Environmentally Assisted Cracking of Duplex and Lean Duplex Stainless Steel Reinforcements in Alkaline Medium Contaminated with Chlorides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Testing Method and Environment
2.3. Characterization Techniques
3. Results
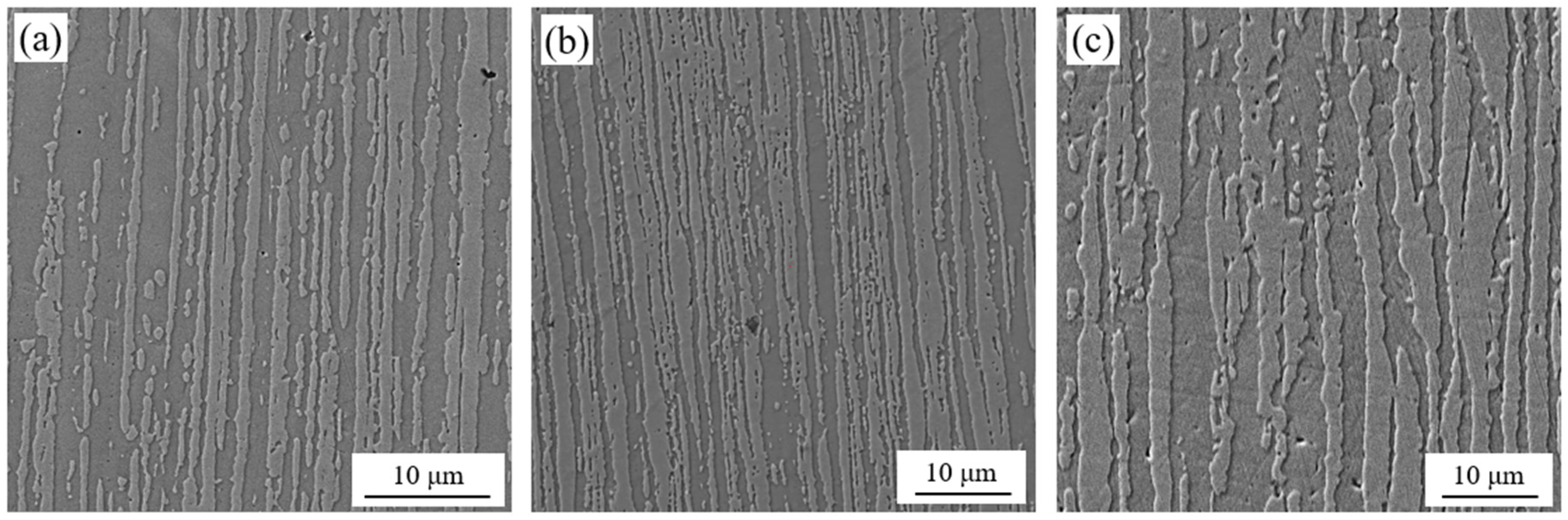
3.1. Microstructure Characterization
3.2. Slow Strain Rate Testing (SSRT)
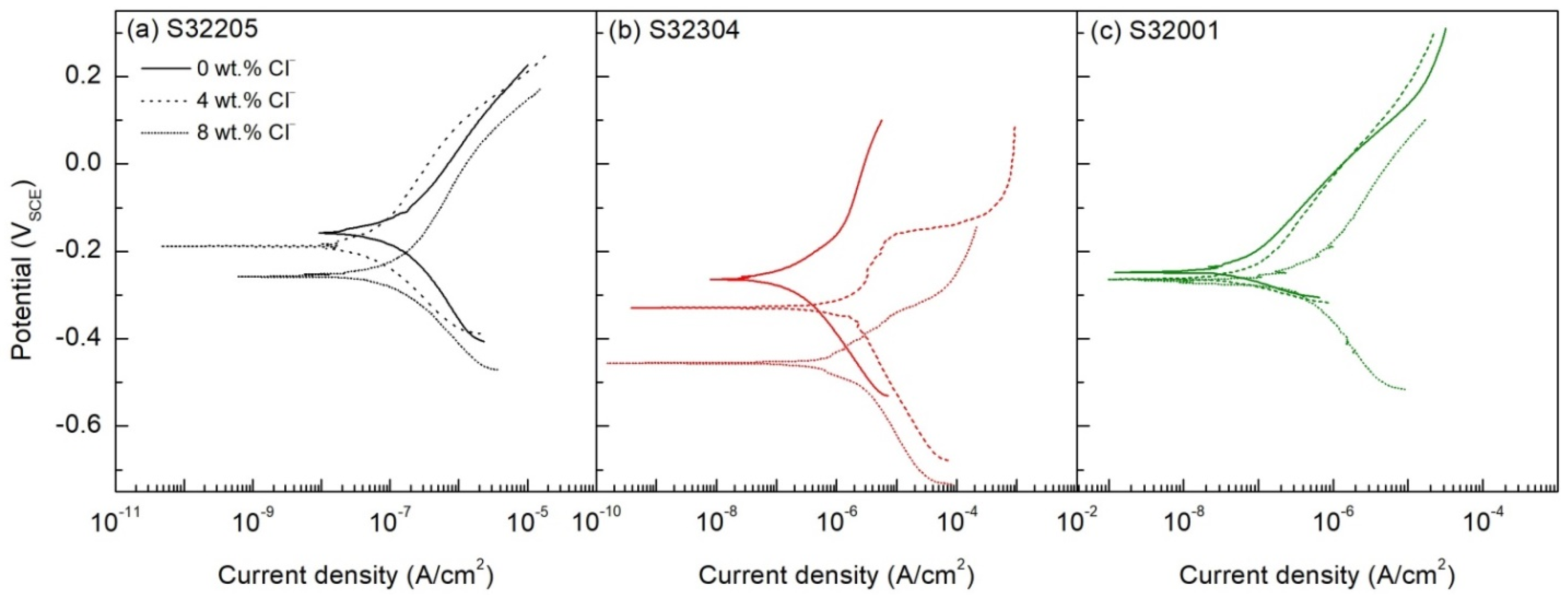
3.3. Potentiodynamic Polarization
4. Discussion
4.1. Phase Quantification by XRD
4.2. Mechanical Properties Degradation Analysis
4.3. Electrochemical Corrosion Kinetics Analysis
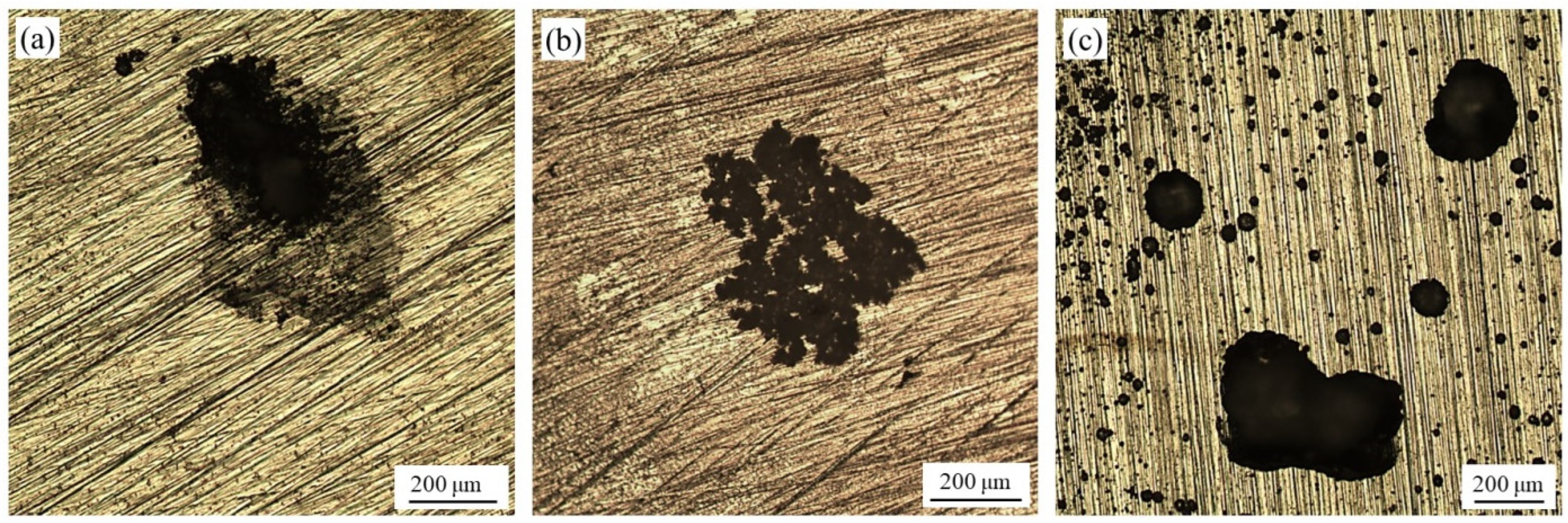
4.4. Pitting Assessment after Potentiodynamic Polarization
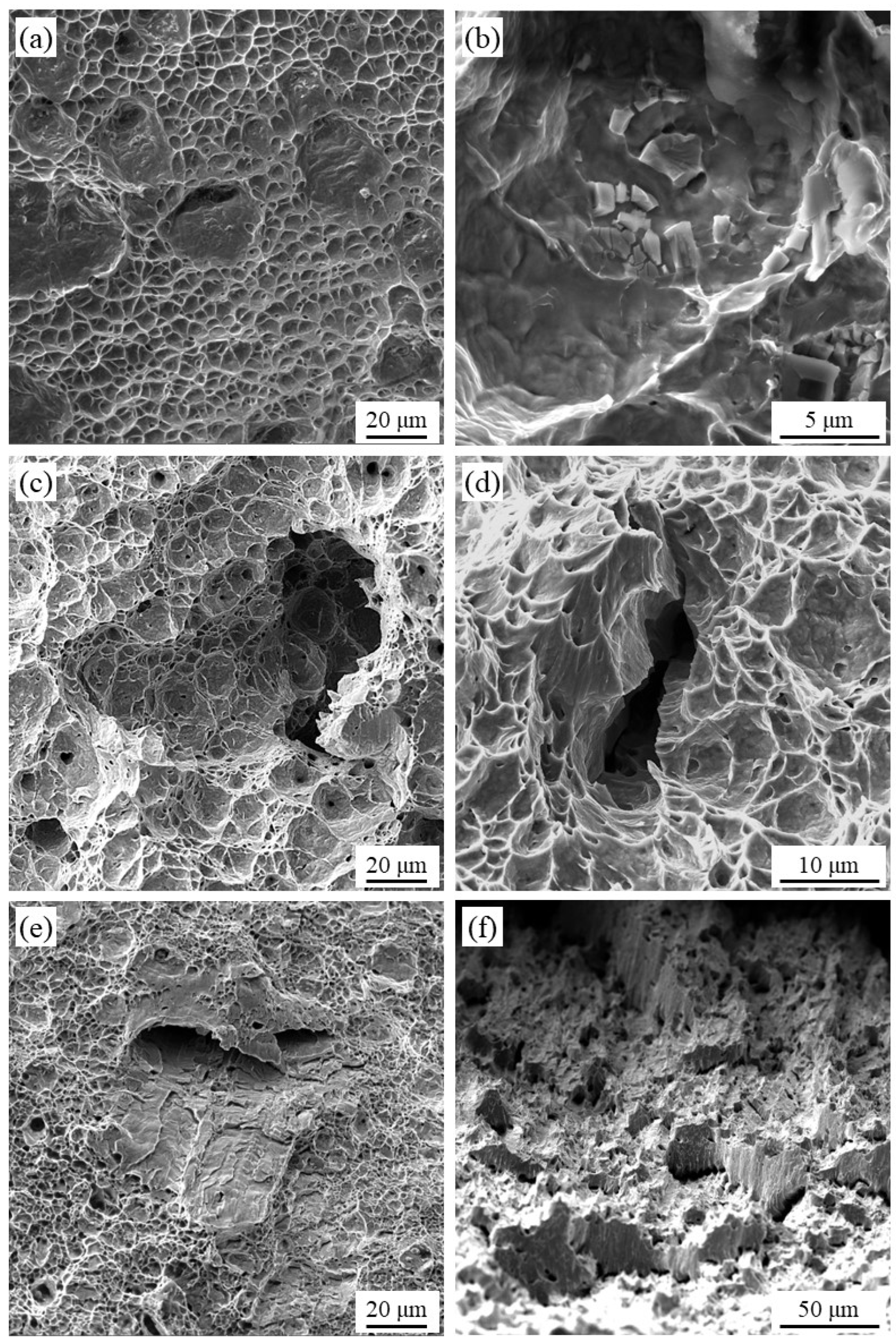
4.5. Fracture Analysis after SSRT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogunsanya, I.G.; Hansson, C.M. Influence of chloride and sulphate anions on the electronic and electrochemical properties of passive films formed on steel reinforcing bars. Materialia 2019, 8, 100491. [Google Scholar] [CrossRef]
- Eurenice, M.; Cronemberger, R.; Nakamatsu, S.; Alberto, C.; Rovere, D. Effect of Cooling Rate on the Corrosion Behavior of As-Cast SAF 2205 Duplex Stainless Steel after Solution Annealing Treatment 2. Exp. Proced. 2015, 18, 138–142. [Google Scholar]
- Bhattacharya, A.; Singh, P.M. Role of microstructure on the corrosion susceptibility of UNS S32101 duplex stainless steel. Corrosion 2008, 64, 532–540. [Google Scholar] [CrossRef]
