Effect of Active Phase Precursor on Structural, Textural and Catalytic Properties of the Model NiOx/CeO2 System Active in Dry Reforming of Methane
Abstract
:1. Introduction
1.1. Main Challenges in CO2 Valorization
1.2. Catalytic Pathways of CO2 Conversion
1.3. Dry Reforming of Methane
1.4. Synthetic Parameters Controlling the Catalytic Performance of Supported Oxide Systems Active in DMR
2. Materials and Methods
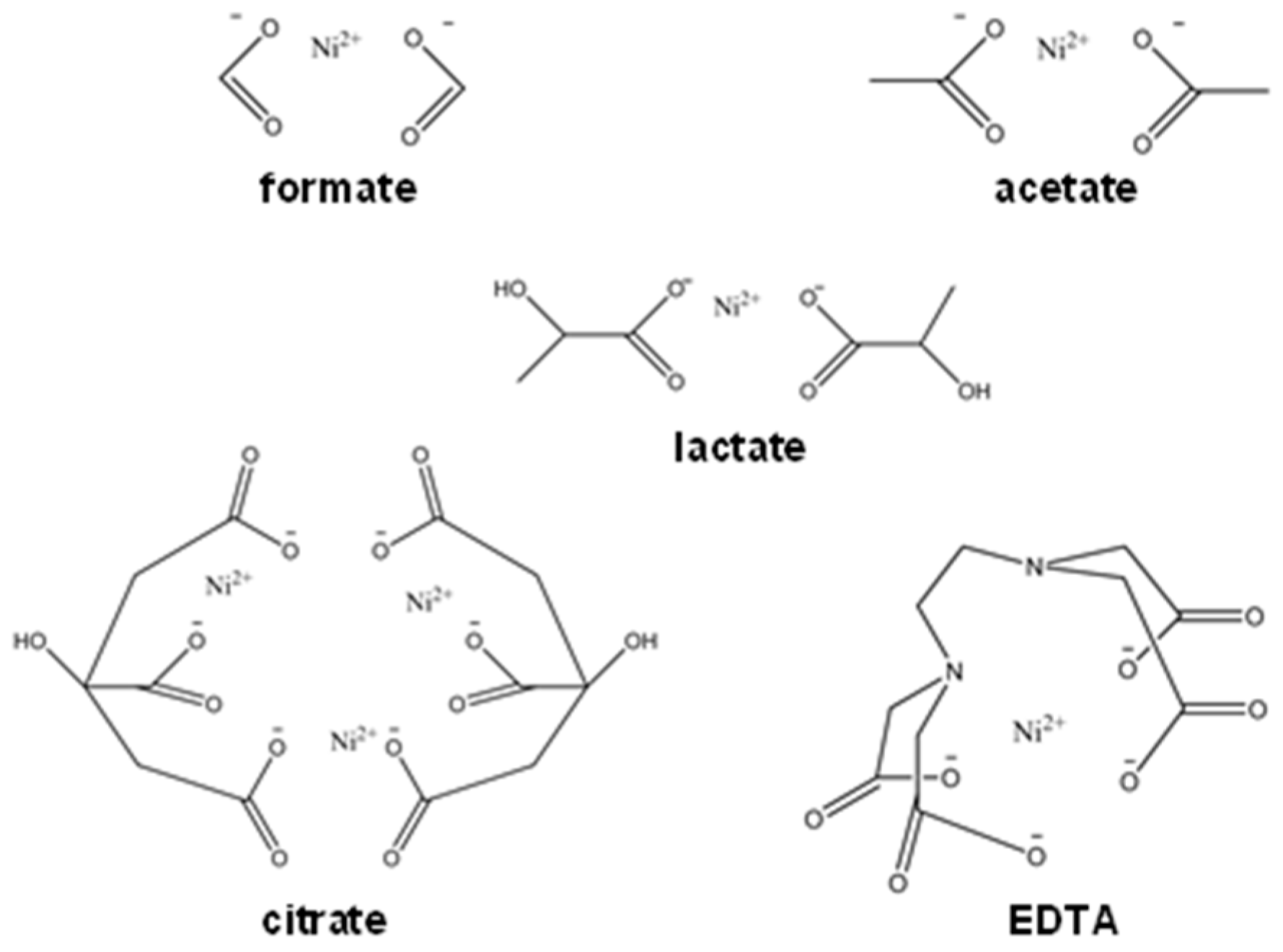
2.1. Synthesis of Catalysts
2.2. Characterization of the Synthesized Ni-Based Catalysts
