Advancements in ZnO-Based Photocatalysts for Water Treatment: A Comprehensive Review
Abstract
1. Introduction
2. Generalities of ZnO
3. Synthesis Methods for ZnO Nanostructures for Photocatalysis
3.1. Hydrothermal Method
3.2. Solvothermal Method
3.3. Chemical Vapor Deposition (CVD)
4. ZnO as a Photocatalyst
4.1. Impurity Presence Effect
4.2. Influence of Specific Surface Area
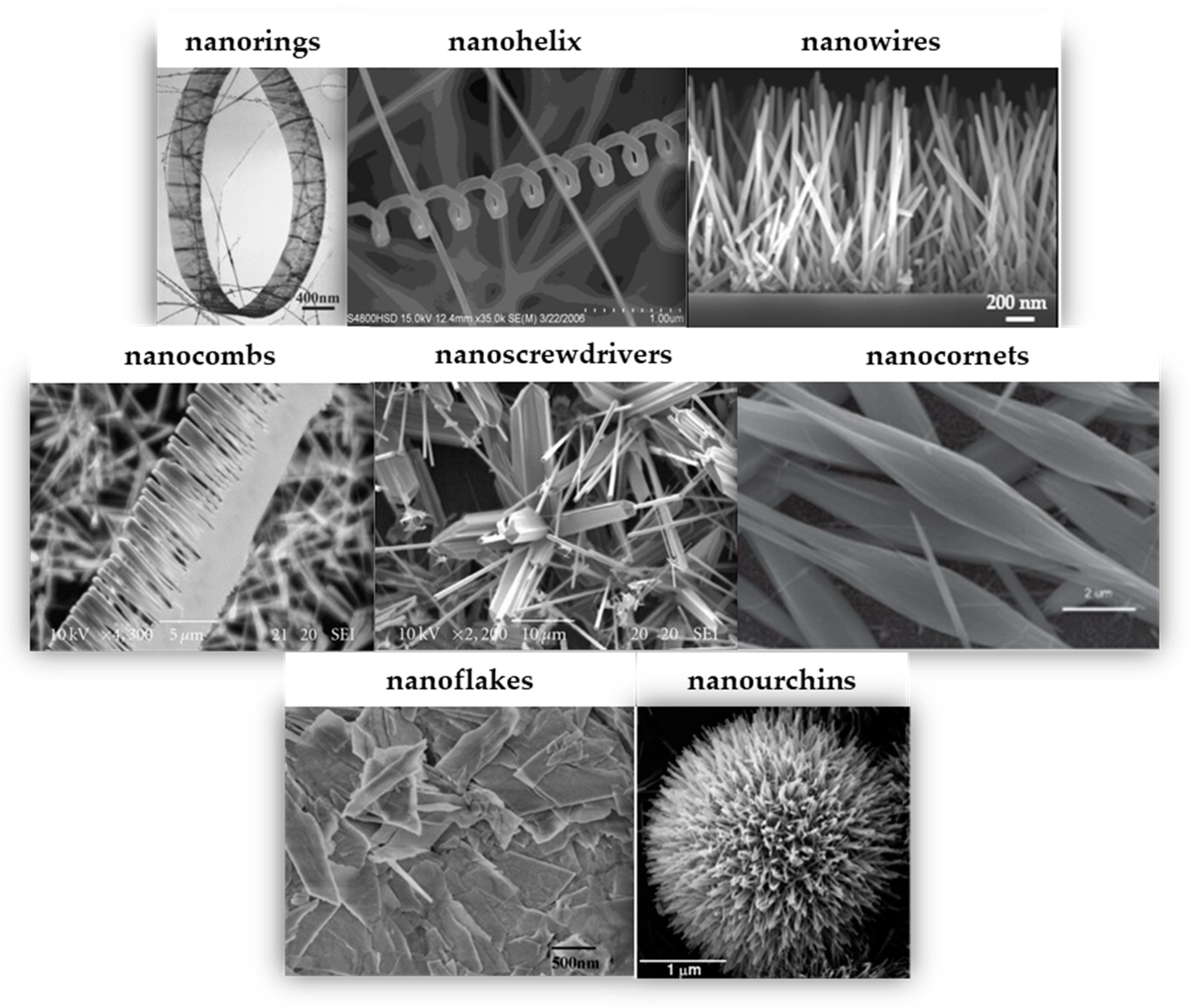
4.3. Influence of Morphology
4.4. Impact of Structural Defects
5. Enhancing the Photocatalytic Efficiency of ZnO
5.1. Doping
5.1.1. Non-Metallic Doping
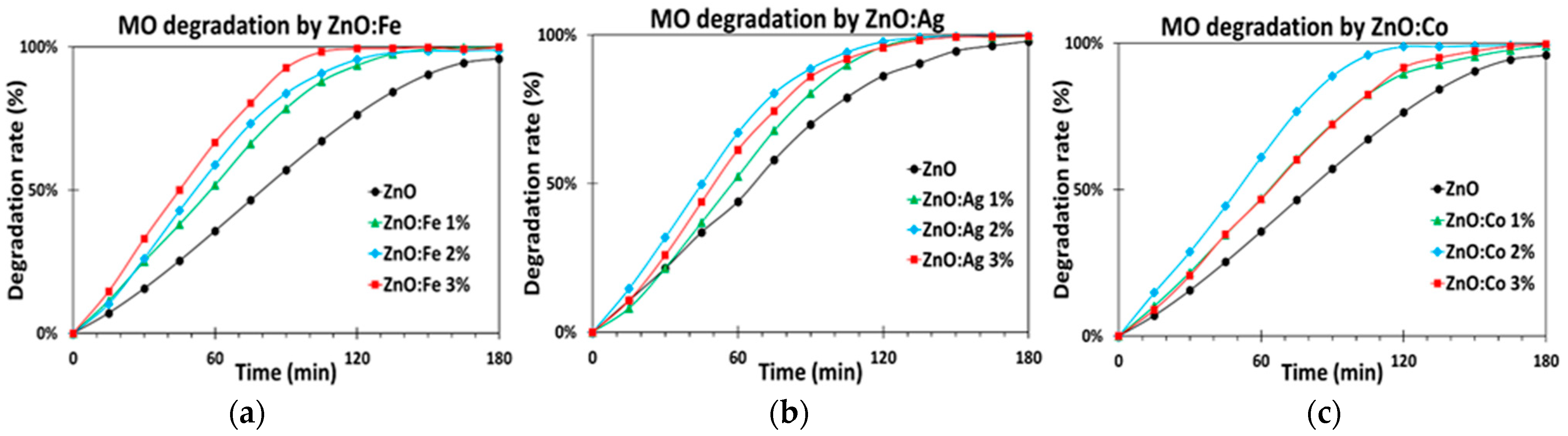
5.1.2. Transition Metal Doping
5.1.3. Rare Earth Metal Doping
5.2. Co-Doping of ZnO
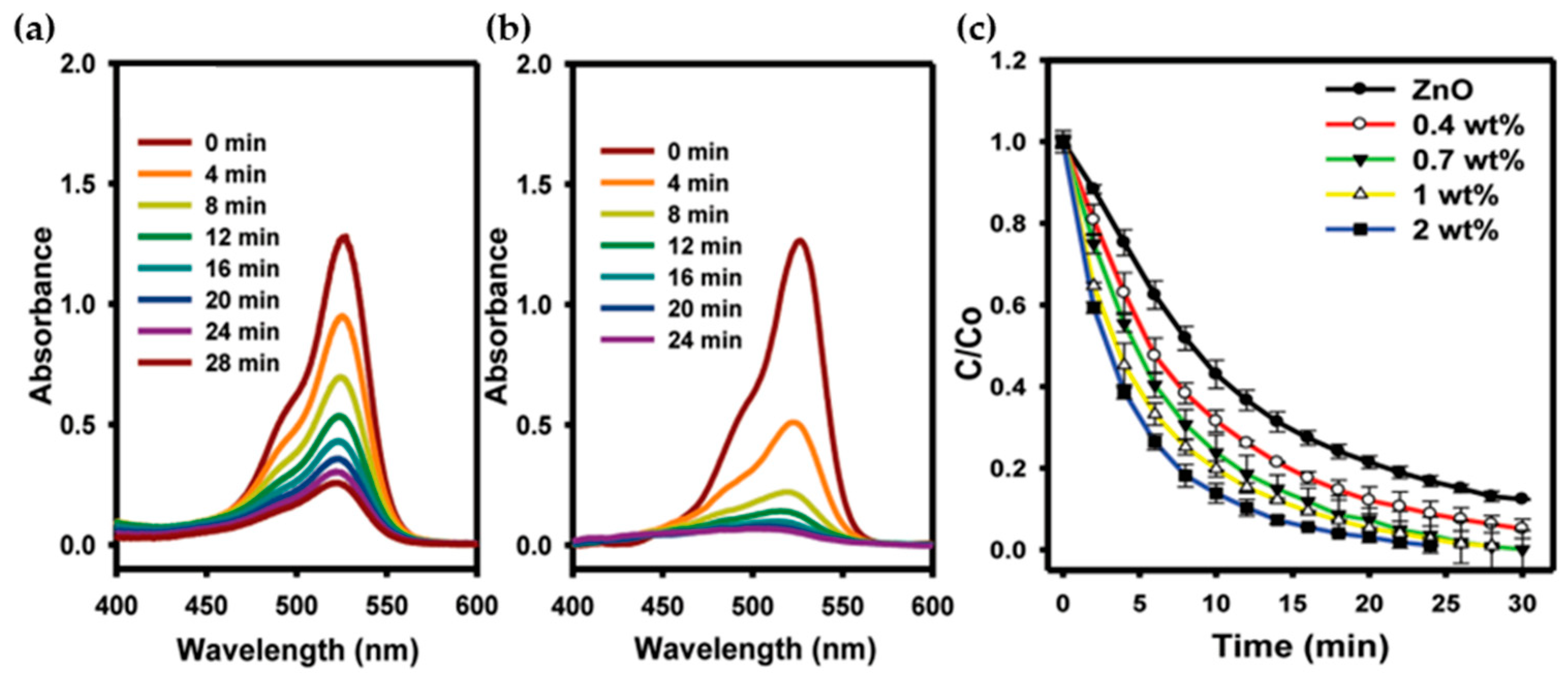
5.3. Noble Metal Deposition on ZnO
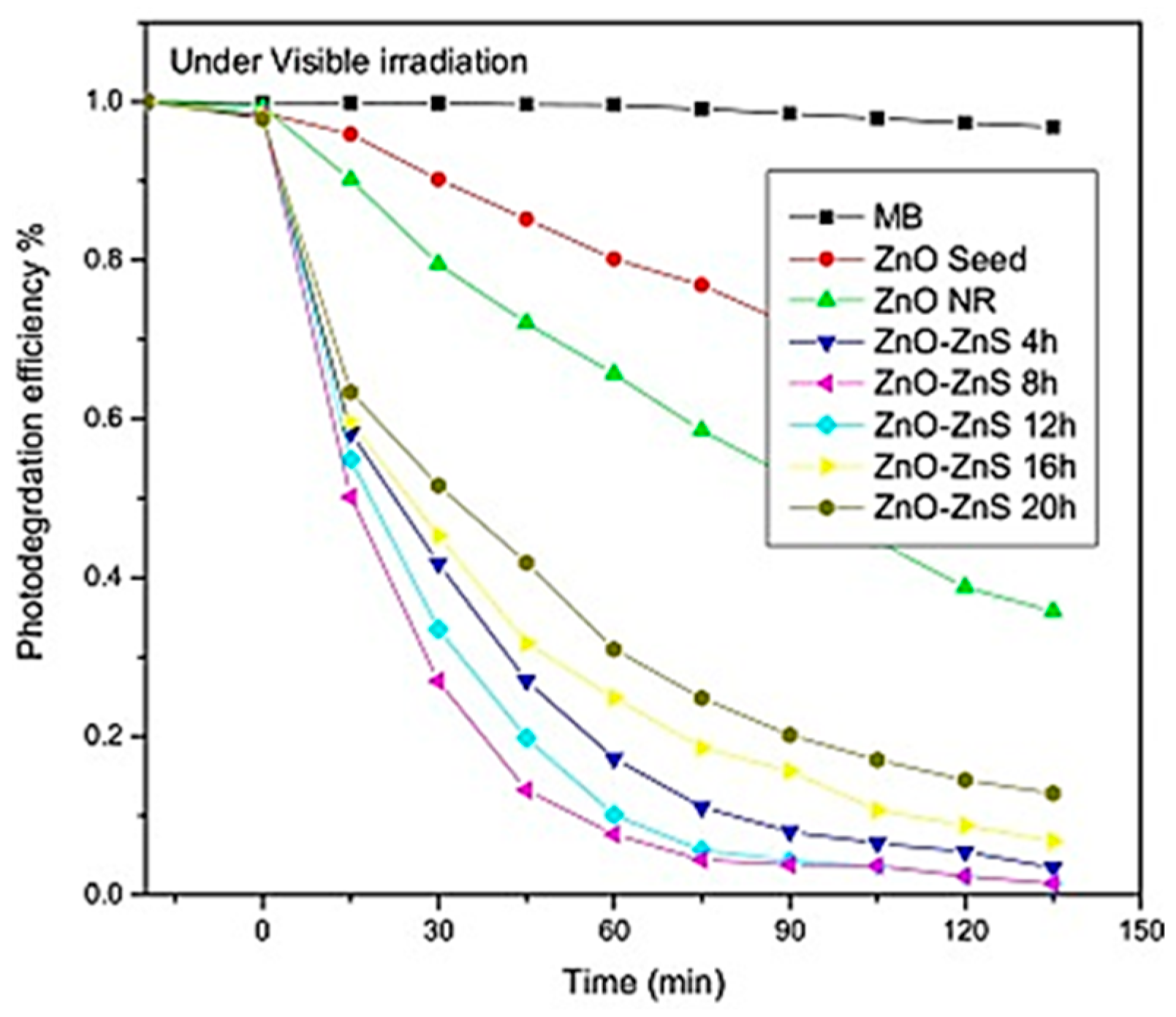
5.4. Semiconductor Composite
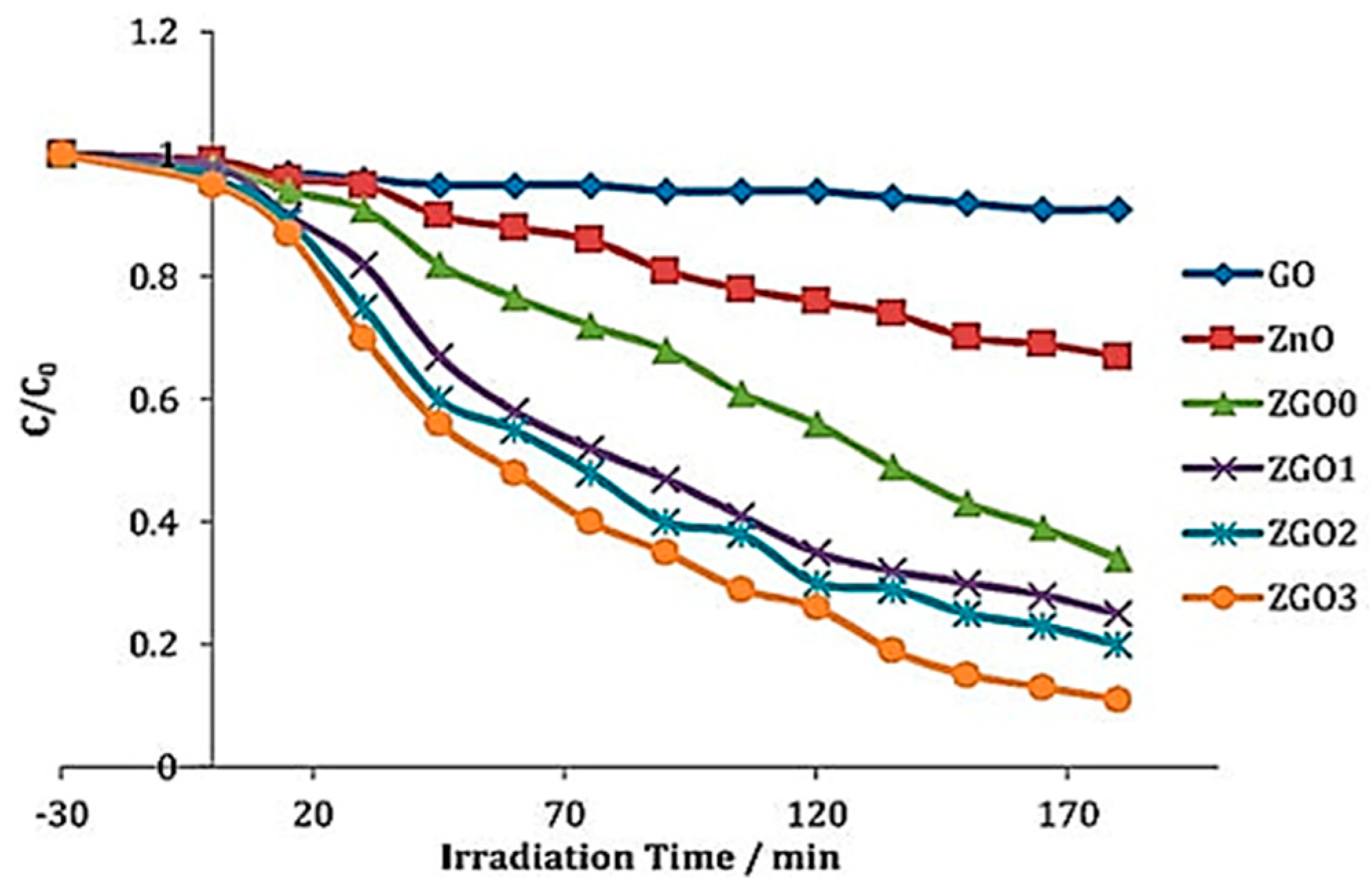
5.5. Coupling with Carbon-Based Materials
6. Role of ZnO in Water Treatment
6.1. Removal of Organic Pollutants
6.2. Disinfection of Water
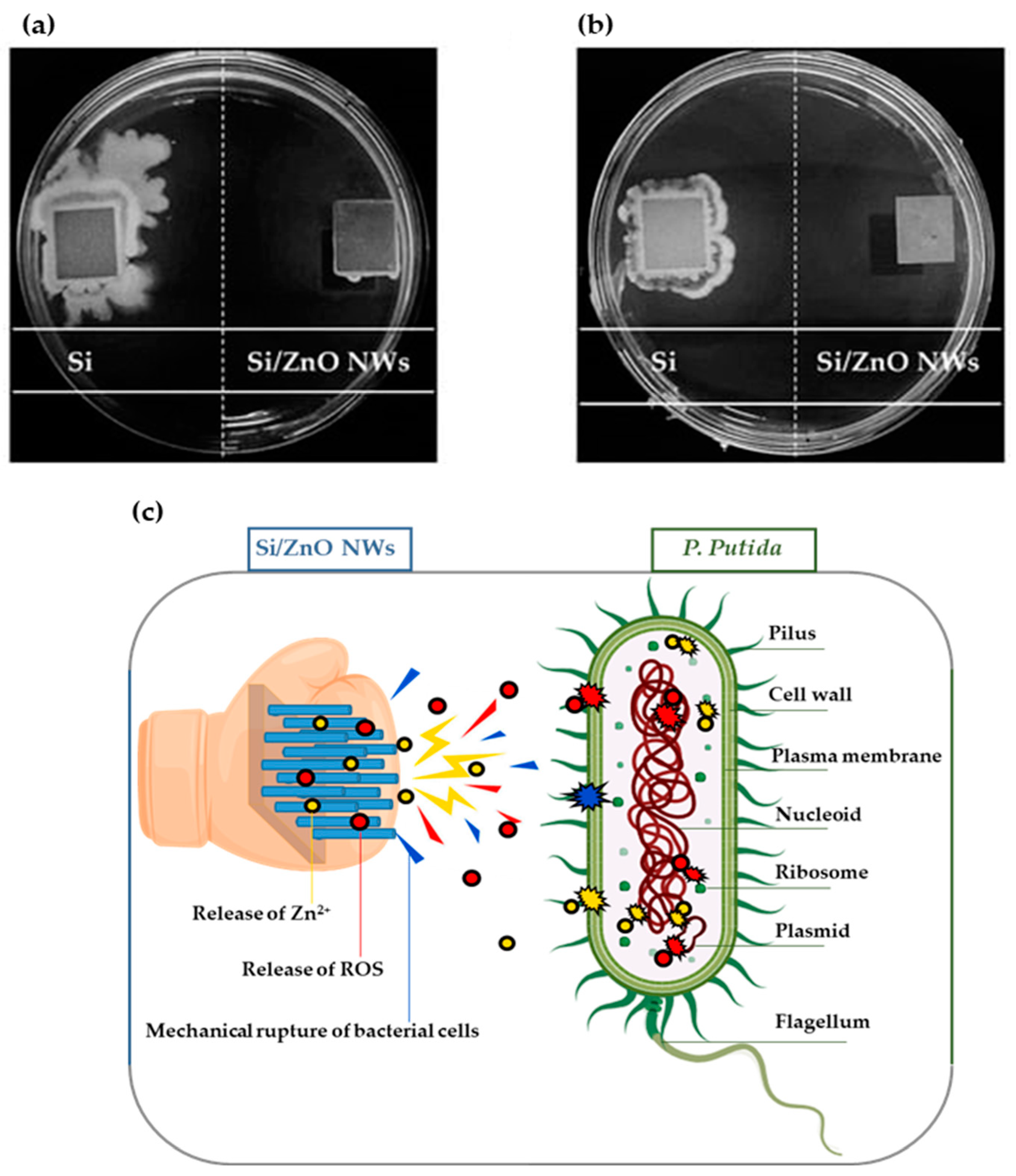
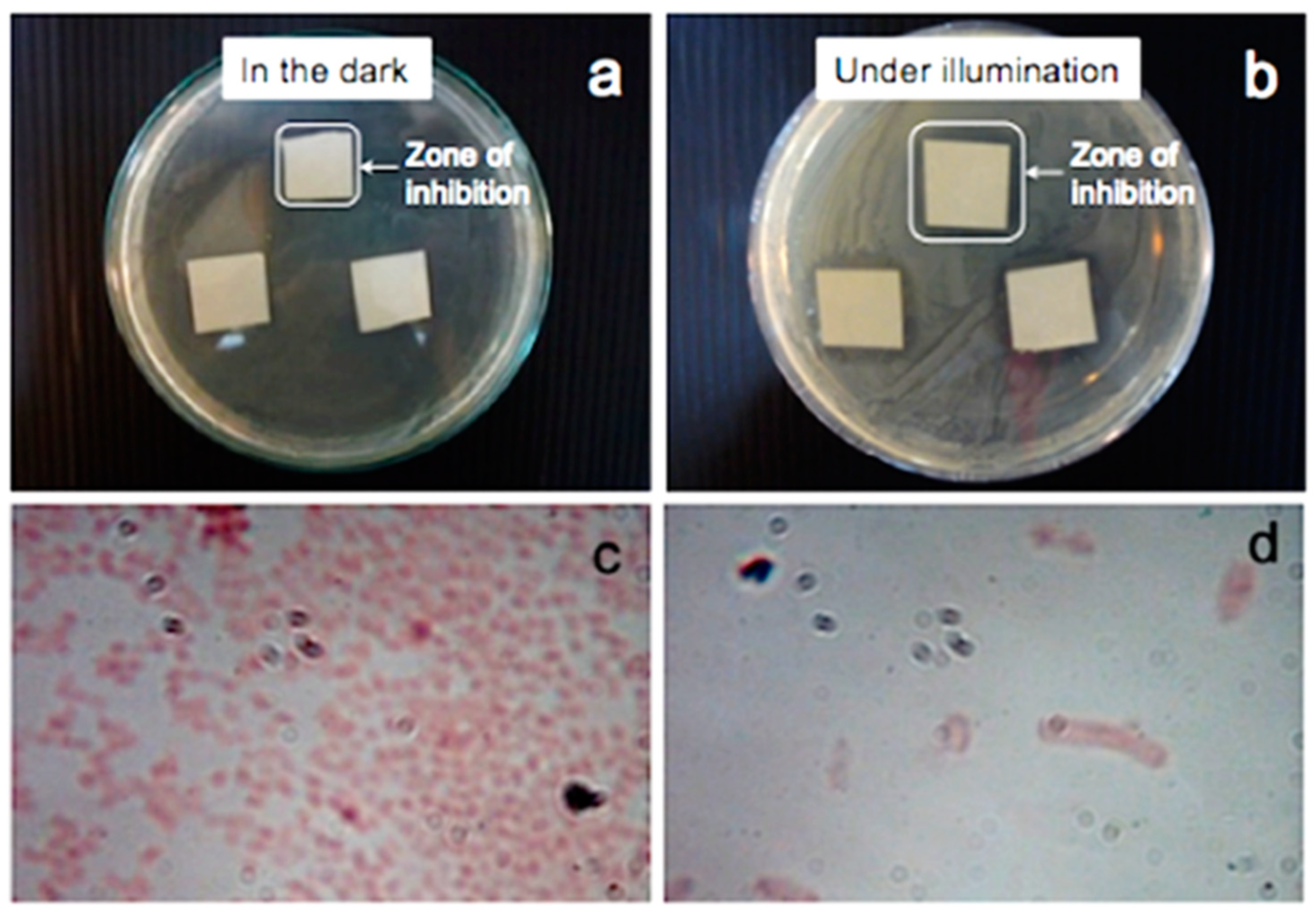
- Release of Reactive Oxygen Species (ROS): The antimicrobial activity of ZnO nanoparticles involves the release of oxygen species from the surface of ZnO, which cause fatal damage to microorganisms. ROS are known to cause oxidative stress by damaging DNA, cell membranes, and cellular proteins. The rupture of the cell wall is due to the surface activity of ZnO, which causes the decomposition of the cell wall and subsequently the cell membrane, the leakage of cell contents, and, eventually, cell death [266,267].
- Release of Zn2+ Ions: Another possible mechanism of ZnO antibacterial activity is the release of Zn2+ ions, which can damage the cell membrane and penetrate the intracellular contents. Studies have suggested that the toxicity of nano ZnO against Escherichia coli and Saccharomyces cerevisiae could result from the solubility of the Zn2+ ions in the microorganism-containing medium [268].
- Direct Contact of Nanoparticles with Cell Membrane: Cell damage does not necessarily result from the entry of the metal oxide particles into the cell. More importantly, the contact between the bacterial cell and the particle causes changes in the microenvironment within the contact area of the organism and particle. This can lead to increased membrane permeability and the subsequent cellular internalization of the nanoparticles [269,270].
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fuzil, N.S.; Othman, N.H.; Alias, N.H.; Marpani, F.; Othman, M.H.D.; Ismail, A.F.; Lau, W.J.; Li, K.; Kusworo, T.D.; Ichinose, I.; et al. A Review on Photothermal Material and Its Usage in the Development of Photothermal Membrane for Sustainable Clean Water Production. Desalination 2021, 517, 115259. [Google Scholar] [CrossRef]
- Tang, W.; Pei, Y.; Zheng, H.; Zhao, Y.; Shu, L.; Zhang, H. Twenty Years of China’s Water Pollution Control: Experiences and Challenges. Chemosphere 2022, 295, 133875. [Google Scholar] [CrossRef]
- Aal, N.A.; Al-Hazmi, F.; Al-Ghamdi, A.A.; Al-Ghamdi, A.A.; El-Tantawy, F.; Yakuphanoglu, F. Novel Rapid Synthesis of Zinc Oxide Nanotubes via Hydrothermal Technique and Antibacterial Properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 871–877. [Google Scholar] [CrossRef]
- Coelho, E.F.; Santos, D.L.; de Lima, L.W.F.; Castricini, A.; Barros, D.L.; Filgueiras, R.; da Cunha, F.F. Water Regimes on Soil Covered with Plastic Film Mulch and Relationships with Soil Water Availability, Yield, and Water Use Efficiency of Papaya Trees. Agric. Water Manag. 2022, 269, 107709. [Google Scholar] [CrossRef]
- Uma Maheswari, B.; Sivakumar, V.M.; Thirumarimurugan, M. Sequester Adsorption of Cr(VI) and Pb(II) Ions from Aqueous Solution by Green Synthesized Nanocomposite (OB/ZnO): Characterization, Kinetics, Isotherm and Mechanism. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100680. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of Hazardous Organics from Water Using Metal-Organic Frameworks (MOFs): Plausible Mechanisms for Selective Adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L.; Qin, X.; Zhao, L. Adsorption Characteristics and the Removal Mechanism of Two Novel Fe-Zn Composite Modified Biochar for Cd(II) in Water. Bioresour. Technol. 2021, 333, 125078. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Wang, Y.; Wang, Z. ZnO/Graphene-Oxide Nanocomposite with Remarkably Enhanced Visible-Light-Driven Photocatalytic Performance. J. Colloid Interface Sci. 2012, 377, 114–121. [Google Scholar] [CrossRef]
- Kumar, N.; Chauhan, N.S.; Mittal, A.; Sharma, S. TiO2 and Its Composites as Promising Biomaterials: A Review. Biometals 2018, 31, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Yadav, S.; Marí, B.; Mittal, A.; Jindal, J. Synthesis and Charcterization of Coupled ZnO/SnO2 Photocatalysts and Their Activity towards Degradation of Cibacron Red Dye. Trans. Indian Ceram. Soc. 2018, 77, 1–7. [Google Scholar] [CrossRef]
- Uribe-López, M.C.; Hidalgo-López, M.C.; López-González, R.; Frías-Márquez, D.M.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M.A. Photocatalytic Activity of ZnO Nanoparticles and the Role of the Synthesis Method on Their Physical and Chemical Properties. J. Photochem. Photobiol. A Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- Neri, G.; Bonavita, A.; Milone, C.; Galvagno, S. Role of the Au Oxidation State in the CO Sensing Mechanism of Au/Iron Oxide-Based Gas Sensors. Sens. Actuators B Chem. 2003, 93, 402–408. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A Review on Exploration of Fe2O3 Photocatalyst towards Degradation of Dyes and Organic Contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef]
- Ng, Y.H.; Iwase, A.; Kudo, A.; Amal, R. Reducing Graphene Oxide on a Visible-Light BiVO4 Photocatalyst for an Enhanced Photoelectrochemical Water Splitting. J. Phys. Chem. Lett. 2010, 1, 2607–2612. [Google Scholar] [CrossRef]
- Kamble, G.S.; Natarajan, T.S.; Patil, S.S.; Thomas, M.; Chougale, R.K.; Sanadi, P.D.; Siddharth, U.S.; Ling, Y.-C. BiVO4 As a Sustainable and Emerging Photocatalyst: Synthesis Methodologies, Engineering Properties, and Its Volatile Organic Compounds Degradation Efficiency. Nanomaterials 2023, 13, 1528. [Google Scholar] [CrossRef]
- Ramanathan, G.; Murali, K.R. Photocatalytic Activity of SnO2 Nanoparticles. J. Appl. Electrochem. 2022, 52, 849–859. [Google Scholar] [CrossRef]
- Kwang Benno Park, H.; Kumar, P.; Kebaili, I.; Boukhris, I.; Hwan Joo, Y.; Hyun Sung, T.; Kumar, A. Optimization and Modelling of Magnesium Oxide (MgO) Photocatalytic Degradation of Binary Dyes Using Response Surface Methodology. Sci. Rep. 2024, 14, 9412. [Google Scholar] [CrossRef]
- Sun, R.; Chen, Z.; Yang, Y.; Peng, J.; Zheng, T. Effects and Mechanism of SiO2 on Photocatalysis and Super Hydrophilicity of TiO2 Films Prepared by Sol-Gel Method. Mater. Res. Express 2019, 6, 46409. [Google Scholar] [CrossRef]
- Cai, J.; Li, D.; Jiang, L.; Yuan, J.; Li, Z.; Li, K. Review on CeO2-Based Photocatalysts for Photocatalytic Reduction of CO2: Progresses and Perspectives. Energy Fuels 2023, 37, 4878–4897. [Google Scholar] [CrossRef]
- Liu, X.; Sayed, M.; Bie, C.; Cheng, B.; Hu, B.; Yu, J.; Zhang, L. Hollow CdS-Based Photocatalysts. J. Mater. 2021, 7, 419–439. [Google Scholar] [CrossRef]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A Review of Its Properties and Conflicting Trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, L.; Wang, H.; Yang, J.; Yuan, X.; Wang, H.; Liang, J.; Li, X.; Li, H.; Bu, Y. Oxygen Vacancies Modified TiO2/O-Terminated Ti3C2 Composites: Unravelling the Dual Effects between Oxygen Vacancy and High-Work-Function Titanium Carbide. Adv. Funct. Mater. 2023, 33, 2307702. [Google Scholar] [CrossRef]
- Şahin, B.; Aydin, R.; Soylu, S.; Türkmen, M.; Kara, M.; Akkaya, A.; Çetin, H.; Ayyıldız, E. The Effect of Thymus Syriacus Plant Extract on the Main Physical and Antibacterial Activities of ZnO Nanoparticles Synthesized by SILAR Method. Inorg. Chem. Commun. 2022, 135, 109088. [Google Scholar] [CrossRef]
- Negi, A.; Gangwar, R.; Kumar Vishwakarma, R.; Singh Negi, D. Antibacterial, Antioxidant and Photodegradation Potential of ZnO Nanoparticles Mediated via Roots of Taraxacum Officinale Radix. Mater. Today Proc. 2022, 57, 2435–2443. [Google Scholar] [CrossRef]
- Abou Zeid, S.; Perez, A.; Bastide, S.; Le Pivert, M.; Rossano, S.; Remita, H.; Hautière, N.; Leprince-Wang, Y. Antibacterial and Photocatalytic Properties of ZnO Nanostructure Decorated Coatings. Coatings 2024, 14, 41. [Google Scholar] [CrossRef]