- Jiang, Y.; Tan, H.; Wang, Z.; Hong, J.; Jiang, L.; Li, J. Influence of Creq/Nieq on pitting corrosion resistance and mechanical properties of UNS S32304 duplex stainless steel welded joints. Corros. Sci. 2013, 70, 252–259. [Google Scholar] [CrossRef]
- Francis, R.; Byrne, G. Duplex Stainless Steels—Alloys for the 21st Century. Metals 2021, 11, 836. [Google Scholar] [CrossRef]
- Charles, J.; Chemelle, P. The history of duplex developments, nowadays DSS properties and duplex market future trends. In Proceedings of the 8th Duplex Stainless Steels Conference, Beaune, France, 13–15 October 2010. [Google Scholar]
- Medina, E.; Medina, J.M.; Cobo, A.; Bastidas, D.M. Evaluation of mechanical and structural behavior of austenitic and duplex stainless steel reinforcements. Constr. Build. Mater. 2015, 78, 1–7. [Google Scholar] [CrossRef]
- Al Kharusi, A.; Rao Saithala, J.; Suryanarayana, M.; Al Nebhani, T.; Al Behlani, N.; Wilms, M.; Yang, B. New material application limits of duplex stainless steel in sour service. In Proceedings of the CORROSION, Nashville, TN, USA, 24–28 March 2019; NACE International: Nashville, TN, USA, 2019; p. 9. [Google Scholar]
- Zanotto, F.; Grassi, V.; Balbo, A.; Monticelli, C.; Melandri, C.; Zucchi, F. Effect of brief thermal aging on stress corrosion cracking susceptibility of LDSS 2101 in the presence of chloride and thiosulphate ions. Corros. Sci. 2018, 130, 22–30. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, J.; Cheng, C.-Q.; Zhang, L.; Li, J.; Liu, B.-J.; Cao, T.-S. The metastable pitting corrosion of 2205 duplex stainless steel under bending deformation. J. Alloys Compd. 2020, 830, 154422. [Google Scholar] [CrossRef]
