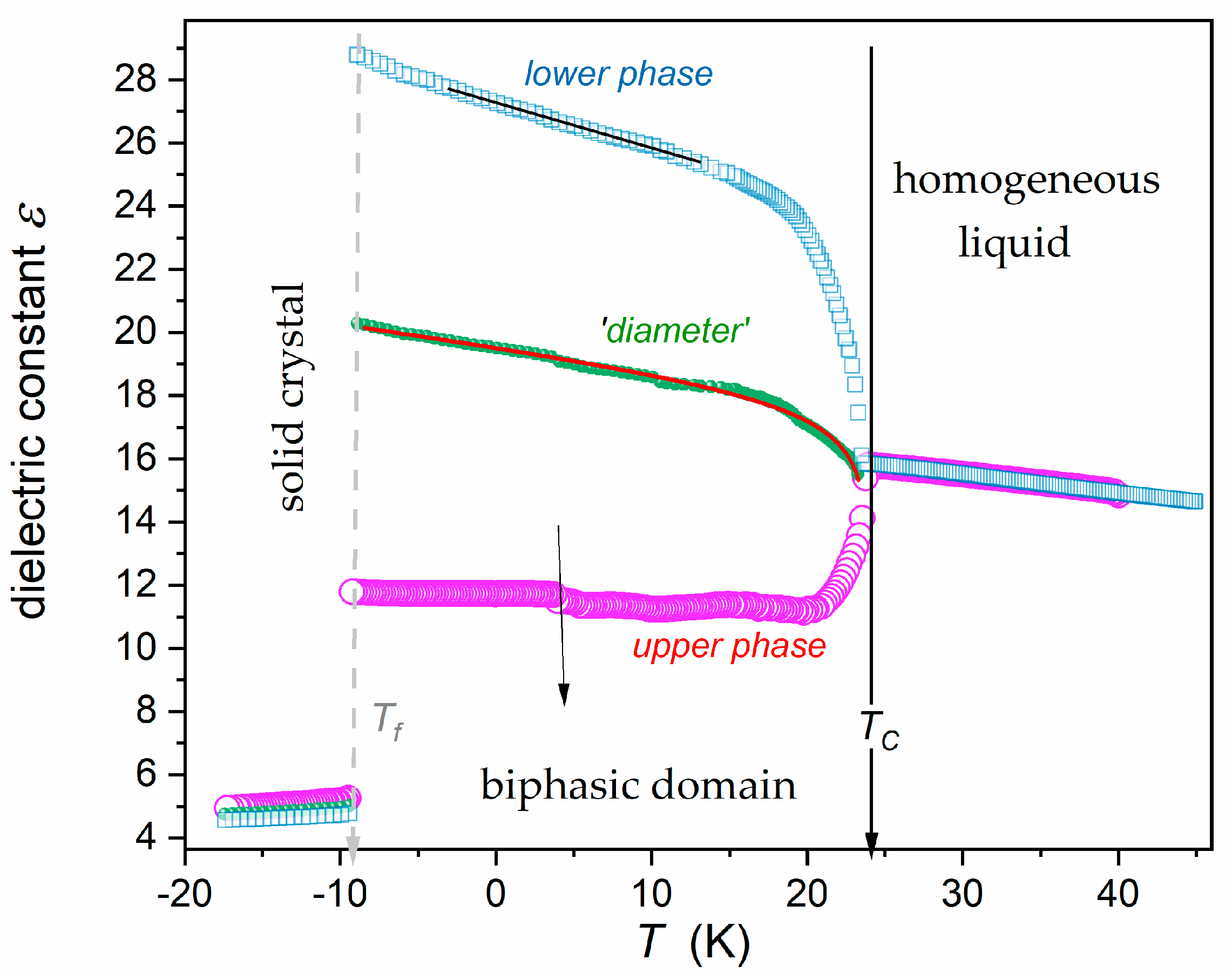
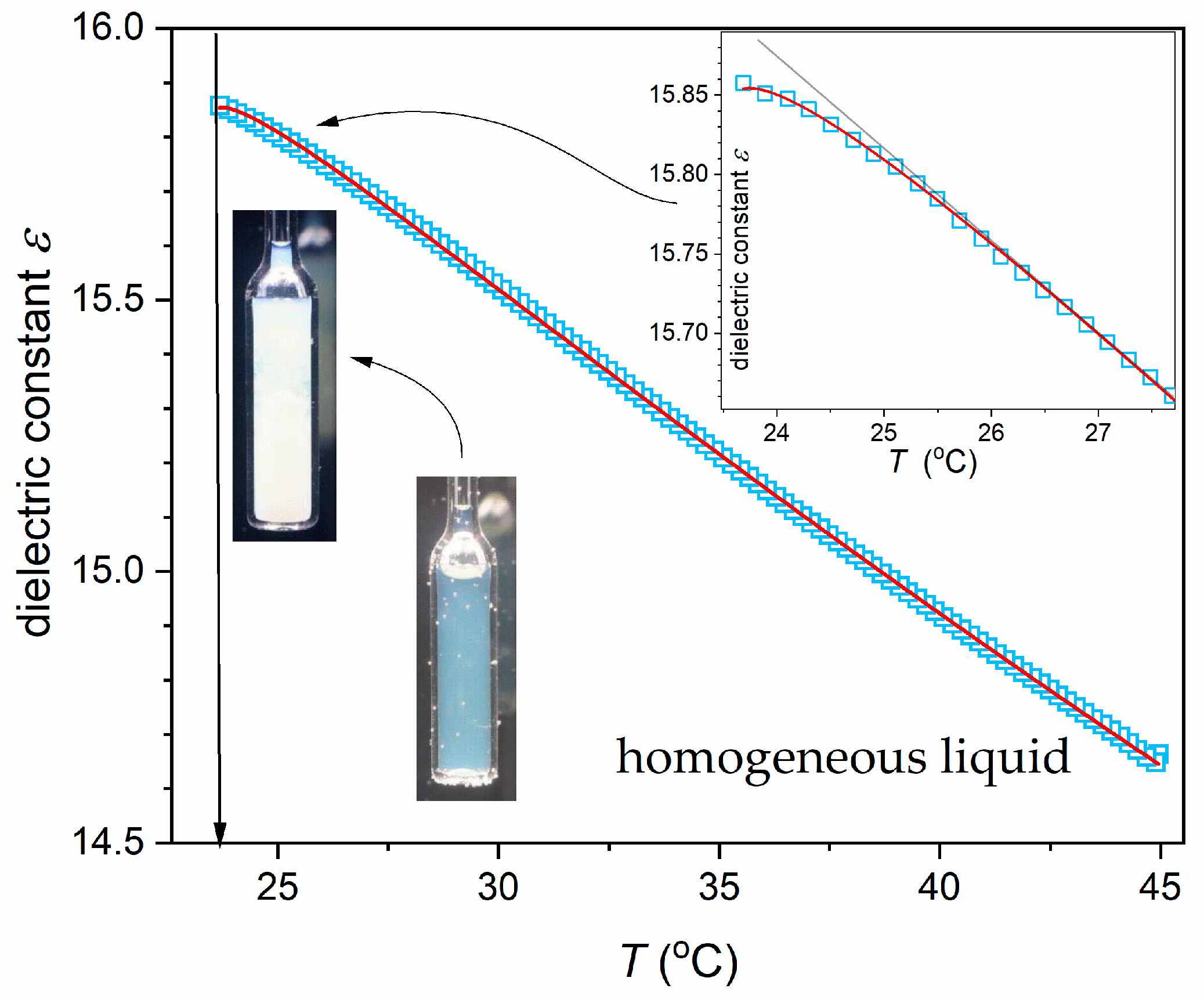

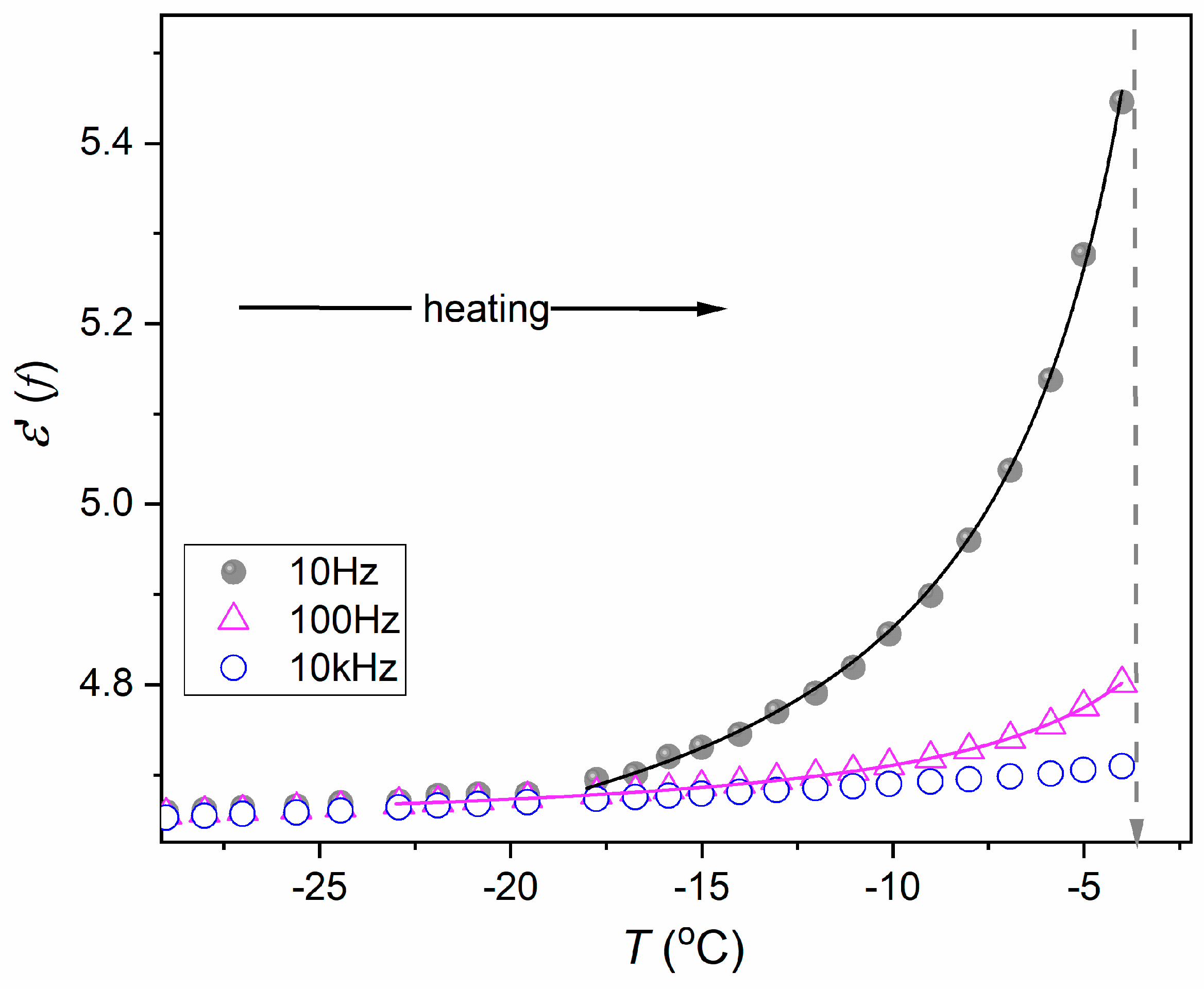
| Phase | Critical Point Parameters | Exponent | Amplitudes | |||
|---|---|---|---|---|---|---|
| (°C) | A | B | C | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalabiński, J.; Drozd-Rzoska, A.; Starzonek, S.; Rzoska, S.J. Pre-Critical and Giant Post-Freezing and Pre-Melting Effects for Dielectric Properties in a Binary Mixture of Limited Miscibility. Crystals 2024, 14, 612. https://doi.org/10.3390/cryst14070612
Kalabiński J, Drozd-Rzoska A, Starzonek S, Rzoska SJ. Pre-Critical and Giant Post-Freezing and Pre-Melting Effects for Dielectric Properties in a Binary Mixture of Limited Miscibility. Crystals. 2024; 14(7):612. https://doi.org/10.3390/cryst14070612
Chicago/Turabian StyleKalabiński, Jakub, Aleksandra Drozd-Rzoska, Szymon Starzonek, and Sylwester J. Rzoska. 2024. "Pre-Critical and Giant Post-Freezing and Pre-Melting Effects for Dielectric Properties in a Binary Mixture of Limited Miscibility" Crystals 14, no. 7: 612. https://doi.org/10.3390/cryst14070612
APA StyleKalabiński, J., Drozd-Rzoska, A., Starzonek, S., & Rzoska, S. J. (2024). Pre-Critical and Giant Post-Freezing and Pre-Melting Effects for Dielectric Properties in a Binary Mixture of Limited Miscibility. Crystals, 14(7), 612. https://doi.org/10.3390/cryst14070612