- Wang, Z.L. Nanostructures of Zinc Oxide. Mater. Today 2004, 7, 26–33. [Google Scholar] [CrossRef]
- Hariharan, C. Photocatalytic Degradation of Organic Contaminants in Water by ZnO Nanoparticles: Revisited. Appl. Catal. A Gen. 2006, 304, 55–61. [Google Scholar] [CrossRef]
- Akyol, A.; Yatmaz, H.C.; Bayramoglu, M. Photocatalytic Decolorization of Remazol Red RR in Aqueous ZnO Suspensions. Appl. Catal. B Environ. 2004, 54, 19–24. [Google Scholar] [CrossRef]
- Colón, G.; Hidalgo, M.C.; Navío, J.A.; Pulido Melián, E.; González Díaz, O.; Doña Rodríguez, J.M. Highly Photoactive ZnO by Amine Capping-Assisted Hydrothermal Treatment. Appl. Catal. B Environ. 2008, 83, 30–38. [Google Scholar] [CrossRef]
- Lizama, C.; Freer, J.; Baeza, J.; Mansilla, H.D. Optimized Photodegradation of Reactive Blue 19 on TiO2 and ZnO Suspensions. Catal. Today 2002, 76, 235–246. [Google Scholar] [CrossRef]
- Chandiran, A.K.; Abdi-Jalebi, M.; Nazeeruddin, M.K.; Grätzel, M. Analysis of Electron Transfer Properties of ZnO and TiO2 Photoanodes for Dye-Sensitized Solar Cells. ACS Nano 2014, 8, 2261–2268. [Google Scholar] [CrossRef]
- Anta, J.A.; Guillén, E.; Tena-Zaera, R. ZnO-Based Dye-Sensitized Solar Cells. J. Phys. Chem. C 2012, 116, 11413–11425. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic Degradation of Azo Dye Acid Red 14 in Water on ZnO as an Alternative Catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Kandavelu, V.; Kastien, H.; Thampi, K.R. Photocatalytic Degradation of Isothiazolin-3-Ones in Water and Emulsion Paints Containing Nanocrystalline TiO2 and ZnO Catalysts. Appl. Catal. B Environ. 2004, 48, 101–111. [Google Scholar] [CrossRef]
- Zhao, S.-W.; Zuo, H.-F.; Guo, Y.; Pan, Q. Carbon-Doped ZnO Aided by Carboxymethyl Cellulose: Fabrication, Photoluminescence and Photocatalytic Applications. J. Alloys Compd. 2017, 695, 1029–1037. [Google Scholar] [CrossRef]
- Rooydell, R.; Brahma, S.; Wang, R.-C.; Modaberi, M.R.; Ebrahimzadeh, F.; Liu, C.-P. Cu Doped ZnO Nanorods with Controllable Cu Content by Using Single Metal Organic Precursors and Their Photocatalytic and Luminescence Properties. J. Alloys Compd. 2017, 691, 936–945. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.; Wang, D.; Wang, L.; Xie, T.; Jiang, T. A High Performance Cobalt-Doped ZnO Visible Light Photocatalyst and Its Photogenerated Charge Transfer Properties. Nano Res. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Güy, N.; Özacar, M. The Influence of Noble Metals on Photocatalytic Activity of ZnO for Congo Red Degradation. Int. J. Hydrogen Energy 2016, 41, 20100–20112. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Lima, M.J.; Baptista, D.L.; Silva, A.M.T.; Silva, C.G.; Faria, J.L. Ag-Loaded ZnO Materials for Photocatalytic Water Treatment. Chem. Eng. J. 2017, 318, 95–102. [Google Scholar] [CrossRef]
- Bao, Z.; Yuan, Y.; Leng, C.; Li, L.; Zhao, K.; Sun, Z. One-Pot Synthesis of Noble Metal/Zinc Oxide Composites with Controllable Morphology and High Catalytic Performance. ACS Appl. Mater. Interfaces 2017, 9, 16417–16425. [Google Scholar] [CrossRef] [PubMed]
- Udawatte, N.; Lee, M.; Kim, J.; Lee, D. Well-Defined Au/ZnO Nanoparticle Composites Exhibiting Enhanced Photocatalytic Activities. ACS Appl. Mater. Interfaces 2011, 3, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Aberoomand-Azar, P.; Raeis-Farshid, S.; Abedini-Khorrami, S.; Givianrad, M.H. The Effect of Different Molar Ratios of ZnO on Characterization and Photocatalytic Activity of TiO2/ZnO Nanocomposite. J. Saudi Chem. Soc. 2016, 20, 373–378. [Google Scholar] [CrossRef]
- Prabhu, Y.T.; Navakoteswara Rao, V.; Shankar, M.V.; Sreedhar, B.; Pal, U. The Facile Hydrothermal Synthesis of CuO@ZnO Heterojunction Nanostructures for Enhanced Photocatalytic Hydrogen Evolution. New J. Chem. 2019, 43, 6794–6805. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Castillo, R.B.; Sillanpaa, M.; Rajendra Kumar, R.T. Effective Shell Wall Thickness of Vertically Aligned ZnO-ZnS Core-Shell Nanorod Arrays on Visible Photocatalytic and Photo Sensing Properties. Appl. Catal. B Environ. 2018, 237, 128–139. [Google Scholar] [CrossRef]
- Rahmah, M.I.; Sabry, R.S.; Aziz, W.J. Preparation of Superhydrophobic Ag/Fe2O3/ZnO Surfaces with Photocatalytic Activity. Surf. Eng. 2021, 37, 1320–1327. [Google Scholar] [CrossRef]
- Gang, R.; Xu, L.; Xia, Y.; Cai, J.; Zhang, L.; Wang, S.; Li, R. Fabrication of MoS2 QDs/ZnO Nanosheet 0D/2D Heterojunction Photocatalysts for Organic Dyes and Gaseous Heavy Metal Removal. J. Colloid Interface Sci. 2020, 579, 853–861. [Google Scholar] [CrossRef]
- Xu, P.; Wang, P.; Wang, Q.; Wei, R.; Li, Y.; Xin, Y.; Zheng, T.; Hu, L.; Wang, X.; Zhang, G. Facile Synthesis of Ag2O/ZnO/RGO Heterojunction with Enhanced Photocatalytic Activity under Simulated Solar Light: Kinetics and Mechanism. J. Hazard. Mater. 2021, 403, 124011. [Google Scholar] [CrossRef]
- Liu, B.; Bie, C.; Zhang, Y.; Wang, L.; Li, Y.; Yu, J. Hierarchically Porous ZnO/g-C3N4 S-Scheme Heterojunction Photocatalyst for Efficient H2O2 Production. Langmuir 2021, 37, 14114–14124. [Google Scholar] [CrossRef]
- Zgura, I.; Preda, N.; Enculescu, M.; Diamandescu, L.; Negrila, C.; Bacalum, M.; Ungureanu, C.; Barbinta-Patrascu, M.E. Cytotoxicity, Antioxidant, Antibacterial, and Photocatalytic Activities of ZnO-CdS Powders. Materials 2020, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Zgura, I.; Preda, N.; Socol, G.; Ghica, C.; Ghica, D.; Enculescu, M.; Negut, I.; Nedelcu, L.; Frunza, L.; Ganea, C.P.; et al. Wet Chemical Synthesis of ZnO-CdS Composites and Their Photocatalytic Activity. Mater. Res. Bull. 2018, 99, 174–181. [Google Scholar] [CrossRef]
- Joseph, J.A.; Nair, S.B.; John, S.S.; Shaji, S.; Philip, R.R. α-Fe2O3/ZnO Heterostructure for Enhanced Photocatalytic and Antibacterial Activity. Semicond. Sci. Technol. 2021, 36, 95007. [Google Scholar] [CrossRef]
- Taufique, M.F.N.; Haque, A.; Karnati, P.; Ghosh, K. ZnO–CuO Nanocomposites with Improved Photocatalytic Activity for Environmental and Energy Applications. J. Electron. Mater. 2018, 47, 6731–6745. [Google Scholar] [CrossRef]
- Jan, T.; Azmat, S.; Mansoor, Q.; Waqas, H.M.; Adil, M.; Ilyas, S.Z.; Ahmad, I.; Ismail, M. Superior Antibacterial Activity of ZnO-CuO Nanocomposite Synthesized by a Chemical Co-Precipitation Approach. Microb. Pathog. 2019, 134, 103579. [Google Scholar] [CrossRef]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of ZnO Decorated Graphene Nanocomposite for Enhanced Photocatalytic Properties. J. Appl. Phys. 2014, 115, 173504. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z.; Li, W.; Hou, B. Highly Efficient Photocatalytic Performance of Graphene–ZnO Quasi-Shell–Core Composite Material. ACS Appl. Mater. Interfaces 2013, 5, 12361–12368. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Voon, S.-Y.; Tan, L.-L.; Goh, B.T.; Yong, S.-T.; Chai, S.-P. Enhanced Daylight-Induced Photocatalytic Activity of Solvent Exfoliated Graphene (SEG)/ZnO Hybrid Nanocomposites toward Degradation of Reactive Black 5. Ind. Eng. Chem. Res. 2014, 53, 17333–17344. [Google Scholar] [CrossRef]
- Ambika, S.; Sundrarajan, M. Antibacterial Behaviour of Vitex Negundo Extract Assisted ZnO Nanoparticles against Pathogenic Bacteria. J. Photochem. Photobiol. B. 2015, 146, 52–57. [Google Scholar] [CrossRef]
- Panigrahi, J.; Behera, D.; Mohanty, I.; Subudhi, U.; Nayak, B.B.; Acharya, B.S. Radio Frequency Plasma Enhanced Chemical Vapor Based ZnO Thin Film Deposition on Glass Substrate: A Novel Approach towards Antibacterial Agent. Appl. Surf. Sci. 2011, 258, 304–311. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO Nanoparticles to Escherichia Coli: Mechanism and the Influence of Medium Components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’Neill, A.J.; York, D.W. Mechanistic Investigation into Antibacterial Behaviour of Suspensions of ZnO Nanoparticles against E. Coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Song, D.; Widenborg, P.; Chin, W.; Aberle, A.G. Investigation of Lateral Parameter Variations of Al-Doped Zinc Oxide Films Prepared on Glass Substrates by Rf Magnetron Sputtering. Sol. Energy Mater. Sol. Cells 2002, 73, 1–20. [Google Scholar] [CrossRef]
- Miccoli, I.; Spampinato, R.; Marzo, F.; Prete, P.; Lovergine, N. DC-Magnetron Sputtering of ZnO:Al Films on (00.1)Al2O3 Substrates from Slip-Casting Sintered Ceramic Targets. Appl. Surf. Sci. 2014, 313, 418–423. [Google Scholar] [CrossRef]
- Winer, I.; Shter, G.E.; Mann-Lahav, M.; Grader, G.S. Effect of Solvents and Stabilizers on Sol–Gel Deposition of Ga-Doped Zinc Oxide TCO Films. J. Mater. Res. 2011, 26, 1309–1315. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Cunha, I.; Pinto, J.V.; Neto, J.P.; Pereira, L.; Fortunato, E.; Martins, R. UV-Responsive Screen-Printed Porous ZnO Nanostructures on Office Paper for Sustainable and Foldable Electronics. Chemosensors 2021, 9, 192. [Google Scholar] [CrossRef]