- Ha, H.Y.; Jang, M.H.; Lee, T.H.; Moon, J. Interpretation of the relation between ferrite fraction and pitting corrosion resistance of commercial 2205 duplex stainless steel. Corros. Sci. 2014, 89, 154–162. [Google Scholar] [CrossRef]
- Cao, X.; Hu, X. The investigation of micro-galvanic corrosion of SAF 2205 duplex stainless steel based on numerical simulation model and immersion test. Corros. Sci. 2022, 207, 110601. [Google Scholar] [CrossRef]
- Fredriksson, W.; Edström, K.; Olsson, C.A. XPS analysis of manganese in stainless steel passive films on 1.4432 and the lean duplex 1.4162. Corros. Sci. 2010, 52, 2505–2510. [Google Scholar] [CrossRef]
- Alvarez, S.M.; Bautista, A.; Velasco, F. Corrosion behaviour of corrugated lean duplex stainless steels in simulated concrete pore solutions. Corros. Sci. 2011, 53, 1748–1755. [Google Scholar] [CrossRef]
- Knyazeva, M.; Pohl, M. Duplex Steels: Part I: Genesis, Formation, Structure. Metallogr. Microstruct. Anal. 2013, 2, 113–121. [Google Scholar] [CrossRef]
- Katona, R.M.; Karasz, E.K.; Schaller, R.F. A Review of the governing factors in pit-to-crack transitions of metallic structures. Corrosion 2023, 79, 72–96. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Cheng, C.Q.; Zhang, D.J.; Zhao, Y.N.; Cao, T.S.; Zhong, S.; Zhang, L.; Zhao, J. Effect of U-bending deformation on pitting corrosion of 2205 duplex stainless steel under wet-dry cycling of chloride salt droplets. Corros. Sci. 2023, 218, 111185. [Google Scholar] [CrossRef]
