A Portable Miniature Cryogenic Environment for In Situ Neutron Diffraction
Abstract
:1. Introduction
2. Materials and Methods
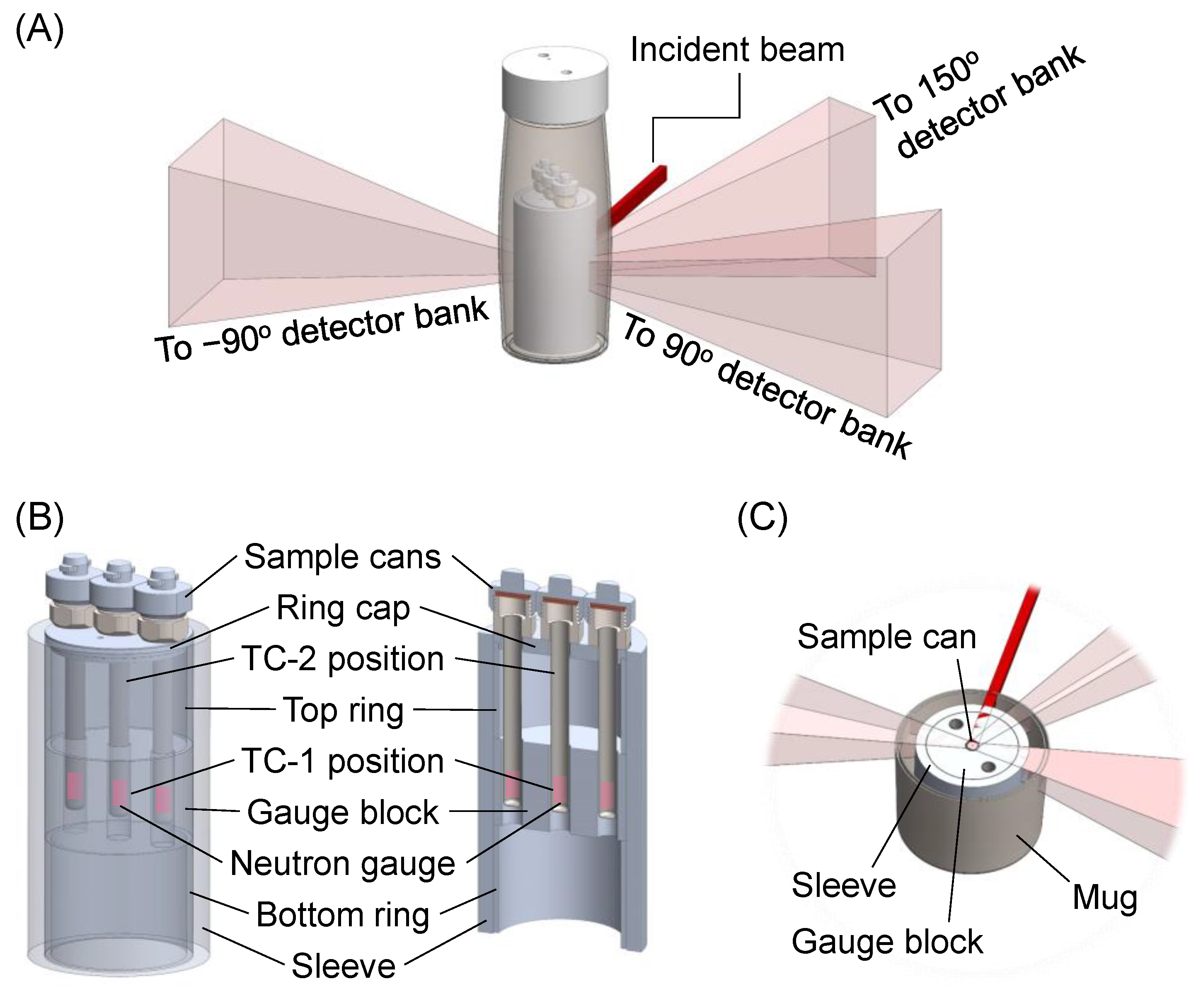
2.1. Design and Material Selection
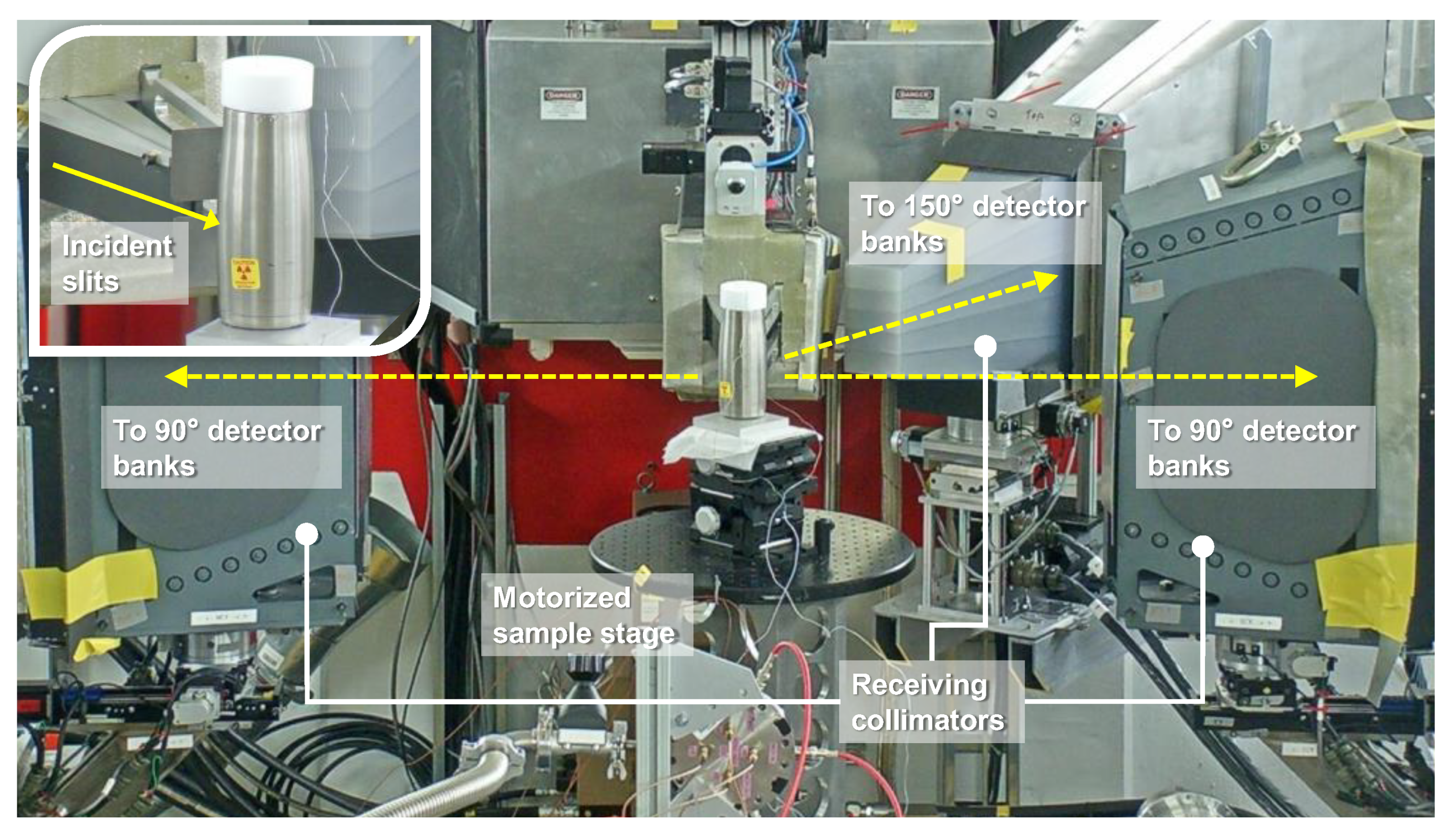
2.2. Experimental Setups
3. Results and Discussion
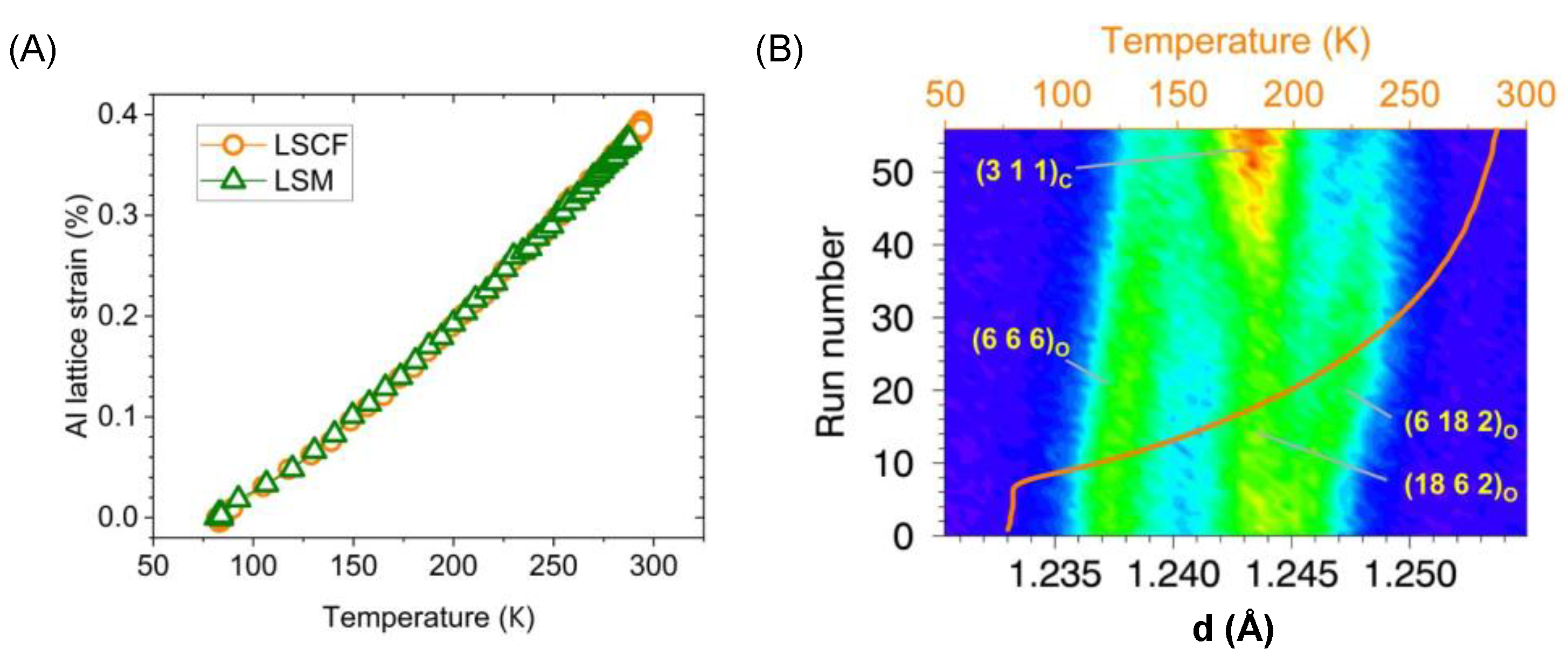
3.1. Temperature Profiles
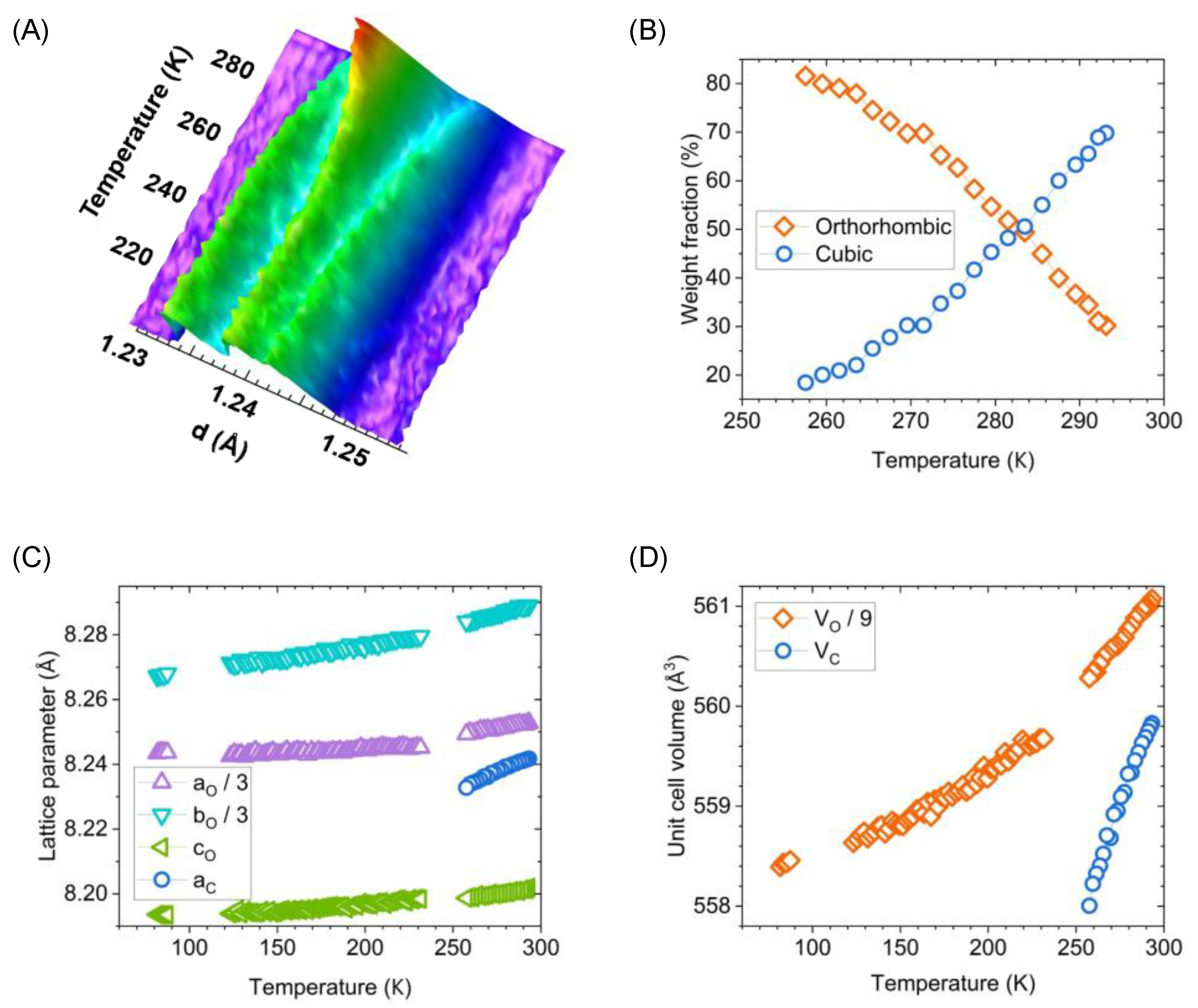
3.2. Cryogenic In Situ Neutron Diffraction of LiMn2O4
3.3. High-Throughput Measurements across Multiple Samples
3.3.1. Feasibility
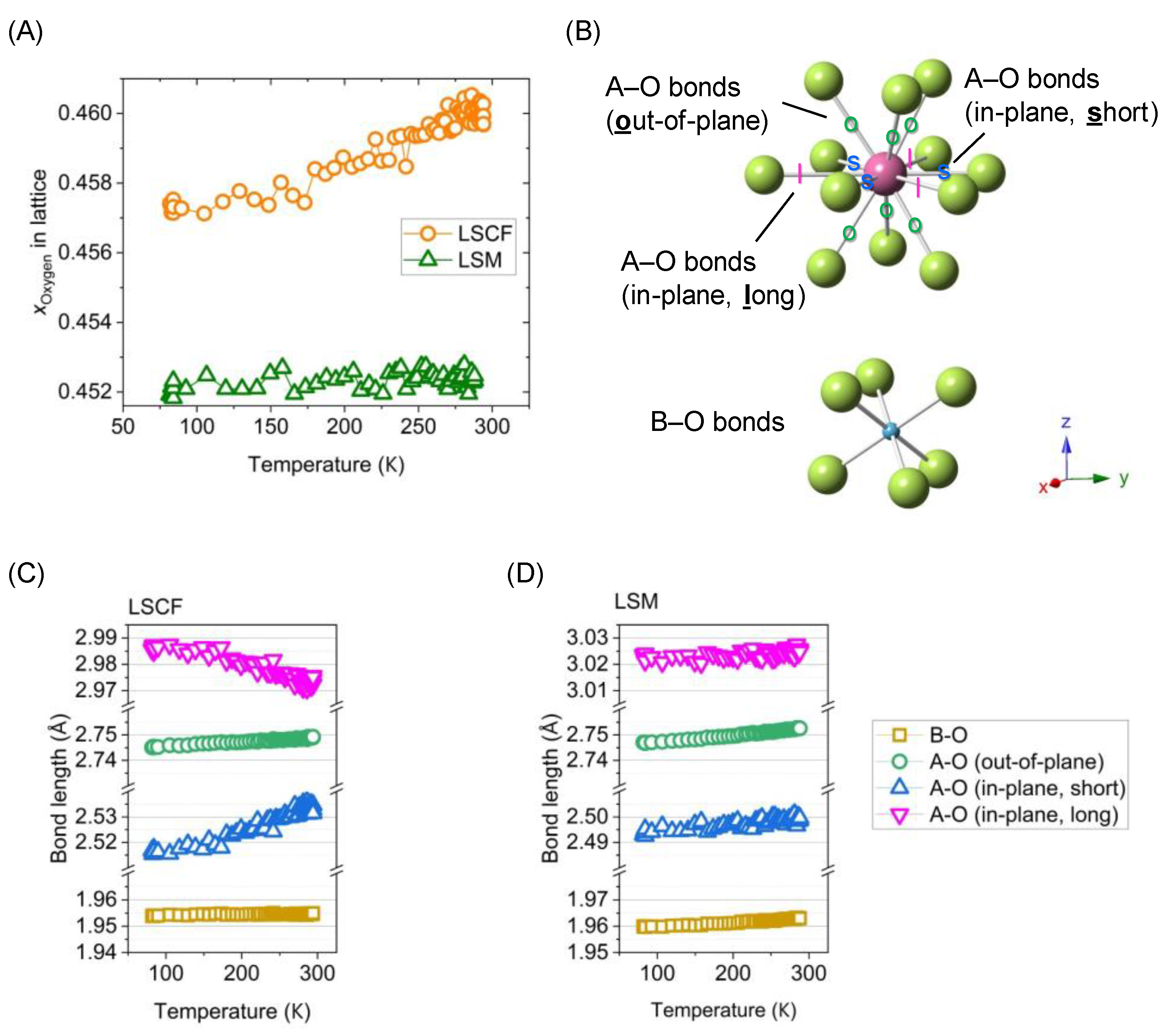
3.3.2. Anisotropic Thermal Expansion in Rhombohedral Perovskites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Börner, H.; Brown, J.; Carlile, C.J.; Cubitt, R.; Currat, R.; Dianoux, A.J.; Farago, B.; Hewat, A.W.; Kulda, J.; Lelièvre-Berna, E.; et al. Neutron Data Booklet, 2nd ed.; Dianoux, A., Lander, G., Eds.; OCP Science: Philadelphia, PA, USA, 2003. [Google Scholar]