- Banerjee, D.; Lao, J.Y.; Wang, D.Z.; Huang, J.Y.; Ren, Z.F.; Steeves, D.; Kimball, B.; Sennett, M. Large-Quantity Free-Standing ZnO Nanowires. Appl. Phys. Lett. 2003, 83, 2061–2063. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Kim, Y.-S.; Seo, H.-K.; Shin, H.-S. Room Temperature Synthesis of Needle-Shaped ZnO Nanorods via Sonochemical Method. Appl. Surf. Sci. 2007, 253, 7622–7626. [Google Scholar] [CrossRef]
- Leprince-Wang, Y.; Martin, N.; Habba, Y.; Le Pivert, M.; Capochichi-Gnambodoe, M. ZnO Nanostructure Based Photocatalysis for Water Purification. NanoWorld J. 2020, 6. [Google Scholar] [CrossRef]
- Chiu, W.S.; Khiew, P.S.; Cloke, M.; Isa, D.; Tan, T.K.; Radiman, S.; Abd-Shukor, R.; Hamid, M.A.A.; Huang, N.M.; Lim, H.N.; et al. Photocatalytic Study of Two-Dimensional ZnO Nanopellets in the Decomposition of Methylene Blue. Chem. Eng. J. 2010, 158, 345–352. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Self-Assembly of Metal Oxides into Three-Dimensional Nanostructures: Synthesis and Application in Catalysis. ACS Nano 2009, 3, 728–736. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Look, D.C.; Jogai, B.; Litton, C.W.; Cantwell, G.; Harsch, W.C. Valence-Band Ordering in ZnO. Phys. Rev. B 1999, 60, 2340–2344. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of Zinc Oxide as a Semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Coleman, V.A.; Bradby, J.E.; Jagadish, C.; Munroe, P.; Heo, Y.W.; Pearton, S.J.; Norton, D.P.; Inoue, M.; Yano, M. Mechanical Properties of ZnO Epitaxial Layers Grown on A- and c-Axis Sapphire. Appl. Phys. Lett. 2005, 86, 203105. [Google Scholar] [CrossRef]
- Dietl, T.; Ohno, H.; Matsukura, F.; Cibert, J.; Ferrand, D. Zener Model Description of Ferromagnetism in Zinc-Blende Magnetic Semiconductors. Science 2000, 287, 1019–1022. [Google Scholar] [CrossRef]
- Jagadish, C.; Pearton, S.J. Zinc Oxide Bulk, Thin Films and Nanostructures Processing, Properties, and Applications, 1st ed.; Jagadish, C., Pearton, S.J., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; ISBN 9780080447223. [Google Scholar]
- Zhang, L.; Li, Y.; Liu, X.; Zhao, L.; Ding, Y.; Povey, M.; Cang, D. The Properties of ZnO Nanofluids and the Role of H2O2 in the Disinfection Activity against Escherichia Coli. Water Res. 2013, 47, 4013–4021. [Google Scholar] [CrossRef]
- Elaaraj, I.; Raouan, S.E.R.; Nakkabi, A.; Es-sounni, B.; Koraichi, I.; El Moualij, N.; Fahim, M. Synthesis, Characterization and Antioxidant, Antibacterial Activity Zn2+, Cu2+, Ni2+ and Co2+, Complexes of Ligand [2-(Thiophen-2-Yl)-1-(Thiophen-2-Ylmethyl)-1H-Benzo[d]Imidazole]. J. Indian Chem. Soc. 2022, 99, 100404. [Google Scholar] [CrossRef]
- Bellanger, X.; Schneider, R.; Dezanet, C.; Arroua, B.; Balan, L.; Billard, P.; Merlin, C. Zn2+ Leakage and Photo-Induced Reactive Oxidative Species Do Not Explain the Full Toxicity of ZnO Core Quantum Dots. J. Hazard. Mater. 2020, 396, 122616. [Google Scholar] [CrossRef]
- Ding, Y.; Kong, X.Y.; Wang, Z.L. Doping and Planar Defects in the Formation of Single-Crystal ZnO Nanorings. Phys. Rev. B 2004, 70, 235408. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Qu, L.S.; Gao, H. Synthesis and Photoluminescence Properties of ZnO Nanohelices. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 12009. [Google Scholar] [CrossRef]
- Peng, D.; Huang, Y.; Yu, K.; Li, L.; Zhu, Z. Synthesis and Field Emission Properties of Hierarchical ZnO Nanostructures. J. Nanomater. 2010, 2010, 153–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Heng, L.; Jiang, L. Chemically Controllable Fabrication of One-Dimensional ZnO Nanostructures and Their Applications in Solar Cells. J. Nanosci. Nanotechnol. 2014, 14, 5597–5613. [Google Scholar] [CrossRef]
- Abdullah, H.; Ariyanto, N.P.; Shaari, S.; Yuliarto, B.; Junaidi, S. Study of Porous Nanoflake ZnO for Dye-Sensitized Solar Cell Application. Am. J. Eng. Appl. Sci. 2009, 2, 236–240. [Google Scholar] [CrossRef][Green Version]
- Gokarna, A.; Parize, R.; Kadiri, H.; Nomenyo, K.; Patriarche, G.; Miska, P.; Lerondel, G. Highly Crystalline Urchin-like Structures Made of Ultra-Thin Zinc Oxide Nanowires. RSC Adv. 2014, 4, 47234–47239. [Google Scholar] [CrossRef]
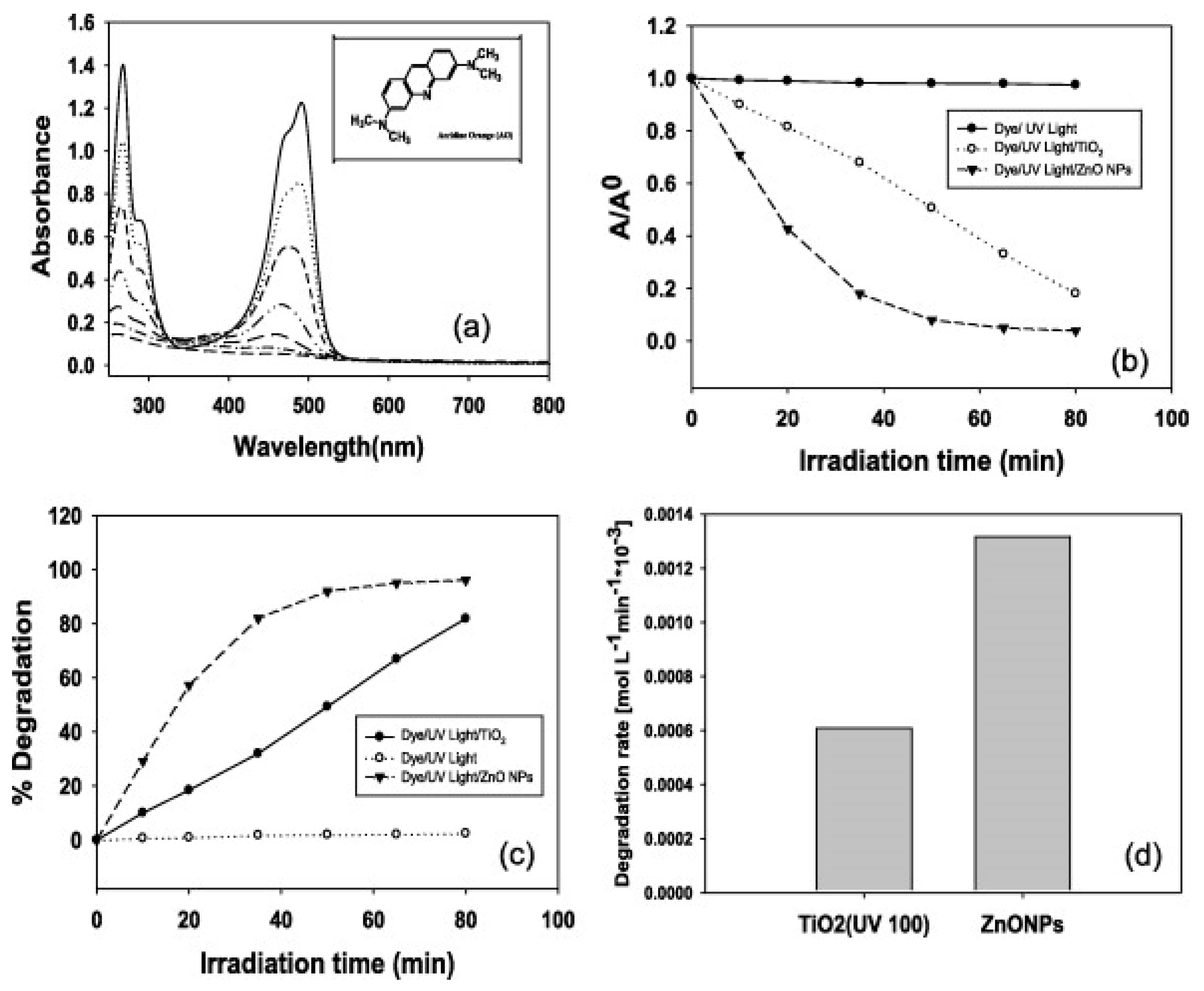
- Chauhan, M.S.; Kumar, R.; Umar, A.; Chauhan, S.; Kumar, G.; Faisal, M.; Hwang, S.W.; Al-Hajry, A. Utilization of ZnO Nanocones for the Photocatalytic Degradation of Acridine Orange. J. Nanosci. Nanotechnol. 2011, 11, 4061–4066. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X. Hydrothermal Synthesis and Photocatalytic Activity of Zinc Oxide Hollow Spheres. Environ. Sci. Technol. 2008, 42, 4902–4907. [Google Scholar] [CrossRef]
- Folawewo, A.D.; Bala, M.D. Nanocomposite Zinc Oxide-Based Photocatalysts: Recent Developments in Their Use for the Treatment of Dye-Polluted Wastewater. Water 2022, 14, 3899. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Mahlambi, M.M.; Ngila, C.J.; Mamba, B.B. Recent Developments in Environmental Photocatalytic Degradation of Organic Pollutants: The Case of Titanium Dioxide Nanoparticles—A Review. J. Nanomater. 2015, 2015, 790173. [Google Scholar] [CrossRef]
- Chen, D.; Jiao, X.; Cheng, G. Hydrothermal Synthesis of Zinc Oxide Powders with Different Morphologies. Solid State Commun. 1999, 113, 363–366. [Google Scholar] [CrossRef]
- Ismail, A.A.; El-Midany, A.; Abdel-Aal, E.A.; El-Shall, H. Application of Statistical Design to Optimize the Preparation of ZnO Nanoparticles via Hydrothermal Technique. Mater. Lett. 2005, 59, 1924–1928. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal Processing of Materials: Past, Present and Future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Chevalier-César, C.; Capochichi-Gnambodoe, M.; Lin, F.; Yu, D.; Leprince-Wang, Y. Effect of Growth Time and Annealing on the Structural Defect Concentration of Hydrothermally Grown ZnO Nanowires. AIMS Mater. Sci. 2016, 3, 562–572. [Google Scholar] [CrossRef]
- Chevalier-César, C.; Capochichi-Gnambodoe, M.; Leprince-Wang, Y. Growth Mechanism Studies of ZnO Nanowire Arrays via Hydrothermal Method. Appl. Phys. A 2014, 115, 953–960. [Google Scholar] [CrossRef]
- Li, T.; Fan, H.M.; Xue, J.M.; Ding, J. Synthesis of Highly-Textured ZnO Films on Different Substrates by Hydrothermal Route. Thin Solid Films 2010, 518, e114–e117. [Google Scholar] [CrossRef]
- Yu, J.; Huang, B.; Qin, X.; Zhang, X.; Wang, Z.; Liu, H. Hydrothermal Synthesis and Characterization of ZnO Films with Different Nanostructures. Appl. Surf. Sci. 2011, 257, 5563–5565. [Google Scholar] [CrossRef]
- Holi, A.M.; Zainal, Z.; Talib, Z.A.; Lim, H.-N.; Yap, C.-C.; Chang, S.-K.; Ayal, A.K. Effect of Hydrothermal Growth Time on ZnO Nanorod Arrays Photoelectrode Performance. Optik 2016, 127, 11111–11118. [Google Scholar] [CrossRef]
- Shrisha, B.V.; Bhat, S.; Kushavah, D.; Gopalakrishna Naik, K. Hydrothermal Growth and Characterization of Al-Doped ZnO Nanorods. Mater. Today Proc. 2016, 3, 1693–1701. [Google Scholar] [CrossRef]
- Wen, X.; Wu, W.; Ding, Y.; Wang, Z.L. Seedless Synthesis of Patterned ZnO Nanowire Arrays on Metal Thin Films (Au, Ag, Cu, Sn) and Their Application for Flexible Electromechanical Sensing. J. Mater. Chem. 2012, 22, 9469–9476. [Google Scholar] [CrossRef]
- Lee, C.Y.; Li, S.Y.; Lin, P.; Tseng, T.Y. ZnO Nanowires Hydrothermally Grown on PET Polymer Substrates and Their Characteristics. J. Nanosci. Nanotechnol. 2005, 5, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.B.; Faisal, M.; Rahman, M.M.; Jamal, A. Low-Temperature Growth of ZnO Nanoparticles: Photocatalyst and Acetone Sensor. Talanta 2011, 85, 943–949. [Google Scholar] [CrossRef] [PubMed]
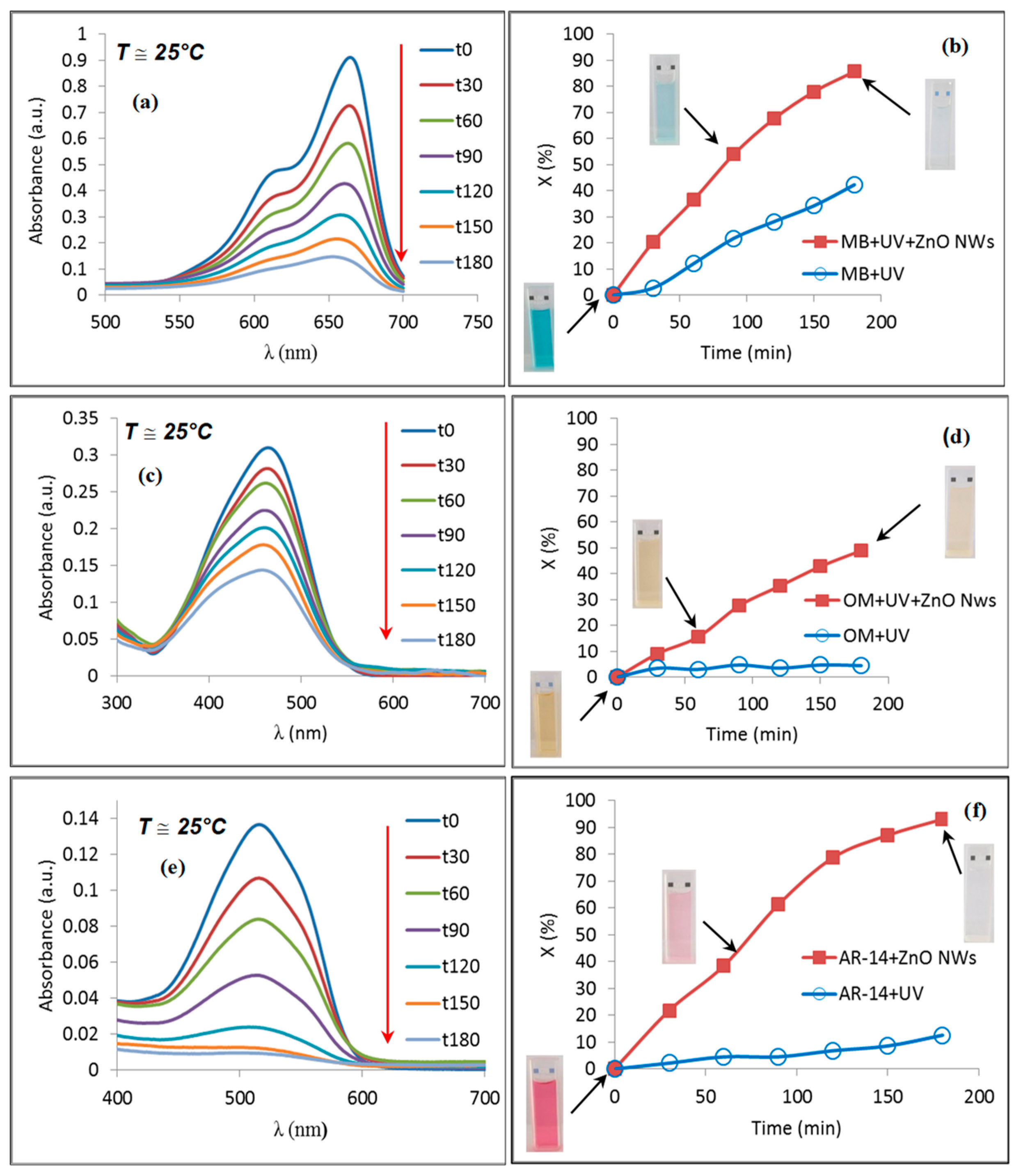
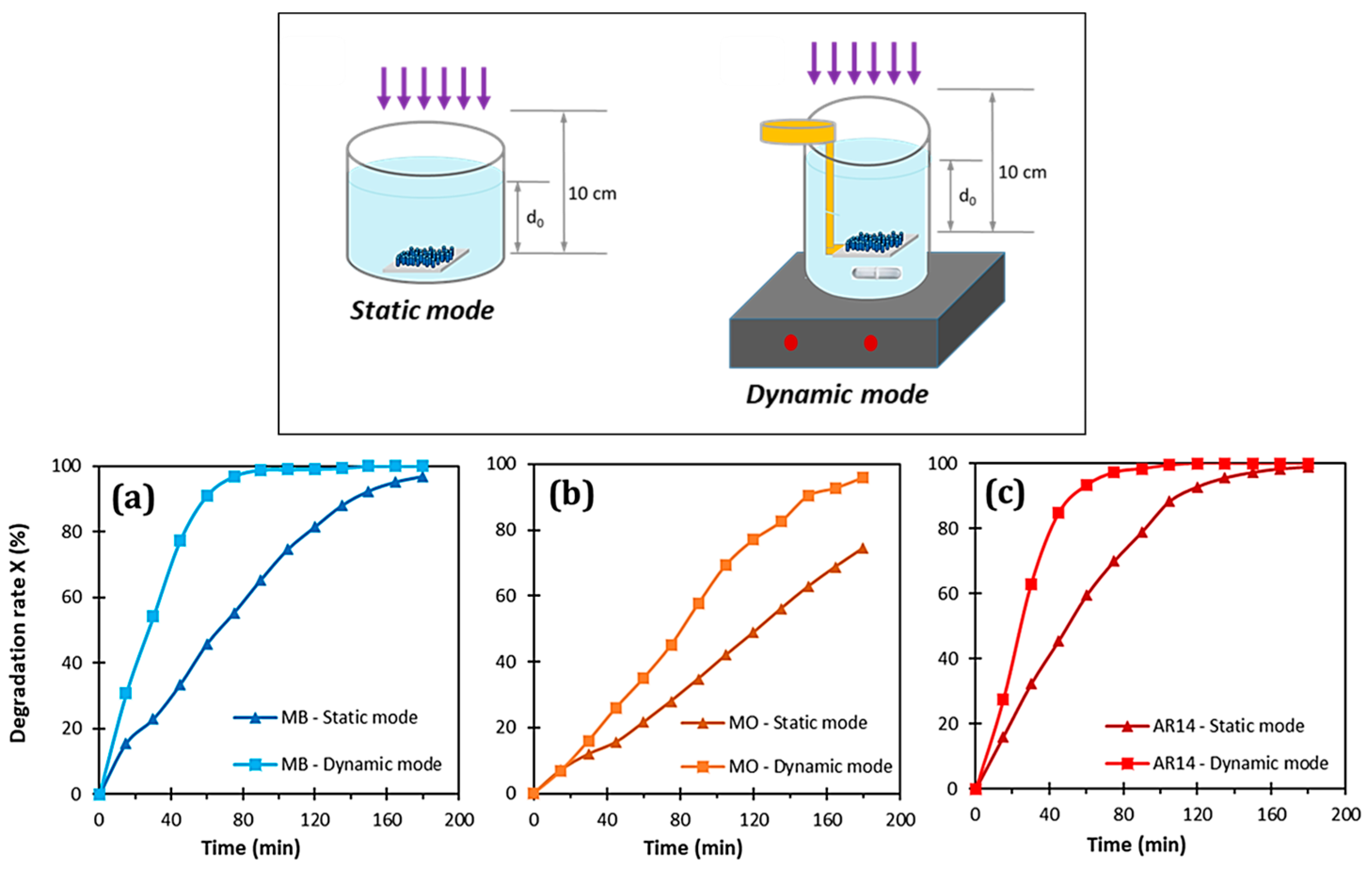
- Habba, Y.G.; Capochichi-Gnambodoe, M.; Serairi, L.; Leprince-Wang, Y. Enhanced Photocatalytic Activity of ZnO Nanostructure for Water Purification. Phys. Status Solidi 2016, 253, 1480–1484. [Google Scholar] [CrossRef]
- Le Pivert, M.; Martin, N.; Leprince-Wang, Y. Hydrothermally Grown ZnO Nanostructures for Water Purification via Photocatalysis. Crystals 2022, 12, 308. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Liu, Y.; Fan, Y.; Dang, F.; Qiu, Y.; Zhou, H.; Wang, W.; Liu, Y. Solvothermal Synthesis of ZnO Nanoparticles for Photocatalytic Degradation of Methyl Orange and P-Nitrophenol. Water 2021, 13, 3224. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.-C.; Vasile, B.-S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Photocatalytic Activity against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.-L.; Pelligra, C.; Chen, C.-H.; Jin, L.; Huang, H.; Sithambaram, S.; Aindow, M.; Joesten, R.; Suib, S.L. ZnO with Different Morphologies Synthesized by Solvothermal Methods for Enhanced Photocatalytic Activity. Chem. Mater. 2009, 21, 2875–2885. [Google Scholar] [CrossRef]