2.3. Activity Tests
3. Results and Discussion
3.1. Catalytic Activity of the Investigated Ni/CeO2 Samples
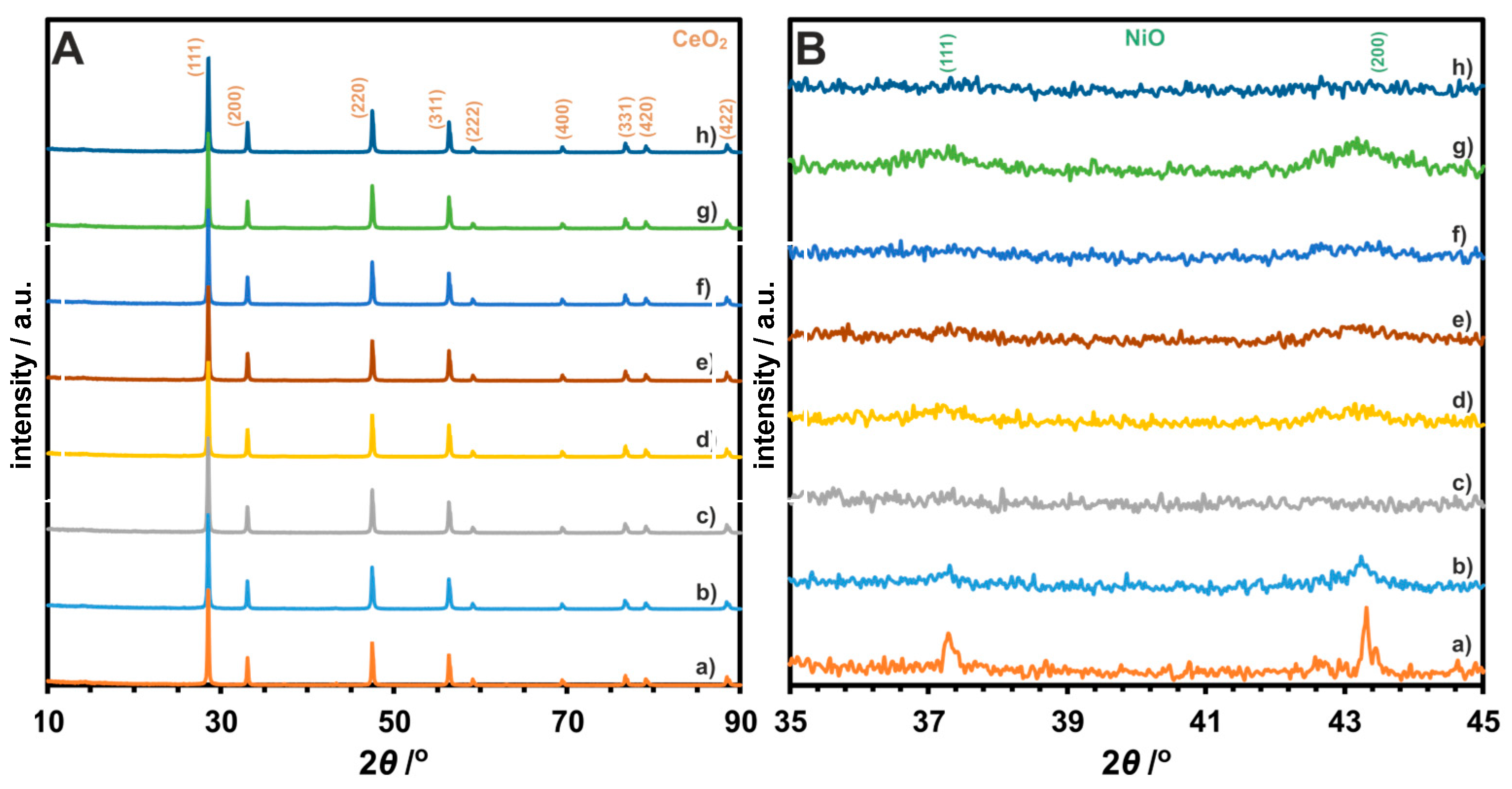
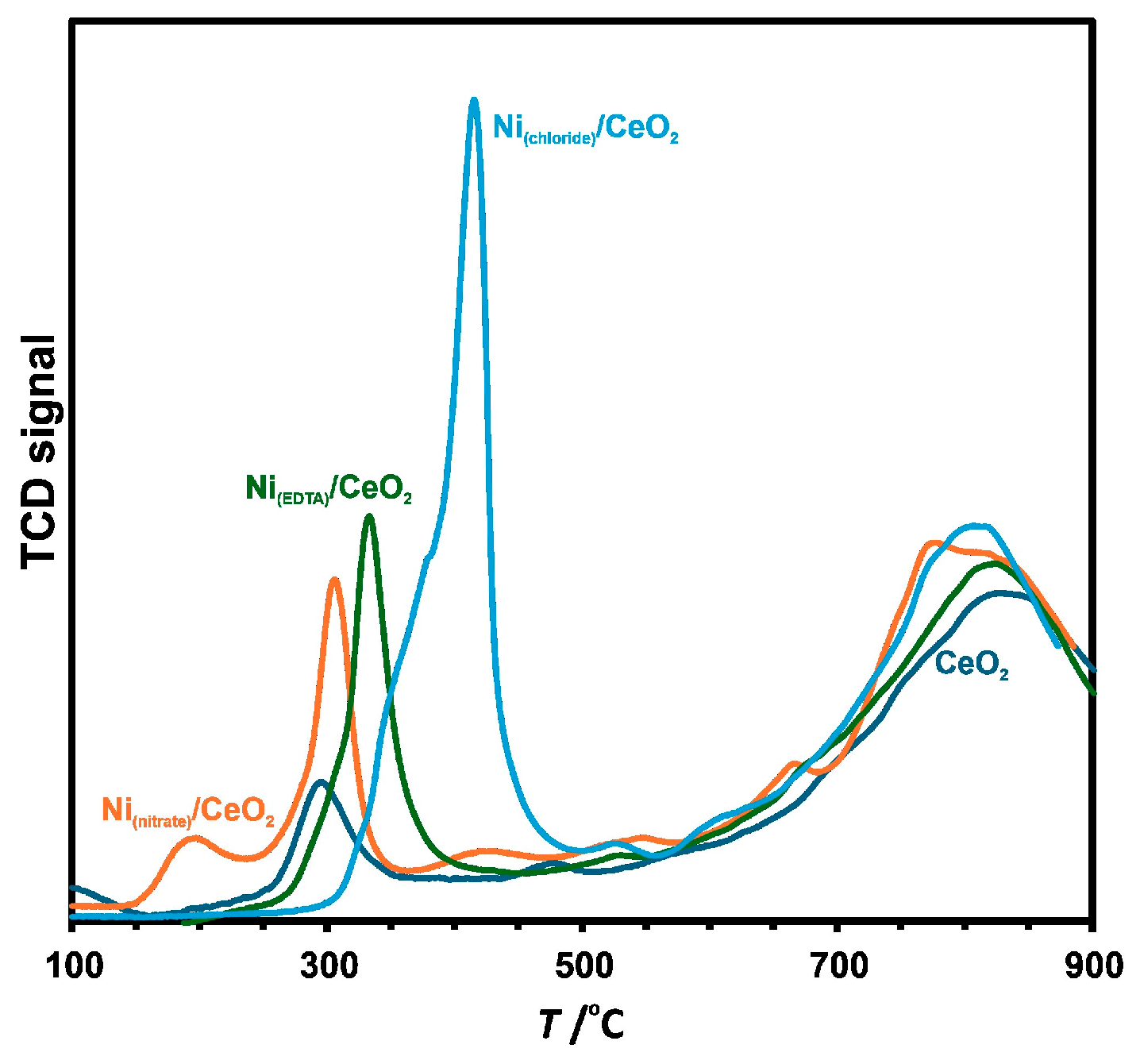
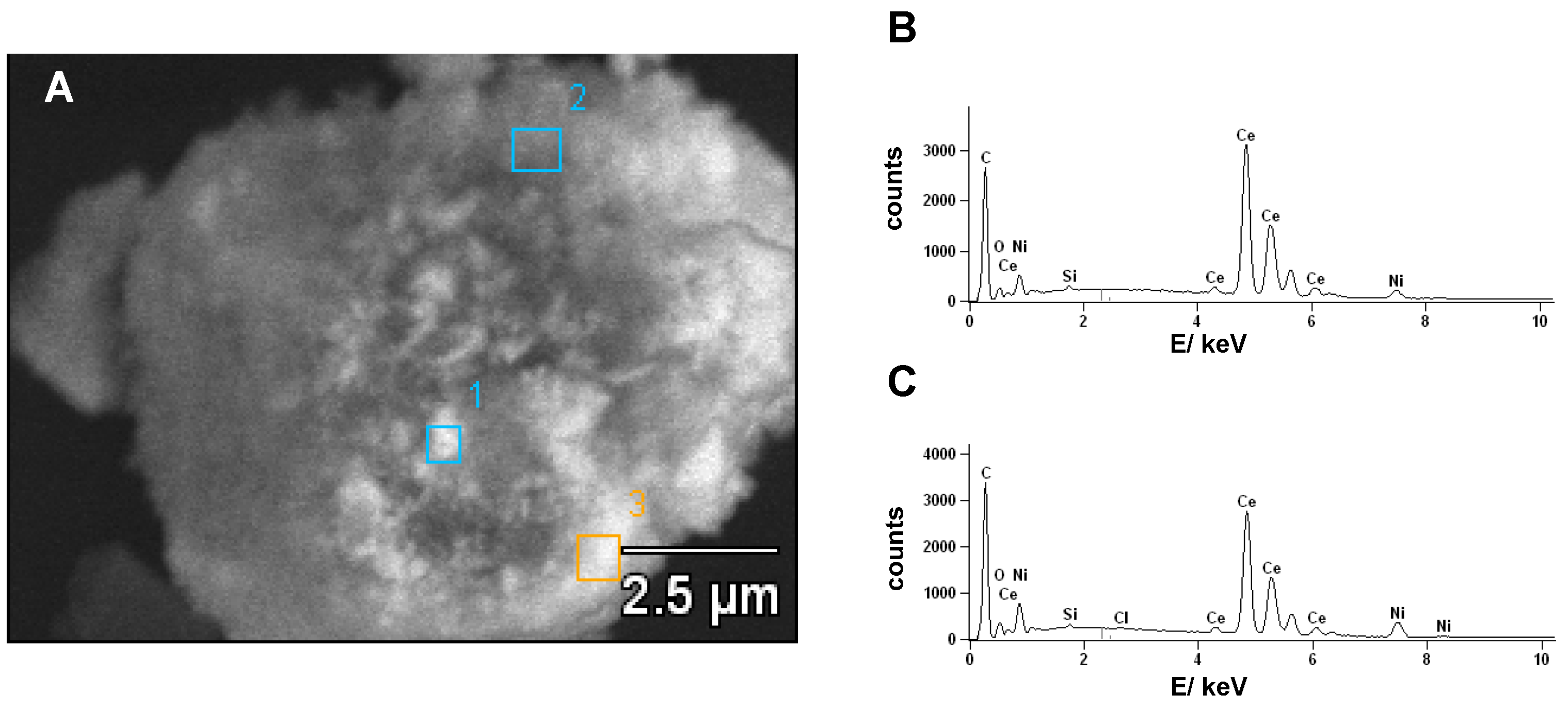
3.2. Structural Properties
3.3. Textural Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchie, H.; Roser, M. CO2 and Greenhouse Gas Emissions. Published Online at OurWorldInData.org 2017. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 27 May 2024).