- Ishikado, M.; Takahashi, R.; Yamauchi, Y.; Nakamura, M.; Ishimaru, S.; Yamauchi, S.; Ohira-Kawamura, S.; Kira, H.; Sakaguchi, Y.; Watanabe, M.; et al. Recent Status of the Cryogenic Sample Environment at the MLF, J-PARC. JPS Conf. Proc. 2024, 41, 011010. [Google Scholar] [CrossRef]
- Koehler, W.C.; Wollan, E.O. Neutron-diffraction study of the magnetic properties of perovskite-like compounds LaBO3. J. Phys. Chem. Solids 1957, 2, 100–106. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hood, Z.D.; Keum, J.K.; Pandian, A.S.; Chi, M.; An, K.; Liang, C.; Sunkara, M.K. Revealing the Structural Stability and Na-Ion Mobility of 3D Superionic Conductor Na3SbS4 at Extremely Low Temperatures. ACS Appl. Energy Mater. 2018, 1, 7028–7034. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Zhao, H.; Sun, G.; Yao, X. Development of a variable temperature mechanical loading device for in situ neutron scattering measurements. J. Mech. Sci. Technol. 2022, 36, 3939–3947. [Google Scholar] [CrossRef]
- Hu, H.; Dou, M.; Zhang, C.; Cheng, H.; He, C.; Ke, Y.; Yuan, B.; Bai, B.; Sun, Y.; Huang, Z.; et al. Development of an automatic sample changer with variable temperature for small-angle neutron scattering at China Spallation Neutron Source. Rev. Sci. Instrum. 2023, 94, 074903. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Lee, E.; Shi, B.; Croy, J.R. Review—From LiMn2O4 to Partially-Disordered Li2MnNiO4: The Evolution of Lithiated-Spinel Cathodes for Li-Ion Batteries. J. Electrochem. Soc. 2022, 169, 020535. [Google Scholar] [CrossRef]
- Lin, Q.; Bian, L.; Liu, C.; Ting, T.; Liu, Z.; Wei, P.; Han, S.; Xu, Y.; Peng, J.; An, S. Improved La0.8Sr0.2MnO3-δ oxygen electrode activity by introducing high oxygen ion conductor oxide for solid oxide steam electrolysis. Int. J. Hydrogen Energy 2024, 49, 616–624. [Google Scholar] [CrossRef]