- Wang, S.L.; Zhu, H.W.; Tang, W.H.; Li, P.G. Propeller-Shaped ZnO Nanostructures Obtained by Chemical Vapor Deposition: Photoluminescence and Photocatalytic Properties. J. Nanomater. 2012, 2012, 594290. [Google Scholar] [CrossRef]
- Hezam, A.; Drmosh, Q.A.; Ponnamma, D.; Bajiri, M.A.; Qamar, M.; Namratha, K.; Zare, M.; Nayan, M.B.; Onaizi, S.A.; Byrappa, K. Strategies to Enhance ZnO Photocatalyst’s Performance for Water Treatment: A Comprehensive Review. Chem. Rec. 2022, 22, e202100299. [Google Scholar] [CrossRef] [PubMed]
- Chijioke-Okere, M.O.; Okorocha, N.J.; Anukam, B.N.; Oguzie, E.E. Photocatalytic Degradation of a Basic Dye Using Zinc Oxide Nanocatalyst. Int. Lett. Chem. Phys. Astron. 2019, 81, 18–26. [Google Scholar] [CrossRef]
- Baruah, S.; Pal, S.K.; Dutta, J. Nanostructured Zinc Oxide for Water Treatment. Nanosci. Nanotechnol.-Asia 2012, 2, 90–102. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the Improvement of the Photocatalytic and Antibacterial Activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Raghupathi, K.R.; St. Pierre, J.; Zhao, D.; Koodali, R.T. Tuning of the Crystallite and Particle Sizes of ZnO Nanocrystalline Materials in Solvothermal Synthesis and Their Photocatalytic Activity for Dye Degradation. J. Phys. Chem. C 2011, 115, 13844–13850. [Google Scholar] [CrossRef]
- Liu, F.; Leung, Y.H.; Djurišić, A.B.; Ng, A.M.C.; Chan, W.K. Native Defects in ZnO: Effect on Dye Adsorption and Photocatalytic Degradation. J. Phys. Chem. C 2013, 117, 12218–12228. [Google Scholar] [CrossRef]
- Gurylev, V.; Perng, T.P. Defect Engineering of ZnO: Review on Oxygen and Zinc Vacancies. J. Eur. Ceram. Soc. 2021, 41, 4977–4996. [Google Scholar] [CrossRef]
- Nicholas, N.J.; Franks, G.V.; Ducker, W.A. Selective Adsorption to Particular Crystal Faces of ZnO. Langmuir 2012, 28, 7189–7196. [Google Scholar] [CrossRef]
- Rao, F.; Zhu, G.; Zhang, W.; Xu, Y.; Cao, B.; Shi, X.; Gao, J.; Huang, Y.; Huang, Y.; Hojamberdiev, M. Maximizing the Formation of Reactive Oxygen Species for Deep Oxidation of NO via Manipulating the Oxygen-Vacancy Defect Position on (BiO)2CO3. ACS Catal. 2021, 11, 7735–7749. [Google Scholar] [CrossRef]
- Flores, N.M.; Pal, U.; Galeazzi, R.; Sandoval, A. Effects of Morphology, Surface Area, and Defect Content on the Photocatalytic Dye Degradation Performance of ZnO Nanostructures. RSC Adv. 2014, 4, 41099–41110. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H. Morphologies of Zinc Oxide Particles and Their Effects on Photocatalysis. Chemosphere 2003, 51, 129–137. [Google Scholar] [CrossRef]
- Li, G.R.; Hu, T.; Pan, G.L.; Yan, T.Y.; Gao, X.P.; Zhu, H.Y. Morphology−Function Relationship of ZnO: Polar Planes, Oxygen Vacancies, and Activity. J. Phys. Chem. C 2008, 112, 11859–11864. [Google Scholar] [CrossRef]
- Thakur, S.; Neogi, S.; Ray, A.K. Morphology-Controlled Synthesis of ZnO Nanostructures for Caffeine Degradation and Escherichia Coli Inactivation in Water. Catalysts 2021, 11, 63. [Google Scholar] [CrossRef]
- Chen, K.; Xiao, J.; Vequizo, J.J.M.; Hisatomi, T.; Ma, Y.; Nakabayashi, M.; Takata, T.; Yamakata, A.; Shibata, N.; Domen, K. Overall Water Splitting by a SrTaO2N-Based Photocatalyst Decorated with an Ir-Promoted Ru-Based Cocatalyst. J. Am. Chem. Soc. 2023, 145, 3839–3843. [Google Scholar] [CrossRef]
- Singh, K.; Nancy; Bhattu, M.; Singh, G.; Mubarak, N.M.; Singh, J. Light-Absorption-Driven Photocatalysis and Antimicrobial Potential of PVP-Capped Zinc Oxide Nanoparticles. Sci. Rep. 2023, 13, 13886. [Google Scholar] [CrossRef]
- El Golli, A.; Contreras, S.; Dridi, C. Bio-Synthesized ZnO Nanoparticles and Sunlight-Driven Photocatalysis for Environmentally-Friendly and Sustainable Route of Synthetic Petroleum Refinery Wastewater Treatment. Sci. Rep. 2023, 13, 20809. [Google Scholar] [CrossRef]
- Umar, A.; Kumar, R.; Kumar, G.; Algarni, H.; Kim, S.H. Effect of Annealing Temperature on the Properties and Photocatalytic Efficiencies of ZnO Nanoparticles. J. Alloys Compd. 2015, 648, 46–52. [Google Scholar] [CrossRef]
- Li, D.; Balek, V.; Ohashi, N.; Mitsuhashi, T.; Hishita, S.; Haneda, H. Self-Assembly Prismatic Aggregates Formed during the Calcination of ZnO Powders: In Situ Monitoring by ETA Technique and Their Photocatalytic Properties. J. Colloid Interface Sci. 2005, 289, 472–478. [Google Scholar] [CrossRef]
- Giraldi, T.R.; Santos, G.V.F.; Mendonça, V.R.; Ribeiro, C.; Weber, I.T. Annealing Effects on the Photocatalytic Activity of ZnO Nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 3635–3640. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; De Marco, I.; Vaiano, V. Zinc Oxide Nanoparticles Obtained by Supercritical Antisolvent Precipitation for the Photocatalytic Degradation of Crystal Violet Dye. Catalysts 2019, 9, 346. [Google Scholar] [CrossRef]
- Casey, P.S.; Rossouw, C.J.; Boskovic, S.; Lawrence, K.A.; Turney, T.W. Incorporation of Dopants into the Lattice of ZnO Nanoparticles to Control Photoactivity. Superlattices Microstruct. 2006, 39, 97–106. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Z.; Sun, X.; Shang, J.; Cai, W. The Surface Properties and Photocatalytic Activities of ZnO Ultrafine Particles. Appl. Surf. Sci. 2001, 180, 308–314. [Google Scholar] [CrossRef]
- Hayat, K.; Gondal, M.A.; Khaled, M.M.; Ahmed, S.; Shemsi, A.M. Nano ZnO Synthesis by Modified Sol Gel Method and Its Application in Heterogeneous Photocatalytic Removal of Phenol from Water. Appl. Catal. A Gen. 2011, 393, 122–129. [Google Scholar] [CrossRef]
- Wolski, L.; Whitten, J.E.; Sobczak, I.; Ziolek, M. The Effect of the Preparation Procedure on the Morphology, Texture and Photocatalytic Properties of ZnO. Mater. Res. Bull. 2017, 85, 35–46. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, H.; Liu, B.; Yang, H. Charge Separation between Wurtzite ZnO Polar {001} Surfaces and Their Enhanced Photocatalytic Activity. Appl. Catal. B Environ. 2015, 163, 189–197. [Google Scholar] [CrossRef]
- Saravanan, R.; Gupta, V.K.; Narayanan, V.; Stephen, A. Comparative Study on Photocatalytic Activity of ZnO Prepared by Different Methods. J. Mol. Liq. 2013, 181, 133–141. [Google Scholar] [CrossRef]
- Daou, I.; Zegaoui, O.; Elghazouani, A. Physicochemical and Photocatalytic Properties of the ZnO Particles Synthesized by Two Different Methods Using Three Different Precursors. Comptes Rendus Chim. 2017, 20, 47–54. [Google Scholar] [CrossRef]
- Akir, S.; Barras, A.; Coffinier, Y.; Bououdina, M.; Boukherroub, R.; Omrani, A.D. Eco-Friendly Synthesis of ZnO Nanoparticles with Different Morphologies and Their Visible Light Photocatalytic Performance for the Degradation of Rhodamine B. Ceram. Int. 2016, 42, 10259–10265. [Google Scholar] [CrossRef]
- Gupta, J.; Barick, K.C.; Bahadur, D. Defect Mediated Photocatalytic Activity in Shape-Controlled ZnO Nanostructures. J. Alloys Compd. 2011, 509, 6725–6730. [Google Scholar] [CrossRef]
- Ahumada-Lazo, R.; Torres-Martínez, L.M.; Ruíz-Gómez, M.A.; Vega-Becerra, O.E.; Figueroa-Torres, M.Z. Photocatalytic Efficiency of Reusable ZnO Thin Films Deposited by Sputtering Technique. Appl. Surf. Sci. 2014, 322, 35–40. [Google Scholar] [CrossRef]
- Jaafar, N.F.; Najman, A.M.M.; Marfur, A.; Jusoh, N.W.C. Strategies for the Formation of Oxygen Vacancies in Zinc Oxide Nanoparticles Used for Photocatalytic Degradation of Phenol under Visible Light Irradiation. J. Photochem. Photobiol. A Chem. 2020, 388, 112202. [Google Scholar] [CrossRef]
- Weerathunga, H.; Tang, C.; Brock, A.J.; Sarina, S.; Wang, T.; Liu, Q.; Zhu, H.-Y.; Du, A.; Waclawik, E.R. Nanostructure Shape-Effects in ZnO Heterogeneous Photocatalysis. J. Colloid Interface Sci. 2022, 606, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Shidpour, R.; Simchi, A.; Ghanbari, F.; Vossoughi, M. Photo-Degradation of Organic Dye by Zinc Oxide Nanosystems with Special Defect Structure: Effect of the Morphology and Annealing Temperature. Appl. Catal. A Gen. 2014, 472, 198–204. [Google Scholar] [CrossRef]
- Jang, E.S.; Won, J.-H.; Hwang, S.-J.; Choy, J.-H. Fine Tuning of the Face Orientation of ZnO Crystals to Optimize Their Photocatalytic Activity. Adv. Mater. 2006, 18, 3309–3312. [Google Scholar] [CrossRef]