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Aranowski, R. Techologies of Envirionmental Protection in Industry and Energy Sector, 1st ed.; (Polish Version); Wyd. Naukowe PWN: Warsaw, Poland, 2016; pp. 80–81. [Google Scholar]
- Rafiee, A.; Khalilpour, K.R.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fuels 2018, 132, 3–16. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.H.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Hepburn, K.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Maeda, C.; Myiazaki, Y.; Ema, T. Recent progress in catalytic conversions of carbon dioxide. Catal. Sci. Technol. 2014, 4, 1482–1497. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Yang, N.; Wang, R. Sustainable technologies for the reclamation of greenhouse gas CO2. J. Clean. Prod. 2015, 103, 784–792. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Dry reforming of methane for syngas production: A review and assessment of catalyst development and efficacy. J. Ind. Chem. Soc. 2021, 98, 100002. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamid, M.Y.S. A review on catalyst development for dry reforming of methane to syngas. Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Matus, E.V.; Shlyakhtina, A.S.; Sukhova, O.B.; Ismagilov, I.Z.; Ushakov, V.A.; Yashnik, S.A.; Nikitin, A.P.; Bharali, P.; Kerzhentsev, M.A.; Ismagilov, Z.R. Effect of preparation methods on the physicochemical and functional properties of Ni/CeO2 catalysts. Kinet. Catal. 2019, 60, 221–230. [Google Scholar] [CrossRef]
- Vos, B.; Poels, E.; Bliek, A. Impact of calcination on the structure of alumina-supported nickel particles. J. Catal. 2001, 198, 77–88. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, X.; Tu, Q.; Shi, B.; Cui, Y.; Chen, C. Influence of preparation conditions on the performance of Ni-based catalysts for glycerol steam reforming. ACS Omega 2018, 3, 13335–13342. [Google Scholar] [CrossRef]
- Legutko, P.; Marzec, M.; Kozieł, M.; Sokołowski, K.; Michalik, M.; Adamski, A. Functional improvement of NiOx/CeO2 model catalyst active in dry methane reforming via optimization of nickel content. Processes 2024, 12, 851. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Nanooxides Derived from Hydrotalcites as Catalysts for Dry Methane Reforming Reaction-Effect of [Ni(EDTA)]2− Adsorption Time. Mater. Sci. Forum 2016, 879, 396–401. [Google Scholar] [CrossRef]
- Tsyganok, A.; Suzuki, K.; Hamakawa, S.; Takehira, K.; Hayakawa, T. Mg–Al Layered Double Hydroxide Intercalated with [Ni(edta)]2− Chelate as a Precursor for an Efficient Catalyst of Methane Reforming with Carbon Dioxide. Catal. Lett. 2001, 77, 75–86. [Google Scholar] [CrossRef]