- Rendón, R.C.; Udayabhaskar, R.; Salvo, C.; Sepúlveda, E.; Rodríguez, J.J.; Camurri, C.P.; Viswanathan, M.R. Evaluation of La0.8Sr0.2MnO3 perovskite prepared by fast solution combustion. Ceram. Int. 2022, 48, 35100–35107. [Google Scholar] [CrossRef]
- Araki, W.; Ikeda, K.; Otomo, N.; Nakazato, Y.; Yokoyama, S.; Arai, Y. Effect of Oxygen Deficiency on Mechanical and Thermomechanical Properties of Ferroic La0.6Sr0.4Co0.2Fe0.8O3−δ. Adv. Eng. Mater. 2022, 24, 2101204. [Google Scholar] [CrossRef]
- Daga, M.; Sanna, C.; Bais, G.; Polentarutti, M.; Massardo, S.; Carnasciali, M.; Holtappels, P.; Costamagna, P.; Pani, M.; Artini, C. Impact of the electrospinning synthesis route on the structural and electrocatalytic features of the LSCF (La0.6Sr0.4Co0.2Fe0.8O3–δ) perovskite for application in solid oxide fuel cells. Solid State Ionics 2024, 413, 116620. [Google Scholar] [CrossRef]
- An, K.; Chen, Y.; Stoica, A.D. VULCAN: A “hammer” for high-temperature materials research. MRS Bull. 2019, 44, 878–885. [Google Scholar] [CrossRef]