- Li, C.; Zhou, H.; Yang, S.; Wei, L.; Han, Z.; Zhang, Y.; Pan, H. Preadsorption of O2 on the Exposed (001) Facets of ZnO Nanostructures for Enhanced Sensing of Gaseous Acetone. ACS Appl. Nano Mater. 2019, 2, 6144–6151. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Yamazaki, M.; Yoshihara, S.; Shirakashi, T. Photocatalytic ZnO Films Prepared by Anodizing. J. Electroanal. Chem. 1998, 442, 1–3. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.-J. ZnO Micro- and Nano-Structures: Microwave-Assisted Solvothermal Synthesis, Morphology Control and Photocatalytic Properties. Appl. Phys. A 2009, 97, 847–852. [Google Scholar] [CrossRef]
- Sun, H.; Yu, Y.; Luo, J.; Ahmad, M.; Zhu, J. Morphology-Controlled Synthesis of ZnO 3D Hierarchical Structures and Their Photocatalytic Performance. CrystEngComm 2012, 14, 8626–8632. [Google Scholar] [CrossRef]
- Nandi, P.; Das, D. Photocatalytic Degradation of Rhodamine-B Dye by Stable ZnO Nanostructures with Different Calcination Temperature Induced Defects. Appl. Surf. Sci. 2019, 465, 546–556. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Singhal, N.; Wang, L.; Gao, W. Comparative Photocatalytic Degradation of Estrone in Water by ZnO and TiO2 under Artificial UVA and Solar Irradiation. Chem. Eng. J. 2012, 213, 150–162. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Sun, B.; Qiao, P.; Ren, L.; Tian, G.; Jiang, B.; Pan, K.; Zhou, W. Surface Oxygen Vacancy Defect-Promoted Electron-Hole Separation for Porous Defective ZnO Hexagonal Plates and Enhanced Solar-Driven Photocatalytic Performance. Chem. Eng. J. 2020, 379, 122295. [Google Scholar] [CrossRef]
- Nie, Q.; Xie, Y.; Ma, J.; Wang, J.; Zhang, G. High Piezo–Catalytic Activity of ZnO/Al2O3 Nanosheets Utilizing Ultrasonic Energy for Wastewater Treatment. J. Clean. Prod. 2020, 242, 118532. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Li, M.; Jiang, J.; Gao, J.; Dong, S. Fabrication of Oxygen Defect-Rich Pencil-like ZnO Nanorods with CDots and Ag Co-Enhanced Photocatalytic Activity for Tetracycline Hydrochloride Degradation. Sep. Purif. Technol. 2021, 266, 118605. [Google Scholar] [CrossRef]
- Singh, J.; Juneja, S.; Palsaniya, S.; Manna, A.K.; Soni, R.K.; Bhattacharya, J. Evidence of Oxygen Defects Mediated Enhanced Photocatalytic and Antibacterial Performance of ZnO Nanorods. Colloids Surf. B. Biointerfaces 2019, 184, 110541. [Google Scholar] [CrossRef]
- Pei, Z.; Ding, L.; Hu, J.; Weng, S.; Zheng, Z.; Huang, M.; Liu, P. Defect and Its Dominance in ZnO Films: A New Insight into the Role of Defect over Photocatalytic Activity. Appl. Catal. B Environ. 2013, 142–143, 736–743. [Google Scholar] [CrossRef]
- Le, T.K.; Nguyen, T.L.; Hoang, C.N.; Nguyen, D.K.A.; Lund, T.; Nguyen, H.K.H.; Huynh, T.K.X. Formation of Surface Defects by Thermal Shock Method for the Improved Photocatalytic Activity of ZnO Nanoparticles. J. Asian Ceram. Soc. 2020, 8, 193–202. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, Z.; Xu, W.; Lu, S.; Yuan, F. First-Principles Study of Dopants and Defects in S-Doped ZnO and Its Effect on Photocatalytic Activity. Comput. Mater. Sci. 2012, 58, 119–124. [Google Scholar] [CrossRef]
- Danilenko, I.; Gorban, O.; Maksimchuk, P.; Viagin, O.; Malyukin, Y.; Gorban, S.; Volkova, G.; Glasunova, V.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; et al. Photocatalytic Activity of ZnO Nanopowders: The Role of Production Techniques in the Formation of Structural Defects. Catal. Today 2019, 328, 99–104. [Google Scholar] [CrossRef]
- He, L.; Tong, Z.; Wang, Z.; Chen, M.; Huang, N.; Zhang, W. Effects of Calcination Temperature and Heating Rate on the Photocatalytic Properties of ZnO Prepared by Pyrolysis. J. Colloid Interface Sci. 2018, 509, 448–456. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, S.; Kim, K.; Kim, C.; Jang, J.H.; Kim, Y.-M.; Lee, H. Multiscale Probing of the Influence of the Defect-Induced Variation of Oxygen Vacancies on the Photocatalytic Activity of Doped ZnO Nanoparticles. J. Mater. Chem. A 2020, 8, 25345–25354. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, M.; You, B.; Zhang, Q.; Yuan, H.; Ostrikov, K. Oxygen Vacancy-Mediated ZnO Nanoparticle Photocatalyst for Degradation of Methylene Blue. Appl. Sci. 2018, 8, 353. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen Vacancy Induced Band-Gap Narrowing and Enhanced Visible Light Photocatalytic Activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, Y.; Dong, Y.; Chen, R.; Xiang, L.; Komarneni, S. Defect-Rich ZnO Nanosheets of High Surface Area as an Efficient Visible-Light Photocatalyst. Appl. Catal. B Environ. 2016, 192, 8–16. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Zhao, H.; Wang, G.; Xiang, L.; Xu, J.; Komarneni, S. Oxygen Defects-Mediated Z-Scheme Charge Separation in g-C3N4/ZnO Photocatalysts for Enhanced Visible-Light Degradation of 4-Chlorophenol and Hydrogen Evolution. Appl. Catal. B Environ. 2017, 206, 406–416. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z.; Ren, T.; Ding, H.; Yao, W.; Zong, R.; Zhu, Y. Influence of Defects on the Photocatalytic Activity of ZnO. J. Phys. Chem. C 2014, 118, 15300–15307. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Dimitropoulos, M.; Govatsi, A.; Sygellou, L.; Tsakiroglou, C.D.; Yannopoulos, S.N. Influence of the Surface-to-Bulk Defects Ratio of ZnO and TiO2 on Their UV-Mediated Photocatalytic Activity. Appl. Catal. B Environ. 2017, 205, 292–301. [Google Scholar] [CrossRef]
- Qamar, M.T.; Aslam, M.; Ismail, I.M.I.; Salah, N.; Hameed, A. The Assessment of the Photocatalytic Activity of Magnetically Retrievable ZnO Coated γ-Fe2O3 in Sunlight Exposure. Chem. Eng. J. 2016, 283, 656–667. [Google Scholar] [CrossRef]
- Gomez-Solís, C.; Ballesteros, J.C.; Torres-Martínez, L.M.; Juárez-Ramírez, I.; Díaz Torres, L.A.; Elvira Zarazua-Morin, M.; Lee, S.W. Rapid Synthesis of ZnO Nano-Corncobs from Nital Solution and Its Application in the Photodegradation of Methyl Orange. J. Photochem. Photobiol. A Chem. 2015, 298, 49–54. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Comparison of Modification Strategies towards Enhanced Charge Carrier Separation and Photocatalytic Degradation Activity of Metal Oxide Semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Chakraborty, M.; Thangavel, R.; Asokan, K. N Doped ZnO and ZnO Nanorods Based P-n Homojunction Fabricated by Ion Implantation. AIP Conf. Proc. 2018, 1953, 50047. [Google Scholar] [CrossRef]
- Wang, M.; Ren, F.; Zhou, J.; Cai, G.; Cai, L.; Hu, Y.; Wang, D.; Liu, Y.; Guo, L.; Shen, S. N Doping to ZnO Nanorods for Photoelectrochemical Water Splitting under Visible Light: Engineered Impurity Distribution and Terraced Band Structure. Sci. Rep. 2015, 5, 12925. [Google Scholar] [CrossRef] [PubMed]
- Panda, N.R.; Acharya, B.S.; Nayak, P. Sonochemical Synthesis of Nitrogen Doped ZnO Nanorods: Effect of Anions on Growth and Optical Properties. J. Mater. Sci. Mater. Electron. 2013, 24, 4043–4049. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, L.-C.; Xie, Y.; Liu, G.; Ma, X.; Cheng, H.-M. Crystallinity-Dependent Substitutional Nitrogen Doping in ZnO and Its Improved Visible Light Photocatalytic Activity. J. Colloid Interface Sci. 2013, 400, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chen, G.; Li, C.; Yin, Y.; Jin, W.; Guo, L.; Ye, H.; Zhu, Y.; Wu, Y. Tailoring the Surface of ZnO Nanorods into Corrugated Nanorods via a Selective Chemical Etch Method. Nanotechnology 2016, 27, 295601. [Google Scholar] [CrossRef] [PubMed]
- Perillo, P.M.; Atia, M.N. C-Doped ZnO Nanorods for Photocatalytic Degradation of p-Aminobenzoic Acid under Sunlight. Nano-Struct. Nano-Objects 2017, 10, 125–130. [Google Scholar] [CrossRef]
- Ratheesh Kumar, P.M.; Sudha Kartha, C.; Vijayakumar, K.P.; Singh, F.; Avasthi, D.K. Effect of Fluorine Doping on Structural, Electrical and Optical Properties of ZnO Thin Films. Mater. Sci. Eng. B 2005, 117, 307–312. [Google Scholar] [CrossRef]
- El Hichou, A.; Bougrine, A.; Bubendorff, J.L.; Ebothé, J.; Addou, M.; Troyon, M. Structural, Optical and Cathodoluminescence Characteristics of Sprayed Undoped and Fluorine-Doped ZnO Thin Films. Semicond. Sci. Technol. 2002, 17, 607. [Google Scholar] [CrossRef]
- Mirzaeifard, Z.; Shariatinia, Z.; Jourshabani, M.; Rezaei Darvishi, S.M. ZnO Photocatalyst Revisited: Effective Photocatalytic Degradation of Emerging Contaminants Using S-Doped ZnO Nanoparticles under Visible Light Radiation. Ind. Eng. Chem. Res. 2020, 59, 15894–15911. [Google Scholar] [CrossRef]
- Khan, A.; Ahmed, M.I.; Adam, A.; Azad, A.-M.; Qamar, M. A Novel Fabrication Methodology for Sulfur-Doped ZnO Nanorods as an Active Photoanode for Improved Water Oxidation in Visible-Light Regime. Nanotechnology 2017, 28, 55602. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, J.; Peng, T. New Insight into the Enhanced Photocatalytic Activity of N-, C- and S-Doped ZnO Photocatalysts. Appl. Catal. B Environ. 2016, 181, 220–227. [Google Scholar] [CrossRef]