- Chaudhary, C.; Manaka, Y.; Nanba, T. Effect of Ni precursors on the catalytic activity of Ni/CeO2 for nitrogen oxide to ammonia reaction. Catal. Commun. 2024, 186, 106816. [Google Scholar] [CrossRef]
- Deraz, N.M. The comparative jurisprudence of catalysts preparation methods: I. precipitation and impregnation methods. J. Ind. Environ. Chem. 2018, 2, 19–21. [Google Scholar]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachrichten Ges. Wiss. Götingen Math. Kl. 1918, 1918, 98–100. [Google Scholar]
- Vargas, W.E.; Niklasson, G.A. Applicability conditions of the Kubelka-Munk theory. Appl. Opt. 1997, 36, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Sajith, N.V.; Suresh, S.; Bindu, M.; Soumya, B.N.; Prathapachandra Kurup, M.R.; Periyat, P. Visible light active Ni2+ doped CeO2 nanoparticles for the removal of methylene blue dye from water. Res. Eng. 2022, 16, 100664. [Google Scholar] [CrossRef]
- Dębek, R.; Zubek, K.; Motak, M.; Da Costa, P.; Grzybek, T. Effect of nickel incorporation into hydrotalcite-based catalyst systems for dry reforming of methane. Res. Chem. Intermediat. 2015, 41, 9485–9495. [Google Scholar] [CrossRef]
- Auxéméry, A.; Botello Frias, B.; Smal, E.; Dziadek, K.; Phillipot, G.; Legutko, P.; Simonov, M.; Thomas, S.; Adamski, A.; Sadykov, V.; et al. Continuous supercritical solvothermal preparation of nanostructuredceria-zirconia as supports for dry methane reforming catalysts. J. Supercrit. Fluids 2020, 162, 104855. [Google Scholar] [CrossRef]
- Adamski, A.; Legutko, P.; Dziadek, K.; Michalik, M.; Sadykov, V. Dry reforming of methane on supported catalysts based on nickel as an example of technology limiting emission level of greenhouse gases. Przem. Chem. (Chem. Ind.) 2019, 98, 1158–1161. [Google Scholar]
- Barrio, L.; Kubacka, A.; Zhou, G.; Estrella, M.; Martínez-Arias, A.; Hanson, J.C.; Fernández-García, M.; Rodriguez, J.A. Unusual Physical and Chemical Properties of Ni in Ce1−xNixO2−y Oxides: Structural Characterization and Catalytic Activity for the Water Gas Shift Reaction. J. Phys. Chem. C 2010, 114, 12689–12697. [Google Scholar] [CrossRef]
- Liu, Z.; Grinter, D.C.; Lustemberg, P.G.; Nguyen-Phan, T.-D.; Zhou, Y.; Luo, S.; Waluyo, I.; Crumlin, E.J.; Stacchiola, D.J.; Zhou, J.; et al. Dry Reforming of Methane on a Highly-ActiveNi-CeO2 Catalyst: Effects of Metal-Support Interactions on C-H Bond Breaking. Angew. Chem. Int. Ed. 2016, 55, 7455–7459. [Google Scholar] [CrossRef]
- Motekaitis, R.J.; Cox, X.B., III; Taylor, P.; Martell, A.E.; Miles, B.; Tvedt, T.J., Jr. Thermal degradation of EDTA chelates in aqueous solution. Can. J. Chem. 1982, 60, 1207–1213. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Asiri, A.M. An investigation of the thermal decomposition of nickel citrate as a precursor for Ni-NiO composite nanoparticles. Therm. Acta 2017, 649, 54–62. [Google Scholar] [CrossRef]