- Sears, V.F. Neutron scattering lengths and cross sections. Neutron News 1992, 3, 26–37. [Google Scholar] [CrossRef]
- Zhang, A.; Li, Y. Thermal Conductivity of Aluminum Alloys—A Review. Materials 2023, 16, 2972. [Google Scholar] [CrossRef] [PubMed]
- Chase, M.W., Jr. NIST-JANAF Thermochemical Tables, Monograph 9, 4th ed.; American Chemical Society: Washington, DC, USA, 1998. [Google Scholar]
- An, K. Data reduction and interactive visualization software for event mode neutron diffraction. In ORNL Report, ORNL-TM-2012; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2012; p. 621. Available online: https://neutrons.ornl.gov/sites/default/files/VDRIVE%20manual_1.pdf (accessed on 1 July 2024).
- Larson, A.C.; Von Dreele, R.B. General structure analysis system (GSAS). In Los Alamos National Laboratory Report LAUR; Los Alamos National Laboratory: Los Alamos, NM, USA, 2004; pp. 86–748. Available online: http://www.ccp14.ac.uk/ccp/ccp14/ftp-mirror/gsas/public/gsas/manual/GSASManual.pdf. (accessed on 1 July 2024).
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Rousse, G.; Masquelier, C.; Rodríguez-Carvajal, J.; Elkaim, E.; Lauriat, J.P.; Martínez, J.L. X-ray Study of the Spinel LiMn2O4 at Low Temperatures. Chem. Mater. 1999, 11, 3629–3635. [Google Scholar] [CrossRef]
- Piszora, P. Temperature dependence of the order and distribution of Mn3+ and Mn4+ cations in orthorhombic LiMn2O4. J. Alloys Compd. 2004, 382, 112–118. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; An, K. Stress-induced charge-ordering process in LiMn2O4. Mater. Res. Lett. 2017, 5, 89–94. [Google Scholar] [CrossRef]
- Wills, A.S.; Raju, N.P.; Greedan, J.E. Low-Temperature Structure and Magnetic Properties of the Spinel LiMn2O4: A Frustrated Antiferromagnet and Cathode Material. Chem. Mater. 1999, 11, 1510–1518. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J.; Rousse, G.; Masquelier, C.; Hervieu, M. Electronic Crystallization in a Lithium Battery Material: Columnar Ordering of Electrons and Holes in the Spinel LiMn2O4. Phys. Rev. Lett. 1998, 81, 4660–4663. [Google Scholar] [CrossRef]
- Gaviko, V.S.; Korolev, A.V.; Arkhipov, V.E.; Bebenin, N.G.; Mukovskii, Y.M. X-ray studies of the (La,Sr)MnO3 perovskite manganite structure. Phys. Solid State 2005, 47, 1299–1305. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yu, D.; An, K. A Portable Miniature Cryogenic Environment for In Situ Neutron Diffraction. Crystals 2024, 14, 640. https://doi.org/10.3390/cryst14070640
Chen Y, Yu D, An K. A Portable Miniature Cryogenic Environment for In Situ Neutron Diffraction. Crystals. 2024; 14(7):640. https://doi.org/10.3390/cryst14070640
Chicago/Turabian StyleChen, Yan, Dunji Yu, and Ke An. 2024. "A Portable Miniature Cryogenic Environment for In Situ Neutron Diffraction" Crystals 14, no. 7: 640. https://doi.org/10.3390/cryst14070640