- Islam, S.E.; Hang, D.-R.; Chen, C.-H.; Chou, M.M.C.; Liang, C.-T.; Sharma, K.H. Rational Design of Hetero-Dimensional C-ZnO/MoS2 Nanocomposite Anchored on 3D Mesoporous Carbon Framework towards Synergistically Enhanced Stability and Efficient Visible-Light-Driven Photocatalytic Activity. Chemosphere 2021, 266, 129148. [Google Scholar] [CrossRef]
- Pan, L.; Muhammad, T.; Ma, L.; Huang, Z.-F.; Wang, S.; Wang, L.; Zou, J.-J.; Zhang, X. MOF-Derived C-Doped ZnO Prepared via a Two-Step Calcination for Efficient Photocatalysis. Appl. Catal. B Environ. 2016, 189, 181–191. [Google Scholar] [CrossRef]
- Rueda-Salaya, L.; Hernández-Ramírez, A.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Villanueva-Rodríguez, M.; Sánchez-Cervantes, E. Solar Photocatalytic Degradation of Diclofenac Aqueous Solution Using Fluorine Doped Zinc Oxide as Catalyst. J. Photochem. Photobiol. A Chem. 2020, 391, 112364. [Google Scholar] [CrossRef]
- Núñez-Salas, R.E.; Rodríguez-Chueca, J.; Hernández-Ramírez, A.; Rodríguez, E.; Maya-Treviño, M. de L. Evaluation of B-ZnO on Photocatalytic Inactivation of Escherichia Coli and Enterococcus Sp. J. Environ. Chem. Eng. 2021, 9, 104940. [Google Scholar] [CrossRef]
- Wang, D.; Jiao, S.; Zhang, S.; Li, H.; Gao, S.; Wang, J.; Ren, J.; Yu, Q.J.; Zhang, Y. Growth Mechanism and Optical Properties of Phosphorus-Doped ZnO Nanorods Synthesized by Hydrothermal Method. Phys. Status Solidi 2017, 214, 1600959. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Abdullah, C.A.C.; Chung, E.L.T.; Andou, Y. Recent Progress in Visible Light-Doped ZnO Photocatalyst for Pollution Control. Int. J. Environ. Sci. Technol. 2023, 20, 5753–5772. [Google Scholar] [CrossRef]
- Palmisano, L.; Augugliaro, V.; Sclafani, A.; Schiavello, M. Activity of Chromium-Ion-Doped Titania for the Dinitrogen Photoreduction to Ammonia and for the Phenol Photodegradation. J. Phys. Chem. 1988, 92, 6710–6713. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Shen, J.; Liu, H.; Li, J.; Wang, A.; Hui, A.; Munir, H.A. Superoxide Anion: Critical Source of High Performance Antibacterial Activity in Co-Doped ZnO QDs. Ceram. Int. 2020, 46, 15822–15830. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, J.; Xiao, C.; Tan, X. Photocatalytic Decolorization of Methylene Blue over Zn1−xCoxO under Visible Light Irradiation. Mater. Sci. Eng. B 2007, 142, 121–125. [Google Scholar] [CrossRef]
- Wu, C.; Shen, L.; Yu, H.; Zhang, Y.-C.; Huang, Q. Solvothermal Synthesis of Cu-Doped ZnO Nanowires with Visible Light-Driven Photocatalytic Activity. Mater. Lett. 2012, 74, 236–238. [Google Scholar] [CrossRef]
- Shah, A.A.; Bhatti, M.A.; Tahira, A.; Chandio, A.D.; Channa, I.A.; Sahito, A.G.; Chalangar, E.; Willander, M.; Nur, O.; Ibupoto, Z.H. Facile Synthesis of Copper Doped ZnO Nanorods for the Efficient Photo Degradation of Methylene Blue and Methyl Orange. Ceram. Int. 2020, 46, 9997–10005. [Google Scholar] [CrossRef]
- Pawar, R.C.; Choi, D.-H.; Lee, J.-S.; Lee, C.S. Formation of Polar Surfaces in Microstructured ZnO by Doping with Cu and Applications in Photocatalysis Using Visible Light. Mater. Chem. Phys. 2015, 151, 167–180. [Google Scholar] [CrossRef]
- Mohan, R.; Krishnamoorthy, K.; Kim, S.-J. Enhanced Photocatalytic Activity of Cu-Doped ZnO Nanorods. Solid State Commun. 2012, 152, 375–380. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Fan, W. Structural, Electronic, and Optical Properties of Ag-Doped ZnO Nanowires: First Principles Study. J. Phys. Chem. C 2011, 115, 3552–3557. [Google Scholar] [CrossRef]
- Chauhan, A.; Verma, R.; Kumari, S.; Sharma, A.; Shandilya, P.; Li, X.; Batoo, K.M.; Imran, A.; Kulshrestha, S.; Kumar, R. Photocatalytic Dye Degradation and Antimicrobial Activities of Pure and Ag-Doped ZnO Using Cannabis Sativa Leaf Extract. Sci. Rep. 2020, 10, 7881. [Google Scholar] [CrossRef]
- Martin, N.C.; Capochichi-Gnambodoe, M.; Le Pivert, M.; Leprince-Wang, Y. A Comparative Study on the Photocatalytic Efficiency of ZnO Nanowires Doped by Different Transition Metals. Acta Phys. Pol. A 2019, 135, 471–474. [Google Scholar] [CrossRef]
- Adam, R.E.; Alnoor, H.; Pozina, G.; Liu, X.; Willander, M.; Nur, O. Synthesis of Mg-Doped ZnO NPs via a Chemical Low-Temperature Method and Investigation of the Efficient Photocatalytic Activity for the Degradation of Dyes under Solar Light. Solid State Sci. 2020, 99, 106053. [Google Scholar] [CrossRef]
- Kadam, S.A.; Thomas, S.A.; Ma, Y.-R.; Maria Jose, L.; Sajan, D.; Aravind, A. Investigation of Adsorption and Photocatalytic Behavior of Manganese Doped Zinc Oxide Nanostructures. Inorg. Chem. Commun. 2021, 134, 108981. [Google Scholar] [CrossRef]
- Guo, J.; Wang, S.; Lin, Z.; Liu, L.; Hui, Y. Ultrasensitive Acetone Sensor Based on Holey Zinc Oxide Nanosheets Doped by Gold Nanoparticles. Mater. Lett. 2021, 302, 130443. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Nguyen, L.T.H.; Duong, A.T.T.; Nguyen, B.D.; Quang Hai, N.; Chu, V.H.; Nguyen, T.D.; Bach, L.G. Preparation, Characterization and Photocatalytic Activity of La-Doped Zinc Oxide Nanoparticles. Materials 2019, 12, 1195. [Google Scholar] [CrossRef]
- Shakir, M.; Faraz, M.; Sherwani, M.A.; Al-Resayes, S.I. Photocatalytic Degradation of the Paracetamol Drug Using Lanthanum Doped ZnO Nanoparticles and Their In-Vitro Cytotoxicity Assay. J. Lumin. 2016, 176, 159–167. [Google Scholar] [CrossRef]
- Shivananjaiah, H.N.; Sailaja Kumari, K.; Geetha, M.S. Green Mediated Synthesis of Lanthanum Doped Zinc Oxide: Study of Its Structural, Optical and Latent Fingerprint Application. J. Rare Earths 2020, 38, 1281–1287. [Google Scholar] [CrossRef]
- Thi, V.H.-T.; Lee, B.-K. Effective Photocatalytic Degradation of Paracetamol Using La-Doped ZnO Photocatalyst under Visible Light Irradiation. Mater. Res. Bull. 2017, 96, 171–182. [Google Scholar] [CrossRef]
- Jia, T.; Wang, W.; Long, F.; Fu, Z.; Wang, H.; Zhang, Q. Fabrication, Characterization and Photocatalytic Activity of La-Doped ZnO Nanowires. J. Alloys Compd. 2009, 484, 410–415. [Google Scholar] [CrossRef]
- Kumar, M.; Negi, K.; Umar, A.; Chauhan, M.S. Photocatalytic and Fluorescent Chemical Sensing Applications of La-Doped ZnO Nanoparticles. Chem. Pap. 2021, 75, 1555–1566. [Google Scholar] [CrossRef]
- Hernández-Carrillo, M.A.; Torres-Ricárdez, R.; García-Mendoza, M.F.; Ramírez-Morales, E.; Rojas-Blanco, L.; Díaz-Flores, L.L.; Sepúlveda-Palacios, G.E.; Paraguay-Delgado, F.; Pérez-Hernández, G. Eu-Modified ZnO Nanoparticles for Applications in Photocatalysis. Catal. Today 2020, 349, 191–197. [Google Scholar] [CrossRef]
- Dash, D.; Panda, N.R.; Sahu, D. Photoluminescence and Photocatalytic Properties of Europium Doped ZnO Nanoparticles. Appl. Surf. Sci. 2019, 494, 666–674. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; De Marco, I.; Sannino, D.; Vaiano, V. Photocatalytic Degradation of Eriochrome Black-T Azo Dye Using Eu-Doped ZnO Prepared by Supercritical Antisolvent Precipitation Route: A Preliminary Investigation. Top. Catal. 2020, 63, 1193–1205. [Google Scholar] [CrossRef]
- Alam, U.; Khan, A.; Ali, D.; Bahnemann, D.; Muneer, M. Comparative Photocatalytic Activity of Sol–Gel Derived Rare Earth Metal (La, Nd, Sm and Dy)-Doped ZnO Photocatalysts for Degradation of Dyes. RSC Adv. 2018, 8, 17582–17594. [Google Scholar] [CrossRef]
- Sordello, F.; Berruti, I.; Gionco, C.; Paganini, M.C.; Calza, P.; Minero, C. Photocatalytic Performances of Rare Earth Element-Doped Zinc Oxide toward Pollutant Abatement in Water and Wastewater. Appl. Catal. B Environ. 2019, 245, 159–166. [Google Scholar] [CrossRef]
- Ujjan, Z.A.; Bhatti, M.A.; Shah, A.A.; Tahira, A.; Shaikh, N.M.; Kumar, S.; Mugheri, A.Q.; Medany, S.S.; Nafady, A.; Alnjiman, F.; et al. Simultaneous Doping of Sulfur and Chloride Ions into ZnO Nanorods for Improved Photocatalytic Properties towards Degradation of Methylene Blue. Ceram. Int. 2022, 48, 5535–5545. [Google Scholar] [CrossRef]
- Iqbal, J.; Liu, X.; Zhu, H.; Wu, Z.B.; Zhang, Y.; Yu, D.; Yu, R. Raman and Highly Ultraviolet Red-Shifted near Band-Edge Properties of LaCe-Co-Doped ZnO Nanoparticles. Acta Mater. 2009, 57, 4790–4796. [Google Scholar] [CrossRef]
- Hannachi, E.; Slimani, Y.; Nawaz, M.; Trabelsi, Z.; Yasin, G.; Bilal, M.; Almessiere, M.A.; Baykal, A.; Thakur, A.; Thakur, P. Synthesis, Characterization, and Evaluation of the Photocatalytic Properties of Zinc Oxide Co-Doped with Lanthanides Elements. J. Phys. Chem. Solids 2022, 170, 110910. [Google Scholar] [CrossRef]