- Marciuš, M.; Ristic, M.; Ivanda, M.; Music, S. Thermal decomposition of hydrated salts of Ni(II)-acetate and Ni(II)-lactate. J. Mol. Struct. 2013, 1044, 231–238. [Google Scholar] [CrossRef]
- Niculescu, M.; Budrugeac, P. Structural characterization of nickel oxide obtained by thermal decomposition of polynuclear coordination compound [Ni2(OH)2(H3CCH(OH)COO−)2(H2O)2 0.5H2O]n. Rev. Roum. Chim. 2013, 58, 381–386. [Google Scholar]
- Qusti, A.H.; Samarkandy, A.A.; Al-Thabaiti, S.; Diefallah, E.H. The kinetics of thermal decomposition of nickel formate dihydrate in air. JKAU Sci. 1997, 9, 73–81. [Google Scholar] [CrossRef]
- Brockner, W.; Ehrhardt, C.; Gjikaj, M. Thermal decomposition of nickel nitrate hexahydrate, Ni(NO3)2 • 6H2O, in comparison to Co(NO3)2 • 6H2O and Ca(NO3)2 • 4H2O. Thermochim. Acta 2007, 456, 64–68. [Google Scholar] [CrossRef]
- Tomaszewicz, E.; Kotfica, M. Mechanism and kinetics of thermal decomposition of nickel(II) sulfate(VI) hexahydrate. J. Therm. Anal. Calorim. 2004, 77, 25–31. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kanungo, S.B. Thermal dehydration and decomposition of nickel chloride hydrate (NiCl2 • xH2O). J. Therm. Anal. 1992, 38, 2417–2436. [Google Scholar] [CrossRef]
- Bensalem, A.; Muller, J.C.; Bozon-Verduraz, F. From bulk CeO2 to supported cerium–oxygen clusters: A diffuse reflectance approach. J. Chem. Soc. Faraday Trans. 1992, 88, 153–154. [Google Scholar] [CrossRef]
- Guo, M.; Lu, J.; Wu, Y.; Wang, Y.; Luo, M. UV and Visible Raman Studies of Oxygen Vacancies in Rare-Earth-Doped Ceria. Langmuir 2011, 27, 3872–3877. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 Catalysts for the Dry Reforming of Methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Iova, F.; Trutia, A. On the structure of the NiO-Al2O3 systems, studied by diffuse-reflectance spectroscopy. Opt. Mater. 2000, 13, 455–458. [Google Scholar] [CrossRef]
- Adamski, A.; Legutko, P.; Dziadek, K.; Parkhomenko, K.; Aymonier, C.; Sadykov, V.A.; Roger, A.-C. Role of CeO2-ZrO2 Support for structural, textural and functional properties of Ni-based catalysts active in dry reforming of methane. E3S Web Conf. 2019, 108, 02018. [Google Scholar] [CrossRef]
- Valente, J.S.; Valle-Orta, M.; Armedaríz-Herrera, H.; Quintana-Solórzano, R.; del Angel, P.; Ramírez-Salgado, J.; Montiel-López, J.R. Controlling the redox properties of nickel in NiO/ZrO2 catalysts synthesized by sol-gel. Catal. Sci. Technol. 2018, 8, 4070–4082. [Google Scholar] [CrossRef]