- Mou, H.; Song, C.; Zhou, Y.; Zhang, B.; Wang, D. Design and Synthesis of Porous Ag/ZnO Nanosheets Assemblies as Super Photocatalysts for Enhanced Visible-Light Degradation of 4-Nitrophenol and Hydrogen Evolution. Appl. Catal. B Environ. 2018, 221, 565–573. [Google Scholar] [CrossRef]
- Muñoz-Fernandez, L.; Sierra-Fernandez, A.; Flores-Carrasco, G.; Milošević, O.; Rabanal, M.E. Solvothermal Synthesis of Ag/ZnO Micro/Nanostructures with Different Precursors for Advanced Photocatalytic Applications. Adv. Powder Technol. 2017, 28, 83–92. [Google Scholar] [CrossRef]
- Faiz, M.B.; Amal, R.; Marquis, C.P.; Harry, E.J.; Sotiriou, G.A.; Rice, S.A.; Gunawan, C. Nanosilver and the Microbiological Activity of the Particulate Solids versus the Leached Soluble Silver. Nanotoxicology 2018, 12, 263–273. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Saranya, K.; Pandikumar, A.; Lin, K.-C. Palladium and Silver Nanoparticles Embedded on Zinc Oxide Nanostars for Photocatalytic Degradation of Pesticides and Herbicides. Chem. Eng. J. 2021, 410, 128434. [Google Scholar] [CrossRef]
- Chang, Y.; Xu, J.; Zhang, Y.; Ma, S.; Xin, L.; Zhu, L.; Xu, C. Optical Properties and Photocatalytic Performances of Pd Modified ZnO Samples. J. Phys. Chem. C 2009, 113, 18761–18767. [Google Scholar] [CrossRef]
- Kim, K.-J.; Kreider, P.B.; Chang, C.-H.; Park, C.-M.; Ahn, H.-G. Visible-Light-Sensitive Nanoscale Au-ZnO Photocatalysts. J. Nanopart. Res. 2013, 15, 1606. [Google Scholar] [CrossRef]
- Chen, Z.H.; Tang, Y.B.; Liu, C.P.; Leung, Y.H.; Yuan, G.D.; Chen, L.M.; Wang, Y.Q.; Bello, I.; Zapien, J.A.; Zhang, W.J.; et al. Vertically Aligned ZnO Nanorod Arrays Sentisized with Gold Nanoparticles for Schottky Barrier Photovoltaic Cells. J. Phys. Chem. C 2009, 113, 13433–13437. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, H.J.; Koutavarapu, R.; Lee, S.H.; Arumugam, M.; Kim, J.H.; Choi, M.Y. ZnO Supported Au/Pd Bimetallic Nanocomposites for Plasmon Improved Photocatalytic Activity for Methylene Blue Degradation under Visible Light Irradiation. Appl. Surf. Sci. 2019, 496, 143665. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, X.; Wang, Z.; Liu, Y.; Zheng, Z.; Qin, X.; Zhang, X.; Wang, P.; Huang, B. ZnO Nanorod Decorated by Au-Ag Alloy with Greatly Increased Activity for Photocatalytic Ethylene Oxidation. Chin. J. Catal. 2020, 41, 1613–1621. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chiang, Y.-J. Preparation of Coupled ZnO/SnO2 Photocatalysts Using a Rotating Packed Bed. Chem. Eng. J. 2012, 181–182, 196–205. [Google Scholar] [CrossRef]
- Chiang, Y.-J.; Lin, C.-C. Photocatalytic Decolorization of Methylene Blue in Aqueous Solutions Using Coupled ZnO/SnO2 Photocatalysts. Powder Technol. 2013, 246, 137–143. [Google Scholar] [CrossRef]
- Yang, G.; Yan, Z.; Xiao, T. Preparation and Characterization of SnO2/ZnO/TiO2 Composite Semiconductor with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2012, 258, 8704–8712. [Google Scholar] [CrossRef]
- Hosseinzadeh, G.; Zinatloo-Ajabshir, S.; Yousefi, A. Innovative Synthesis of a Novel ZnO/ZnBi2O4/Graphene Ternary Heterojunction Nanocomposite Photocatalyst in the Presence of Tragacanth Mucilage as Natural Surfactant. Ceram. Int. 2022, 48, 6078–6086. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Xu, B.-Q.; Zhao, J.; Mai, B.; Peng, P.; Sheng, G.; Fu, J. Enhanced Photocatalytic Performance of Nanosized Coupled ZnO/SnO2 Photocatalysts for Methyl Orange Degradation. J. Photochem. Photobiol. A Chem. 2004, 168, 47–52. [Google Scholar] [CrossRef]
- Shaveisi, Y.; Sharifnia, S.; Karamian, E. Application of Mixture Experimental Design for Photocatalytic Ammonia Degradation by Sunlight-Driven WO3-Ag3PO4-ZnO Ternary Photocatalysts. Int. J. Energy Res. 2019, 43, 4879–4897. [Google Scholar] [CrossRef]
- Tau, O.; Lovergine, N.; Prete, P. Adsorption and Decomposition Steps on Cu(111) of Liquid Aromatic Hydrocarbon Precursors for Low-Temperature CVD of Graphene: A DFT Study. Carbon 2023, 206, 142–149. [Google Scholar] [CrossRef]
- Paul, R.; Wang, M.; Roy, A. Transparent Graphene/BN-Graphene Stacked Nanofilms for Electrocatalytic Oxygen Evolution. ACS Appl. Nano Mater. 2020, 3, 10418–10426. [Google Scholar] [CrossRef]
- Fung, C.-M.; Tang, J.-Y.; Tan, L.-L.; Mohamed, A.R.; Chai, S.-P. Recent Progress in Two-Dimensional Nanomaterials for Photocatalytic Carbon Dioxide Transformation into Solar Fuels. Mater. Today Sustain. 2020, 9, 100037. [Google Scholar] [CrossRef]
- Liu, J.; Bai, H.; Wang, Y.; Liu, Z.; Zhang, X.; Sun, D.D. Self-Assembling TiO2 Nanorods on Large Graphene Oxide Sheets at a Two-Phase Interface and Their Anti-Recombination in Photocatalytic Applications. Adv. Funct. Mater. 2010, 20, 4175–4181. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Wang, P.; Xing, L.; Fang, Y.; Zhai, Y.; Dong, S. One-Pot, Water-Phase Approach to High-Quality Graphene/TiO2 Composite Nanosheets. Chem. Commun. 2010, 46, 7148–7150. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Joshi, B.N.; Lee, M.W.; Kim, N.Y.; Yoon, S.S. Electrospun Graphene-ZnO Nanofiber Mats for Photocatalysis Applications. Appl. Surf. Sci. 2014, 294, 24–28. [Google Scholar] [CrossRef]
- Weng, B.; Yang, M.-Q.; Zhang, N.; Xu, Y.-J. Toward the Enhanced Photoactivity and Photostability of ZnO Nanospheres via Intimate Surface Coating with Reduced Graphene Oxide. J. Mater. Chem. A 2014, 2, 9380–9389. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Baynosa, M.L.; Nguyen, V.H.; Tuma, D.; Lee, Y.R.; Shim, J.-J. Solvent-Driven Morphology-Controlled Synthesis of Highly Efficient Long-Life ZnO/Graphene Nanocomposite Photocatalysts for the Practical Degradation of Organic Wastewater under Solar Light. Appl. Surf. Sci. 2019, 486, 37–51. [Google Scholar] [CrossRef]
- Tayebi, M.; Tayyebi, A.; Masoumi, Z.; Lee, B.-K. Photocorrosion Suppression and Photoelectrochemical (PEC) Enhancement of ZnO via Hybridization with Graphene Nanosheets. Appl. Surf. Sci. 2020, 502, 144189. [Google Scholar] [CrossRef]
- Mohamed, H.H. Sonochemical Synthesis of ZnO Hollow Microstructure/Reduced Graphene Oxide for Enhanced Sunlight Photocatalytic Degradation of Organic Pollutants. J. Photochem. Photobiol. A Chem. 2018, 353, 401–408. [Google Scholar] [CrossRef]
- Nenavathu, B.P.; Kandula, S.; Verma, S. Visible-Light-Driven Photocatalytic Degradation of Safranin-T Dye Using Functionalized Graphene Oxide Nanosheet (FGS)/ZnO Nanocomposites. RSC Adv. 2018, 8, 19659–19667. [Google Scholar] [CrossRef]
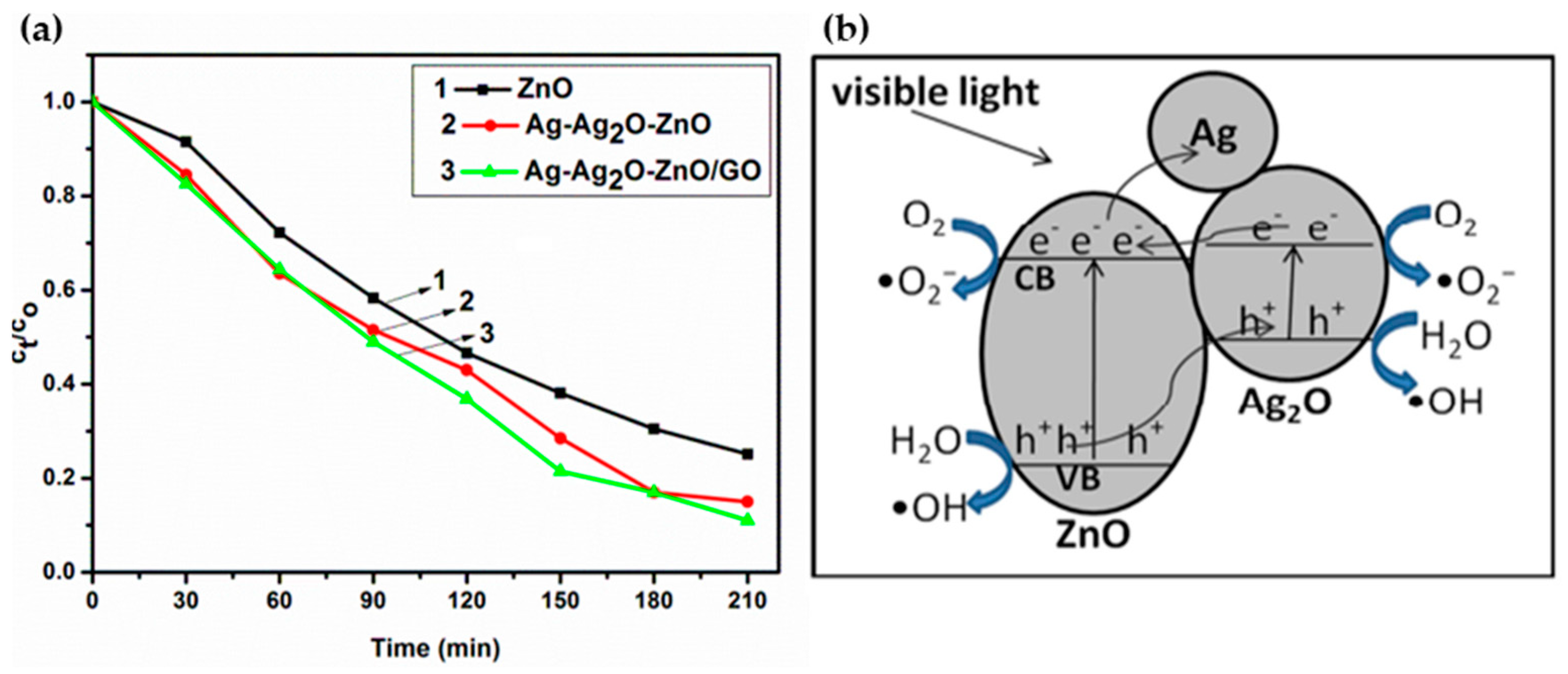
- Umukoro, E.H.; Peleyeju, M.G.; Ngila, J.C.; Arotiba, O.A. Photocatalytic Degradation of Acid Blue 74 in Water Using Ag–Ag2O–Zno Nanostuctures Anchored on Graphene Oxide. Solid State Sci. 2016, 51, 66–73. [Google Scholar] [CrossRef]
- Geetha, D.; Nagarajan, E.R. Chapter 3—Impact and Issues of Organic Pollutants. In Management of Contaminants of Emerging Concern (CEC) in Environment; Singh, P., Hussain, C.M., Rajkhowa, S.B.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 93–126. ISBN 978-0-12-822263-8. [Google Scholar]
- Mohamed Isa, E.D.; Che Jusoh, N.W.; Hazan, R.; Shameli, K. Photocatalytic Degradation of Methyl Orange Using Pullulan-Mediated Porous Zinc Oxide Microflowers. Environ. Sci. Pollut. Res. 2021, 28, 5774–5785. [Google Scholar] [CrossRef] [PubMed]
- Inderyas, A.; Bhatti, I.A.; Ashar, A.; Ashraf, M.; Ghani, A.; Yousaf, M.; Mohsin, M.; Ahmad, M.; Rafique, S.; Masood, N.; et al. Synthesis of Immobilized ZnO over Polyurethane and Photocatalytic Activity Evaluation for the Degradation of Azo Dye under UV and Solar Light Irardiation. Mater. Res. Express 2020, 7, 25033. [Google Scholar] [CrossRef]
- Niranjani, R.; AnchanaDevi, C. Photocatalytic Degradation of Pesticide Phorate Using Zinc Oxide Nanoparticles. Int. J. Acad. Res. Dev. 2017, 2, 35–40. [Google Scholar]
- Khan, S.H.; Pathak, B.; Fulekar, M.H. Synthesis, Characterization and Photocatalytic Degradation of Chlorpyrifos by Novel Fe: ZnO Nanocomposite Material. Nanotechnol. Environ. Eng. 2018, 3, 13. [Google Scholar] [CrossRef]
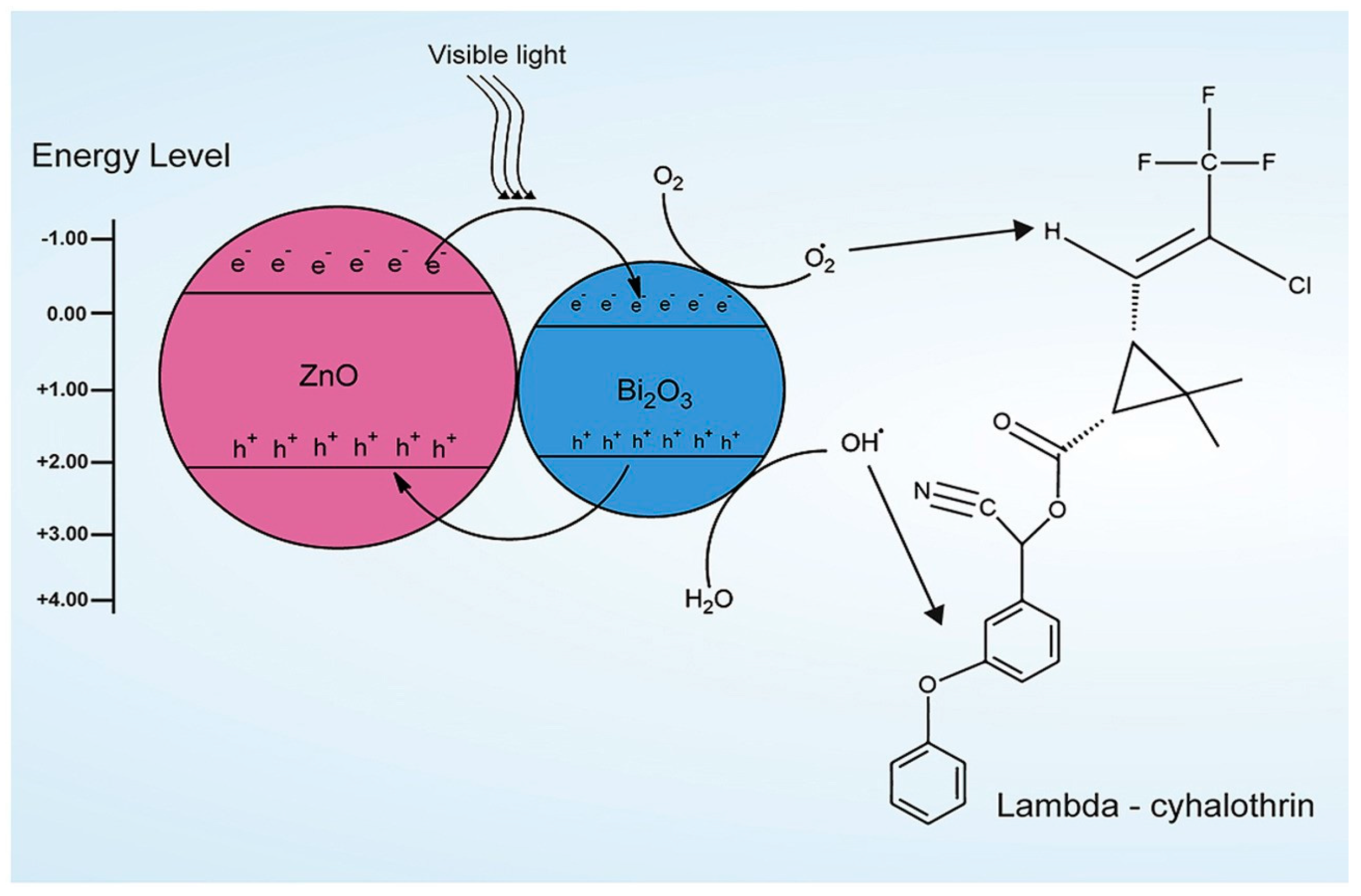
- Premalatha, N.; Rose Miranda, L. Surfactant Modified ZnO–Bi2O3 Nanocomposite for Degradation of Lambda- Cyhalothrin Pesticide in Visible Light: A Study of Reaction Kinetics and Intermediates. J. Environ. Manag. 2019, 246, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kumar, N.; Kumari, V.; Mittal, A.; Sharma, S. Photocatalytic Degradation of Triclopyr, a Persistent Pesticide by ZnO/SnO2 Nano-Composities. Mater. Today Proc. 2019, 19, 642–645. [Google Scholar] [CrossRef]
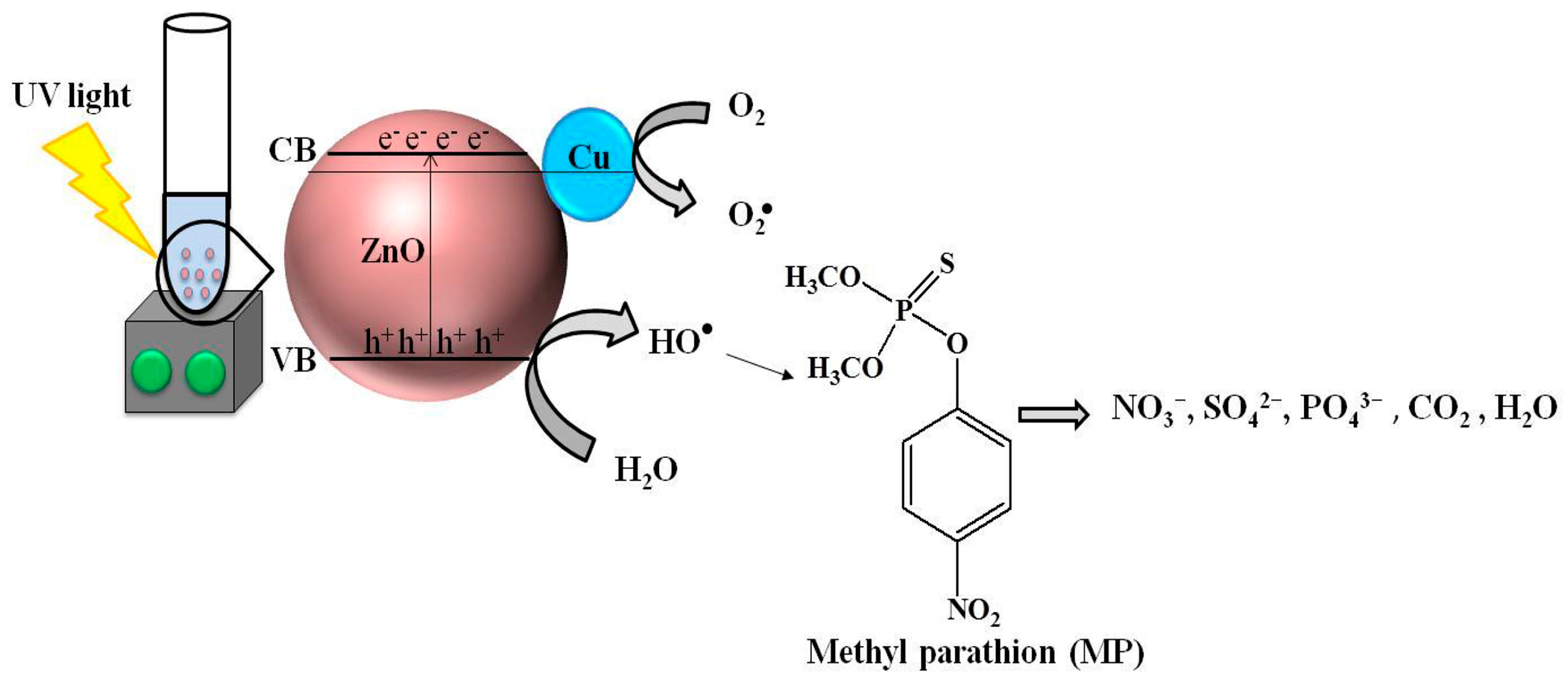