- Makhado, K.P.; Mphahlele-Makgwane, M.M.; Kumar, N.; Baker, P.G.L.; Makgwane, P.R. Current updates on p-type nickel oxide (NiO) based photocatalysts towards decontamination of organic pollutants from wastewater. Mater. Today Sust. 2024, 25, 100664. [Google Scholar] [CrossRef]
- Ferriz, R.M.; Egami, T.; Wong, G.S.; Vohs, J.M. Reaction of NO on CeO2 and Rh/CeO2 thin films supported on a-Al2O3 (0001) and YSZ (100). Catal. Sci. Technol. 2001, 476, 9–21. [Google Scholar] [CrossRef]
- Sanchis, R.; García, T.; Dejoz, A.M.; Vázquez, I.; Lopis, F.J.; Solsona, B. Easy Method for the Transformation of Levulinic Acid into Gamma-Valerolactone Using a Nickel Catalyst Derived from Nanocasted Nickel Oxide. Materials 2019, 12, 2918. [Google Scholar] [CrossRef]
- Feng, G.; Han, W.; Wang, Z.; Li, F.; Xue, W. Highly Reducible Nanostructured CeO2 for CO Oxidation. Catalysts 2018, 8, 535. [Google Scholar] [CrossRef]
- Li, L.; Jiang, B.; Tang, D.; Zheng, Z.; Zhao, C. Hydrogen Production from Chemical Looping Reforming of Ethanol Using Ni/CeO2 Nanorod Oxygen Carrier. Catalysts 2018, 8, 257. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Hao, J.; Li, L.; Gao, Y.; Gu, Y.; Cao, Z.; Liu, J. Strong Metal−Support Interactions of Ni-CeO2 Effectively Improve the Performance of a Molten Hydroxide Direct Carbon Fuel Cell. ACS Omega 2022, 7, 24646–24655. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]


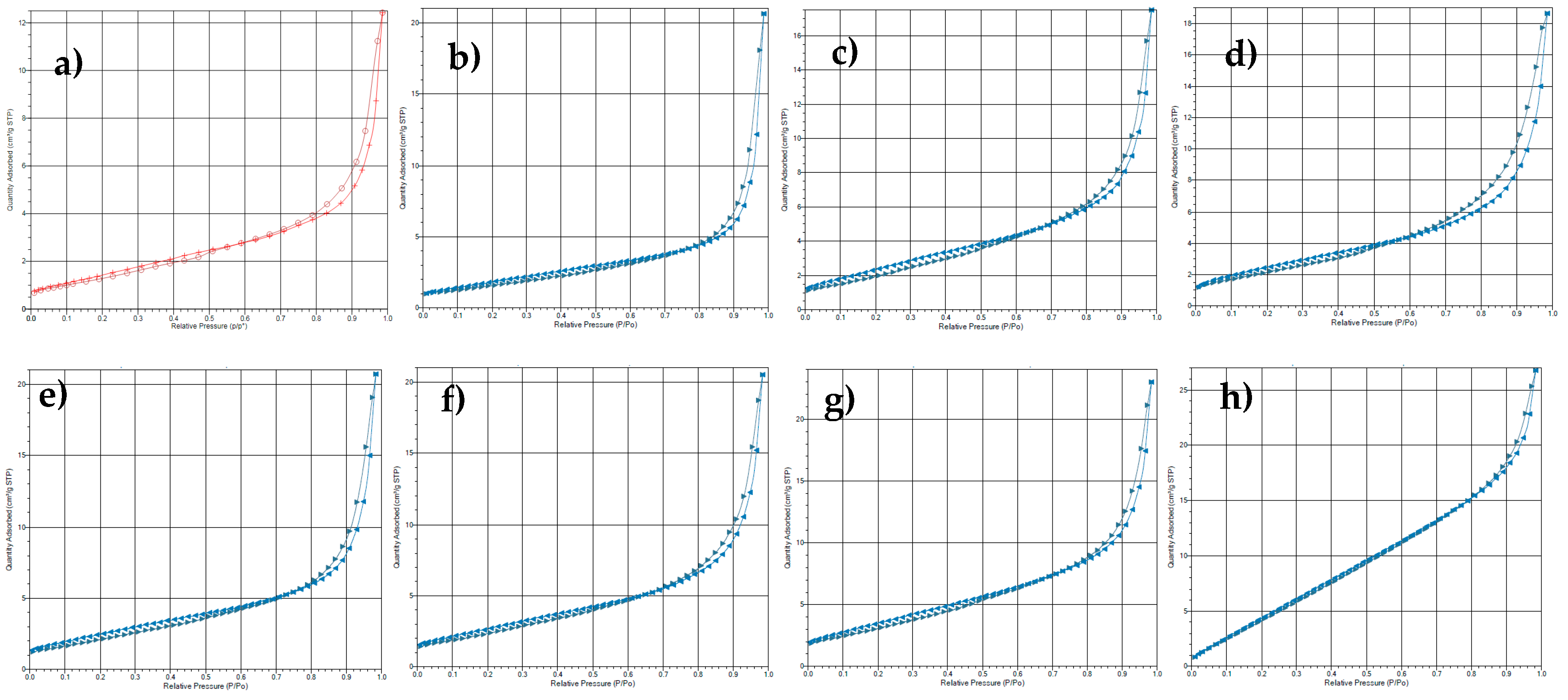

| Sample/Ni Precursor | SSA/m2g−1 | Vpore/cm3g−1 | dpore/Å |
|---|---|---|---|
| Nitrate | 6.01 | 0.019 | 127.91 |
| EDTA | 7.26 | 0.032 | 175.91 |
| Formate | 9.61 | 0.027 | 112.77 |
| Sulfate | 9.63 | 0.029 | 119.85 |
| Acetate | 9.84 | 0.032 | 130.38 |
| Lactate | 10.47 | 0.032 | 121.60 |
| Citrate | 13.78 | 0.036 | 103.48 |
| Chloride | 27.09 | 0.041 | 61.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legutko, P.; Kozieł, M.; Kowalczyk, A.; Michalik, M.; Adamski, A. Effect of Active Phase Precursor on Structural, Textural and Catalytic Properties of the Model NiOx/CeO2 System Active in Dry Reforming of Methane. Crystals 2024, 14, 634. https://doi.org/10.3390/cryst14070634
Legutko P, Kozieł M, Kowalczyk A, Michalik M, Adamski A. Effect of Active Phase Precursor on Structural, Textural and Catalytic Properties of the Model NiOx/CeO2 System Active in Dry Reforming of Methane. Crystals. 2024; 14(7):634. https://doi.org/10.3390/cryst14070634
Chicago/Turabian StyleLegutko, Piotr, Marcin Kozieł, Andrzej Kowalczyk, Marek Michalik, and Andrzej Adamski. 2024. "Effect of Active Phase Precursor on Structural, Textural and Catalytic Properties of the Model NiOx/CeO2 System Active in Dry Reforming of Methane" Crystals 14, no. 7: 634. https://doi.org/10.3390/cryst14070634