- Aulakh, M.K.; Kaur, S.; Pal, B.; Singh, S. Morphological Influence of ZnO Nanostructures and Their Cu Loaded Composites for Effective Photodegradation of Methyl Parathion. Solid State Sci. 2020, 99, 106045. [Google Scholar] [CrossRef]
- Korake, P.V.; Sridharkrishna, R.; Hankare, P.P.; Garadkar, K.M. Photocatalytic Degradation of Phosphamidon Using Ag-Doped ZnO Nanorods. Toxicol. Environ. Chem. 2012, 94, 1075–1085. [Google Scholar] [CrossRef]
- Devipriya, S.; Yesodharan, S. Photocatalytic Degradation of Pesticide Contaminants in Water. Sol. Energy Mater. Sol. Cells 2005, 86, 309–348. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc Oxide Based Photocatalytic Degradation of Persistent Pesticides: A Comprehensive Review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of Pharmaceuticals and Endocrine Disrupting Compounds from Water by Zinc Oxide-Based Photocatalytic Degradation: A Review. Sustain. Cities Soc. 2016, 27, 407–418. [Google Scholar] [CrossRef]
- Majumder, S.; Chatterjee, S.; Basnet, P.; Mukherjee, J. ZnO Based Nanomaterials for Photocatalytic Degradation of Aqueous Pharmaceutical Waste Solutions—A Contemporary Review. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100386. [Google Scholar] [CrossRef]
- Eskandari, M.; Goudarzi, N.; Moussavi, S.G. Application of Low-Voltage UVC Light and Synthetic ZnO Nanoparticles to Photocatalytic Degradation of Ciprofloxacin in Aqueous Sample Solutions. Water Environ. J. 2018, 32, 58–66. [Google Scholar] [CrossRef]
- Malakootian, M.; Mahdizadeh, H.; Dehdarirad, A.; Amiri Gharghani, M. Photocatalytic Ozonation Degradation of Ciprofloxacin Using ZnO Nanoparticles Immobilized on the Surface of Stones. J. Dispers. Sci. Technol. 2019, 40, 846–854. [Google Scholar] [CrossRef]
- Kaur, A.; Gupta, G.; Ibhadon, A.O.; Salunke, D.B.; Sinha, A.S.K.; Kansal, S.K. A Facile Synthesis of Silver Modified ZnO Nanoplates for Efficient Removal of Ofloxacin Drug in Aqueous Phase under Solar Irradiation. J. Environ. Chem. Eng. 2018, 6, 3621–3630. [Google Scholar] [CrossRef]
- Choina, J.; Bagabas, A.; Fischer, C.; Flechsig, G.-U.; Kosslick, H.; Alshammari, A.; Schulz, A. The Influence of the Textural Properties of ZnO Nanoparticles on Adsorption and Photocatalytic Remediation of Water from Pharmaceuticals. Catal. Today 2015, 241, 47–54. [Google Scholar] [CrossRef]
- Ranjith Kumar, D.; Ranjith, K.S.; Haldorai, Y.; Kandasami, A.; Rajendra Kumar, R.T. Nitrogen-Implanted ZnO Nanorod Arrays for Visible Light Photocatalytic Degradation of a Pharmaceutical Drug Acetaminophen. ACS Omega 2019, 4, 11973–11979. [Google Scholar] [CrossRef]
- Lin, C.-F.; Shiu, Y.-J.; Kuo, C.-S.; Lin, A.Y.-C.; Wu, C.-H.; Hong, P.-K.A. Photocatalytic Degradation of Morphine, Methamphetamine, and Ketamine by Illuminated TiO2 and ZnO. React. Kinet. Mech. Catal. 2013, 110, 559–574. [Google Scholar] [CrossRef]
- Elhalil, A.; Elmoubarki, R.; Farnane, M.; Machrouhi, A.; Sadiq, M.; Mahjoubi, F.Z.; Qourzal, S.; Barka, N. Photocatalytic Degradation of Caffeine as a Model Pharmaceutical Pollutant on Mg Doped ZnO-Al2O3 Heterostructure. Environ. Nanotechnol. Monit. Manag. 2018, 10, 63–72. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Ray, S.S.; Onyango, M.S.; Momba, M.N.B. Preparation and Antibacterial Activity of Chitosan-Based Nanocomposites Containing Bentonite-Supported Silver and Zinc Oxide Nanoparticles for Water Disinfection. Appl. Clay Sci. 2015, 114, 330–339. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.-S.; Mendoza, R.M.O.; Lu, M.-C. Zinc Oxide Nanoparticles for Water Disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced Bioactivity of ZnO Nanoparticles-an Antimicrobial Study. Sci. Technol. Adv. Mater. 2008, 9, 35004. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Kasemets, K.; Ivask, A.; Dubourguier, H.-C.; Kahru, A. Toxicity of Nanoparticles of ZnO, CuO and TiO2 to Yeast Saccharomyces Cerevisiae. Toxicol. Vitr. 2009, 23, 1116–1122. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of Nanosized and Bulk ZnO, CuO and TiO2 to Bacteria Vibrio Fischeri and Crustaceans Daphnia Magna and Thamnocephalus Platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological Impact Studies Based on Escherichia Coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Navale, G.; Late, D. Antimicrobial Activity of ZnO Nanoparticles against Pathogenic Bacteria and Fungi. JSM Nanotechnol. Nanomed. 2015, 3, 1033. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative Eco-Toxicity of Nanoscale TiO2, SiO2, and ZnO Water Suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial Activity of ZnO Nanoparticle Suspensions on a Broad Spectrum of Microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Gondal, M.A.; Dastageer, M.A.; Khalil, A.; Hayat, K.; Yamani, Z.H. Nanostructured ZnO Synthesis and Its Application for Effective Disinfection of Escherichia Coli Micro Organism in Water. J. Nanopart. Res. 2011, 13, 3423–3430. [Google Scholar] [CrossRef]
- Narayanan, P.M.; Wilson, W.S.; Abraham, A.T.; Sevanan, M. Synthesis, Characterization, and Antimicrobial Activity of Zinc Oxide Nanoparticles Against Human Pathogens. Bionanoscience 2012, 2, 329–335. [Google Scholar] [CrossRef]
- Tayel, A.A.; El-Tras, W.F.; Moussa, S.; El-baz, A.F.; Mahrous, H.; Salem, M.F.; Brimer, L. Antibacterial Action of Zinc Oxide Nanoparticles against Foodborne Pathogens. J. Food Saf. 2011, 31, 211–218. [Google Scholar] [CrossRef]
- Barnes, R.J.; Molina, R.; Xu, J.; Dobson, P.J.; Thompson, I.P. Comparison of TiO2 and ZnO Nanoparticles for Photocatalytic Degradation of Methylene Blue and the Correlated Inactivation of Gram-Positive and Gram-Negative Bacteria. J. Nanopart. Res. 2013, 15, 1432. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal Activity of Zinc Oxide Nanoparticles against Botrytis Cinerea and Penicillium Expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of Commercial Metal Oxide Nanoparticles in Water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef]
- Tso, C.; Zhung, C.; Shih, Y.; Tseng, Y.-M.; Wu, S.; Doong, R. Stability of Metal Oxide Nanoparticles in Aqueous Solutions. Water Sci. Technol. 2010, 61, 127–133. [Google Scholar] [CrossRef]
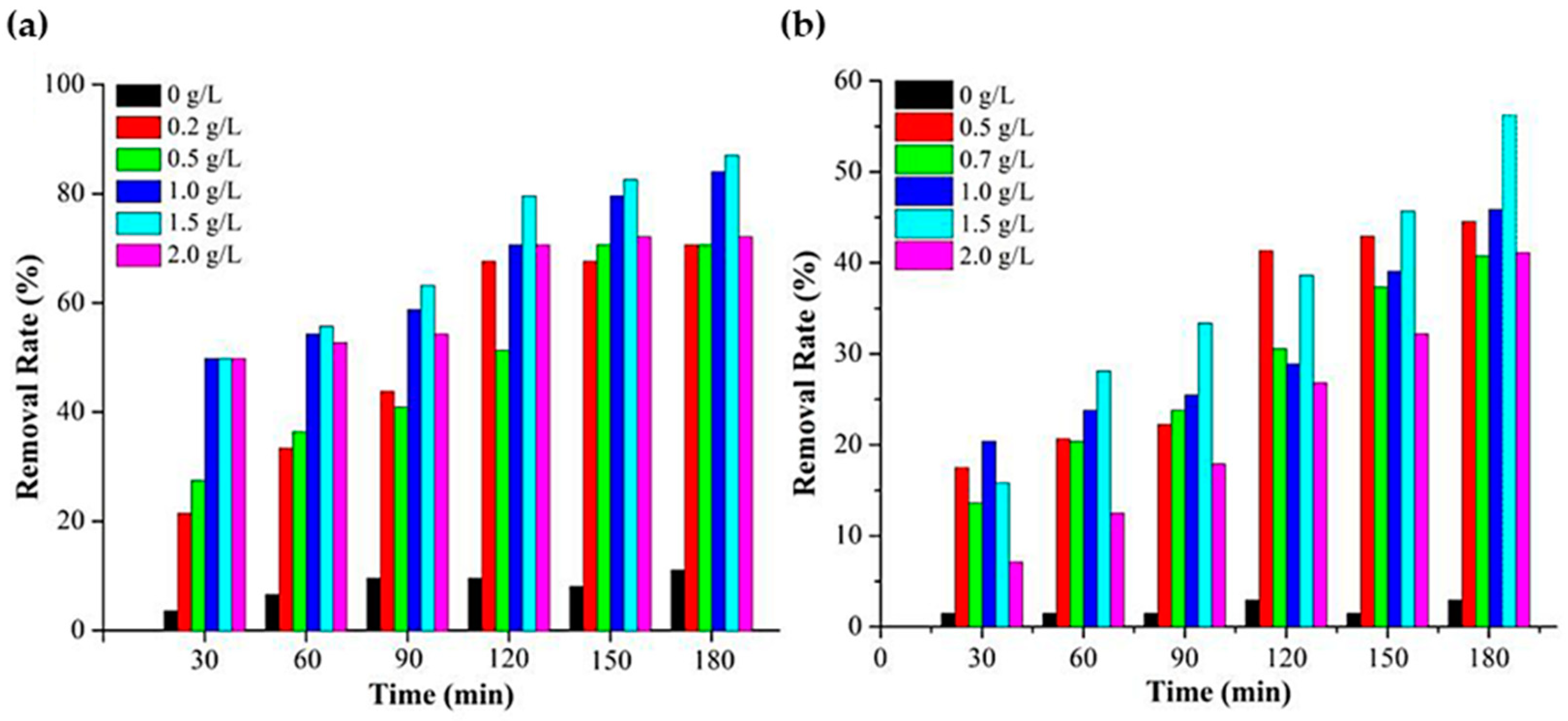
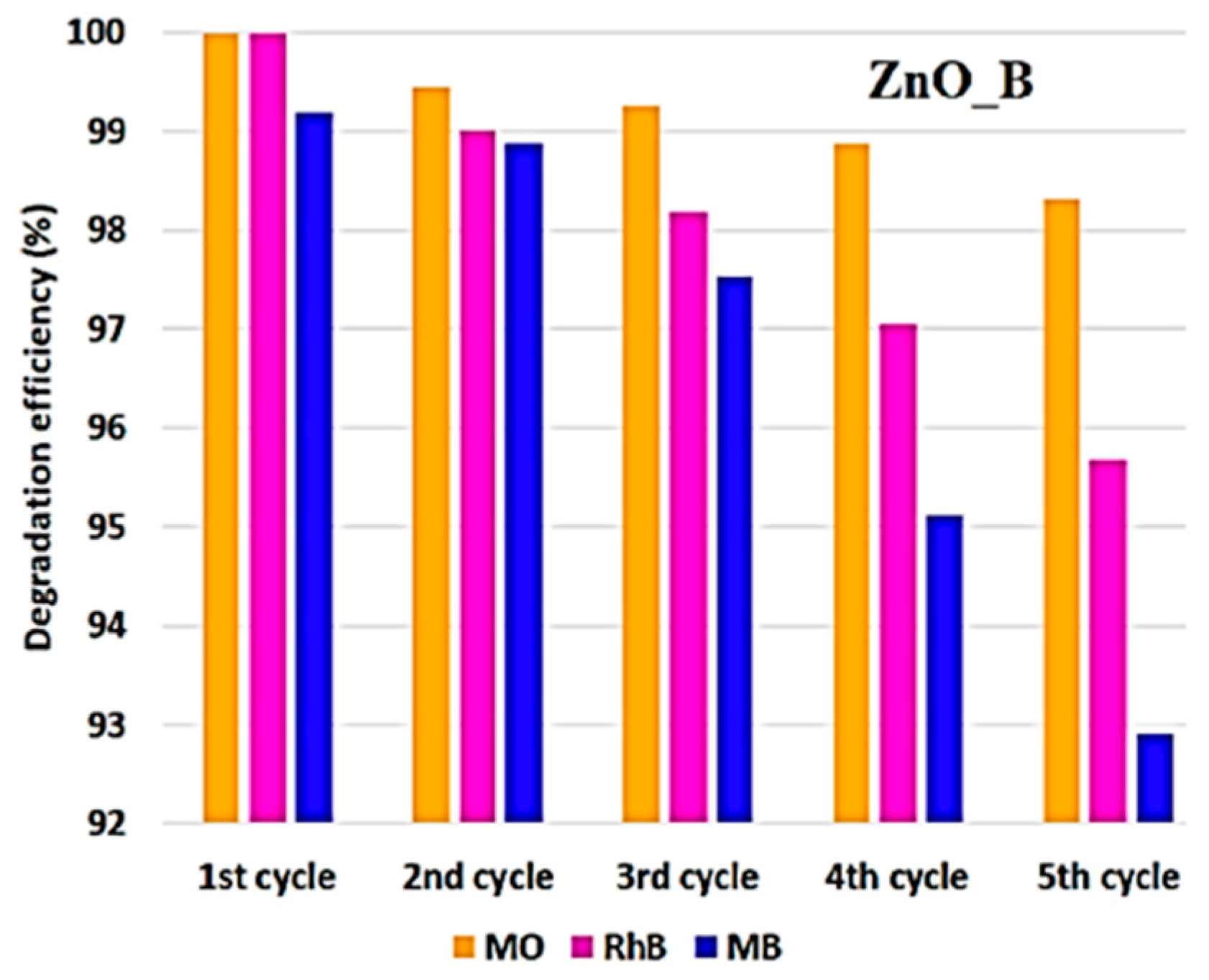
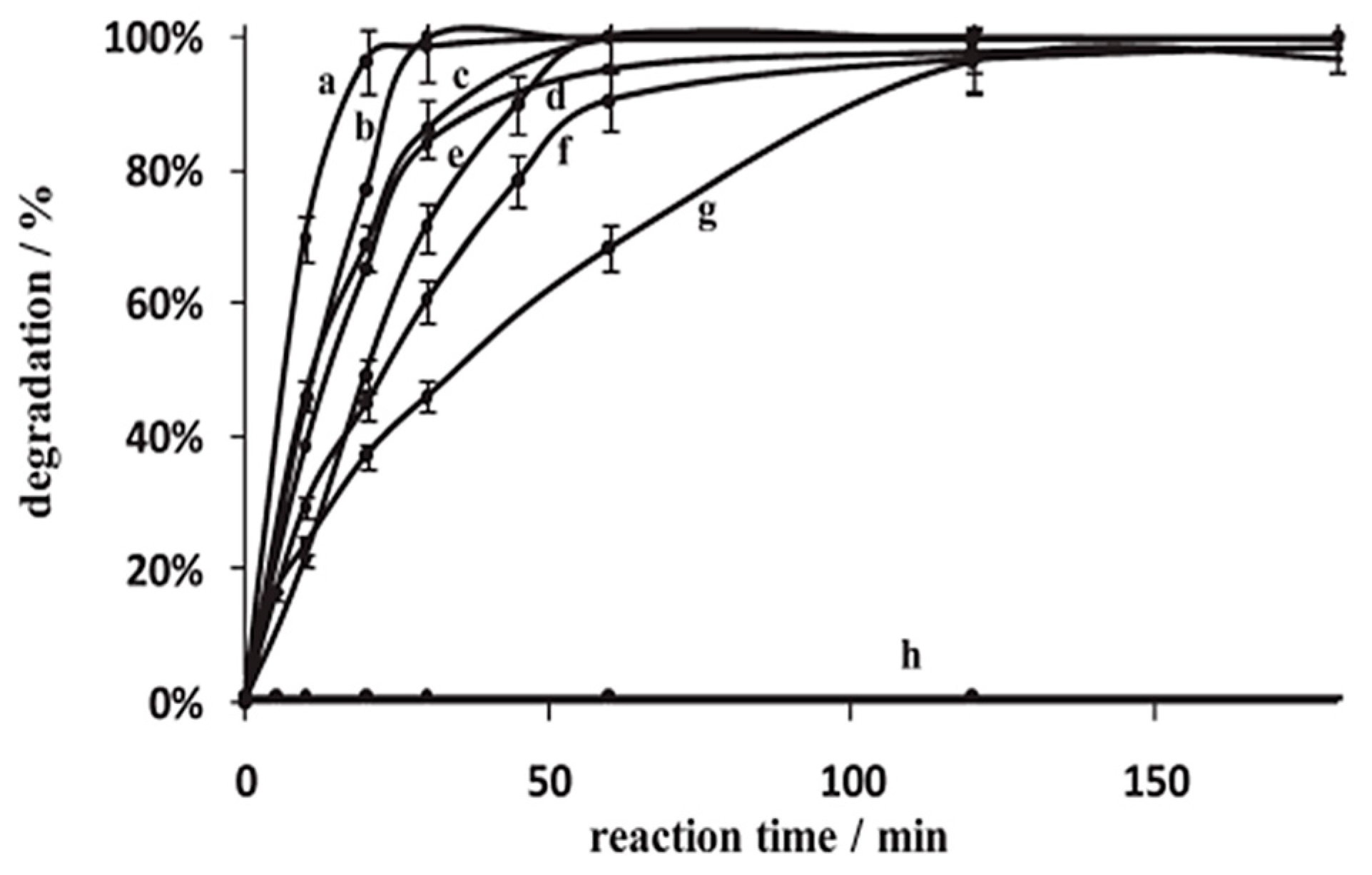
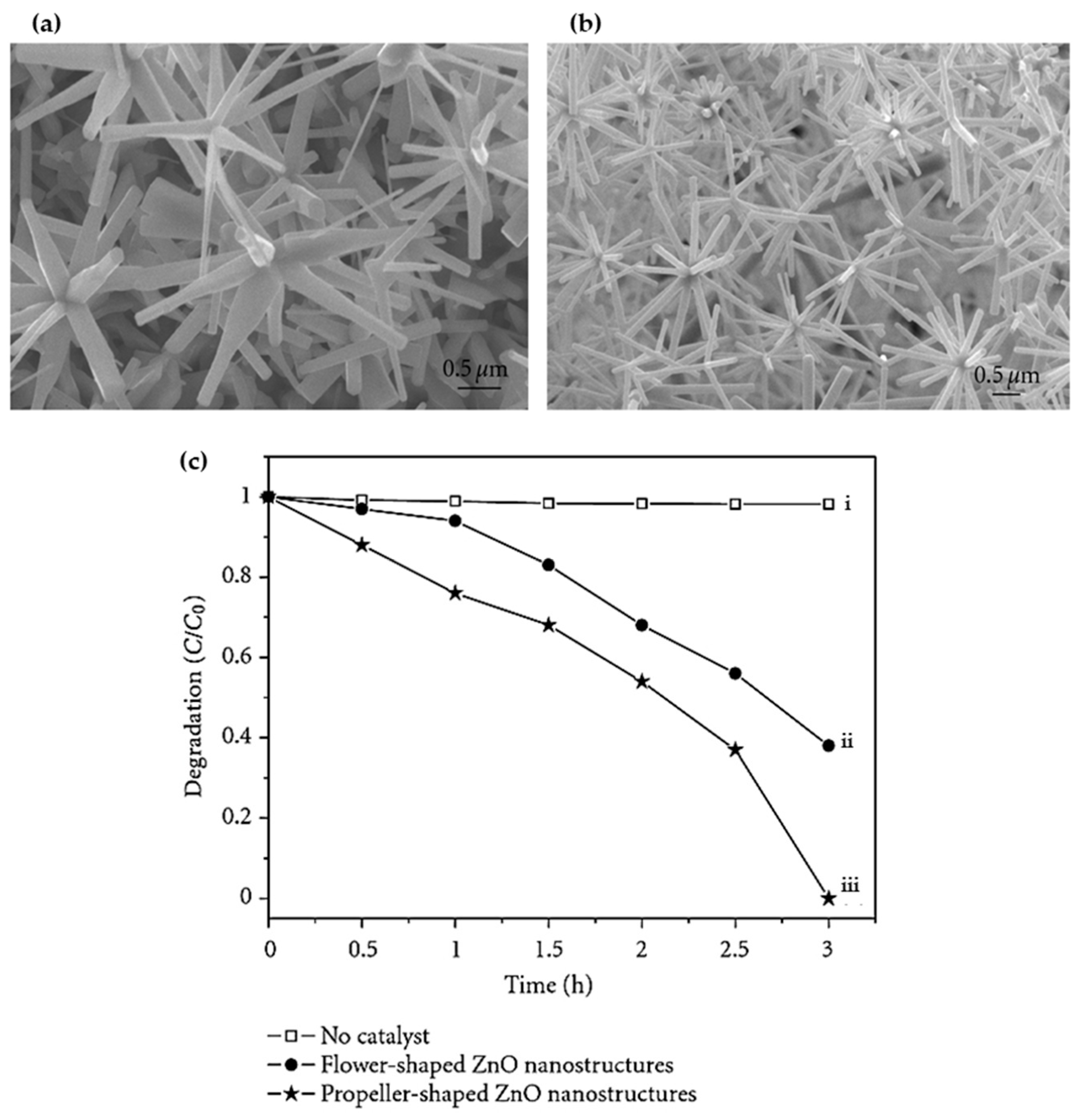

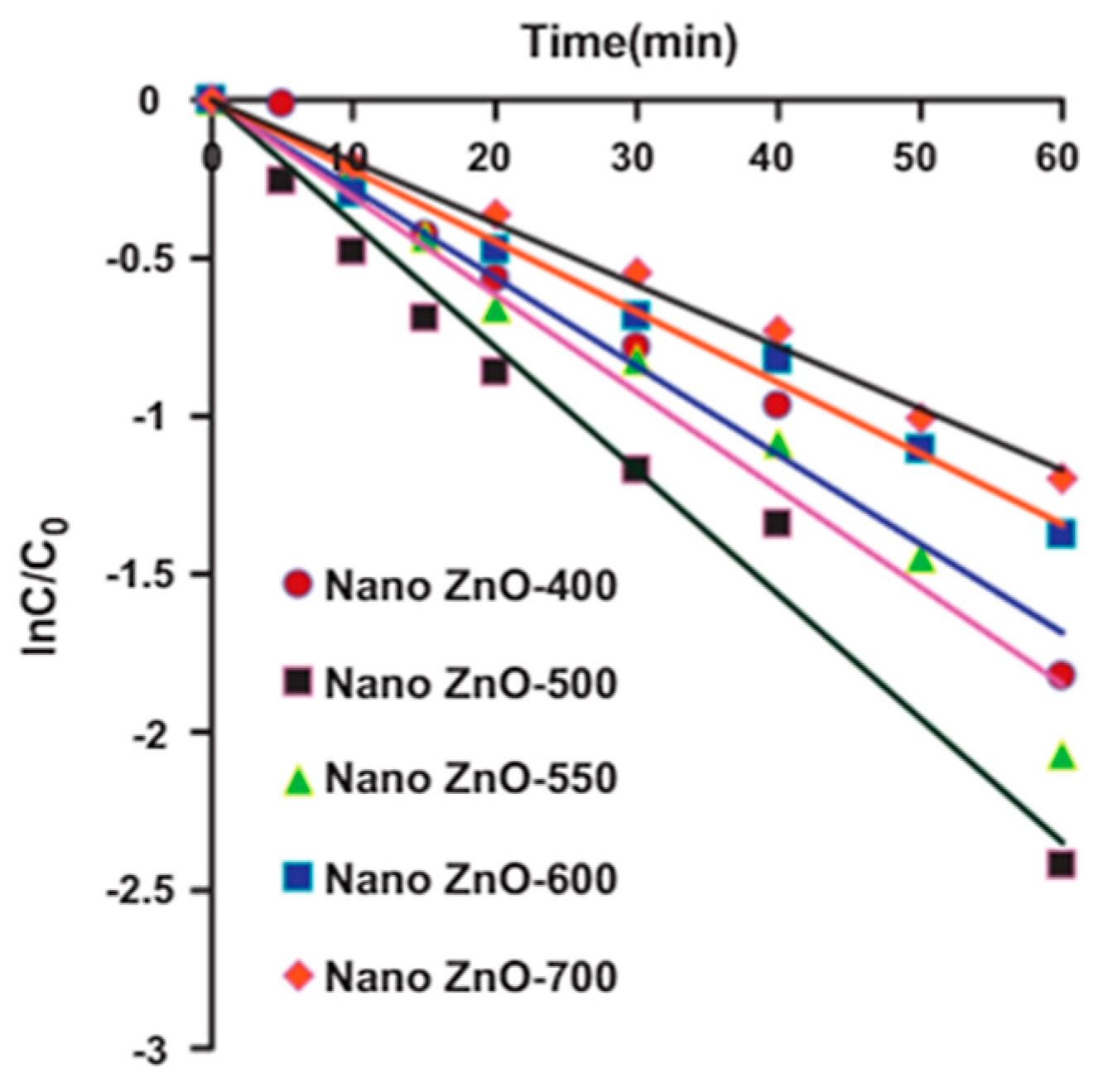
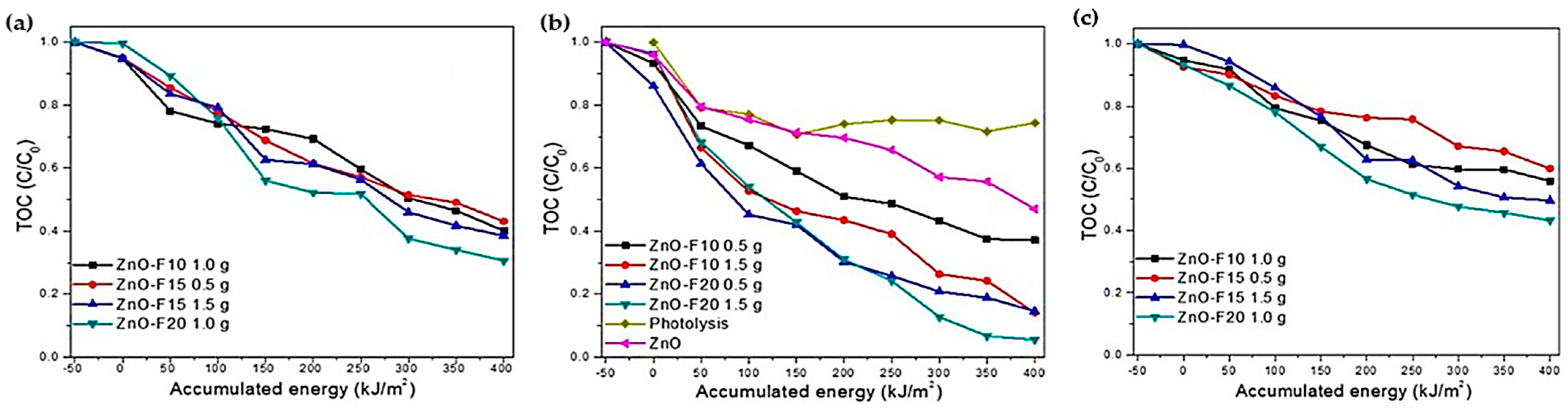
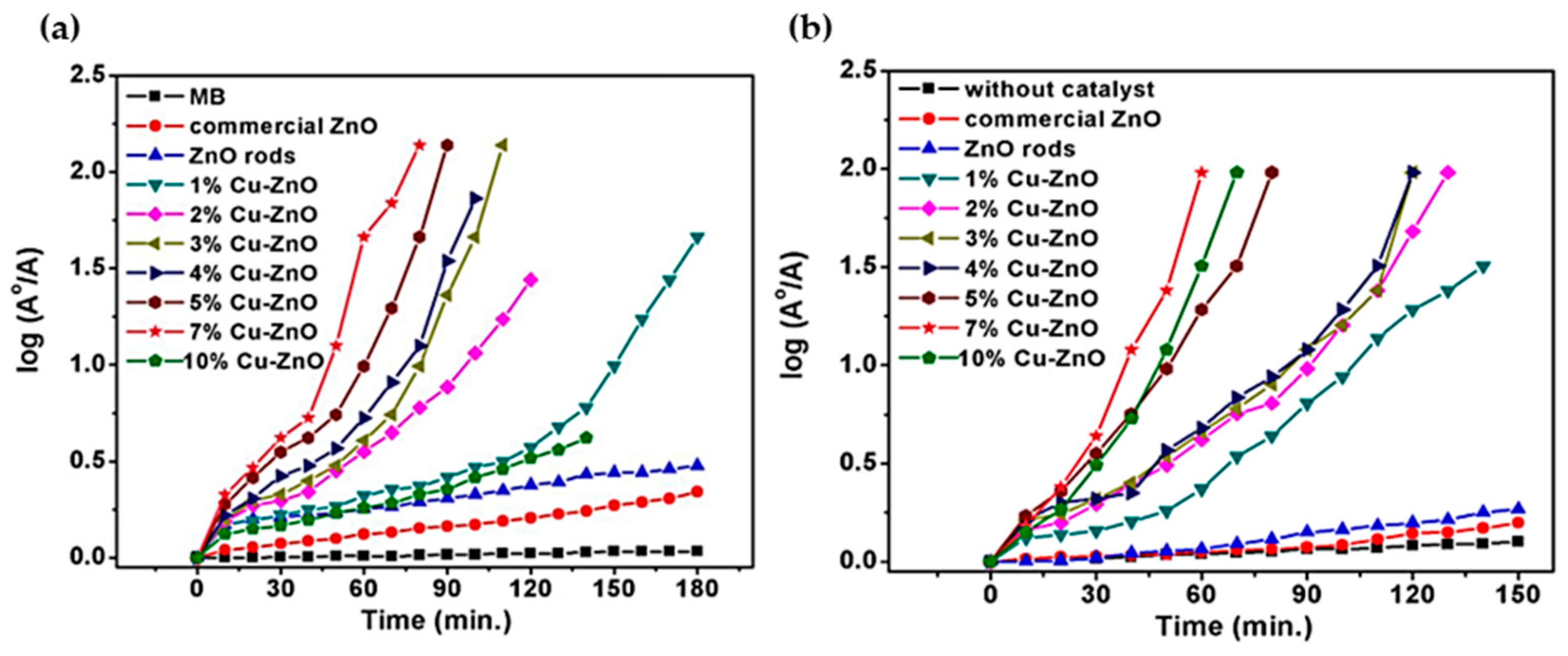
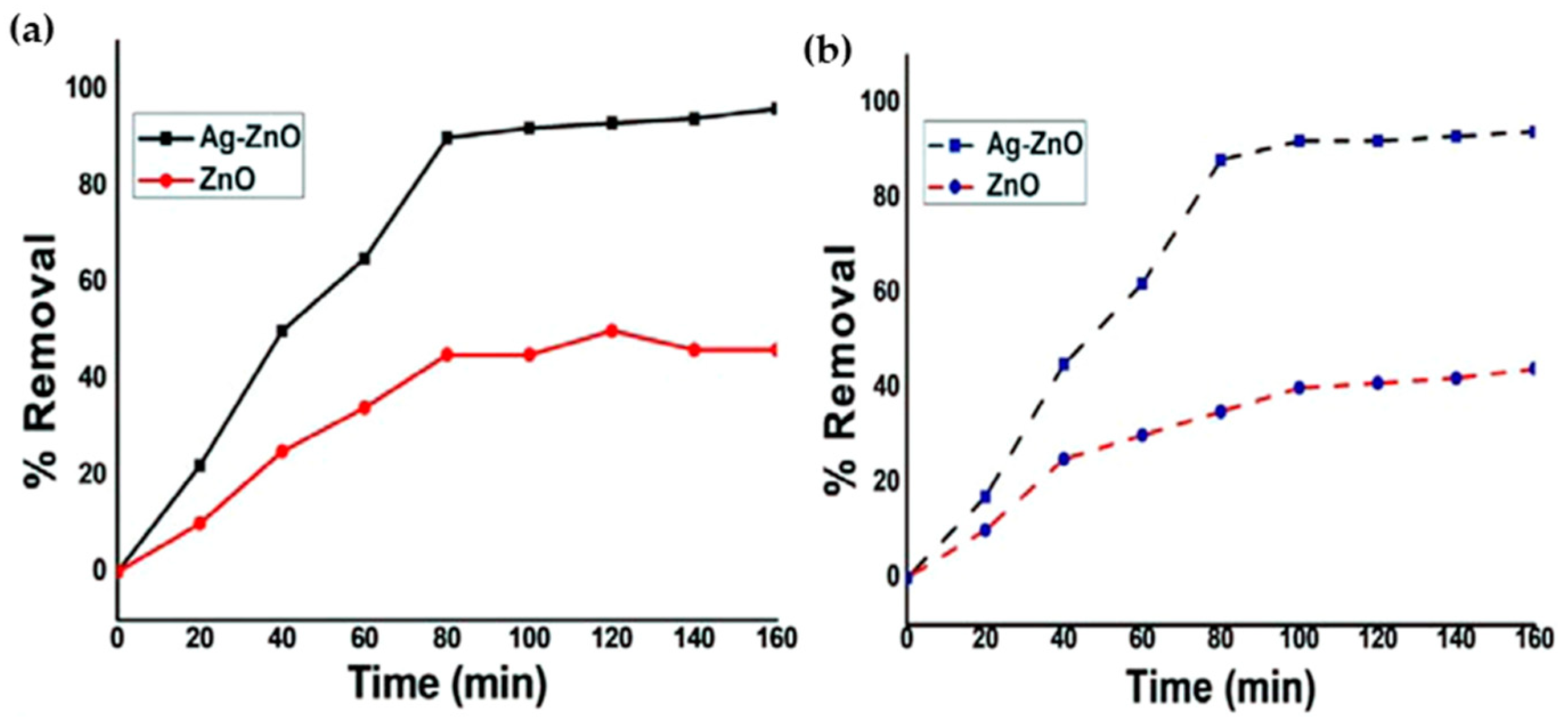




| Annealing Temp. | λmax. (nm) | Band Gap (eV) | Particle Size (nm) (from FESEM) | Crystallite Size (nm) (from XRD) |
|---|---|---|---|---|
| 400 | 374.4 | 3.317 | 40–45 | 24.94 |
| 500 | 376.8 | 3.296 | 55–60 | 28.16 |
| 600 | 378.0 | 3.285 | 85–90 | 31.19 |
| 700 | 379.2 | 3.275 | 100–110 | 37.90 |
| 800 | 381.6 | 3.254 | 115–120 | 48.53 |
| Compound and System | Catalyst Dosage (g/L) | Removal Efficiency (%) in 30 min | Reaction Rate, k (min−1) | t1/2 (min) |
|---|---|---|---|---|
| Ketamine, UV lamp/ZnO | 0.01 | 98.5 | 0.14 | 5.90 |
| 0.04 | 98.7 | 0.15 | 5.30 | |
| 0.05 | 99.9 | 0.23 | 3.83 | |
| 0.10 | 99.9 | 0.26 | 3.11 | |
| 0.40 | 99.9 | 0.43 | 2.25 | |
| 0.70 | 99.9 | 0.25 | 3.54 | |
| 1.00 | 99.9 | 0.27 | 2.72 | |
| Methamphetamine, UV lamp/ZnO | 0.01 | 81.4 | 0.06 | 14.76 |
| 0.04 | 98.8 | 0.15 | 7.46 | |
| 0.05 | 99.9 | 0.23 | 6.27 | |
| 0.10 | 99.9 | 0.32 | 4.70 | |
| 0.40 | 99.9 | 0.38 | 2.58 | |
| 0.70 | 99.9 | 0.14 | 5.44 | |
| 1.00 | 99.9 | 0.22 | 3.53 | |
| Morphine, UV lamp/ZnO | 0.01 | 99.7 | 0.19 | 4.26 |
| 0.04 | 99.9 | 0.21 | 3.61 | |
| 0.05 | 99.9 | 0.21 | 3.39 | |
| 0.10 | 99.9 | 0.37 | 2.38 | |
| 0.40 | 99.9 | 0.68 | 1.06 | |
| 0.70 | 99.9 | 0.48 | 1.52 | |
| 1.00 | 99.9 | 0.60 | 1.14 |
| System | Dosage (g/L) | Ketamine (min) | Methamphetamine (min) | Morphine (min) |
|---|---|---|---|---|
| UV lamp/ZnO | 0.4 | 17 | 19 | 10 |
| UVLED/ZnO | 0.4 | 48 | 95 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Zeid, S.; Leprince-Wang, Y. Advancements in ZnO-Based Photocatalysts for Water Treatment: A Comprehensive Review. Crystals 2024, 14, 611. https://doi.org/10.3390/cryst14070611
Abou Zeid S, Leprince-Wang Y. Advancements in ZnO-Based Photocatalysts for Water Treatment: A Comprehensive Review. Crystals. 2024; 14(7):611. https://doi.org/10.3390/cryst14070611
Chicago/Turabian StyleAbou Zeid, Souad, and Yamin Leprince-Wang. 2024. "Advancements in ZnO-Based Photocatalysts for Water Treatment: A Comprehensive Review" Crystals 14, no. 7: 611. https://doi.org/10.3390/cryst14070611
APA StyleAbou Zeid, S., & Leprince-Wang, Y. (2024). Advancements in ZnO-Based Photocatalysts for Water Treatment: A Comprehensive Review. Crystals, 14(7), 611. https://doi.org/10.3390/cryst14070611