- Hirayama, R.; Haruyama, S. Electrochemical impedance for degraded coated steel having pores. Corrosion 1991, 47, 952–958. [Google Scholar] [CrossRef]
- Rajaguru, J.; Arunachalam, N. Effect of machined surface integrity on the stress corrosion cracking behaviour of super duplex stainless steel. Eng. Fail. Anal. 2021, 125, 105411. [Google Scholar] [CrossRef]
- Örnek, C.; Engelberg, D.L. Towards understanding the effect of deformation mode on stress corrosion cracking susceptibility of grade 2205 duplex stainless steel. Mater. Sci. Eng. A 2016, 666, 269–279. [Google Scholar] [CrossRef]
- Martin, U.; Bastidas, D.M. Stress corrosion cracking mechanisms of UNS S32205 duplex stainless steel in carbonated solution induced by chlorides. Metals 2023, 13, 567. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, T.; Hu, J.; Jiang, Y.; Jiang, L.; Li, J. Microstructure evolution and pitting corrosion resistance of the Gleeble-simulated heat-affected zone of a newly developed lean duplex stainless steel 2002. J. Alloys Compd. 2016, 658, 1031–1040. [Google Scholar] [CrossRef]
- Briz, E.; Martin, U.; Biezma, M.V.; Calderon-Uriszar-Aldaca, I.; Bastidas, D.M. Evaluation of the mechanical behavior of 2001 LDSS and 2205 DSS reinforcements exposed to simultaneous load and corrosion in chloride contained concrete pore solution. J. Build. Eng. 2020, 31, 101456. [Google Scholar] [CrossRef]
- ASTM E407-23; Standard Practice for Microetching Metals and Alloys. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- ASTM G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM G129-21; Standard Practice for Slow Strain Rate Testing to Evaluate the Susceptibility of Metallic Materials to Environmentally Assisted Cracking. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Sandim, M.J.R.; Souza Filho, I.R.; Mota, C.F.G.S.; Zilnyk, K.D.; Sandim, H.R.Z. Microstructural and magnetic characterization of a lean duplex steel: Strain- induced martensite formation and austenite reversion. J. Magn. Magn. Mater. 2021, 517, 167370. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, Y.; Gao, Z.; Jiang, Z. Mechanical behavior of special-shaped double-web steel-reinforced concrete column joints. Metals 2023, 13, 601. [Google Scholar] [CrossRef]
- Yu, X.; Al-Saadi, S.; Kohli, I.; Zhao, X.; Singh Raman, R.K. Austenitic Stainless-Steel Reinforcement for Seawater Sea Sand Concrete: Investigation of Stress Corrosion Cracking. Metals 2021, 11, 500. [Google Scholar] [CrossRef]
- Ren, Z.; Fang, L.; Wang, H.; Ding, P.; Zeng, X. Seawater corrosion resistance of duplex stainless steel and the axial compressivestiffness of its reinforced concrete columns. Materials 2023, 16, 7249. [Google Scholar] [CrossRef]
- Hammood, A.S. Biomineralization of 2304 duplex stainless steel with surface modification by electrophoretic deposition. J. Appl. Biomater. Funct. Mater. 2020, 18, 228080001989621. [Google Scholar] [CrossRef]
- Örnek, C.; Leygraf, C.; Pan, J. Passive film characterisation of duplex stainless steel using scanning Kelvin probe force microscopy in combination with electrochemical measurements. NPJ Mater. Degrad. 2019, 3, 8. [Google Scholar] [CrossRef]
- Ponciano Gomes, J.A.C.; Silva, S.C.; Campos, T. Stress corrosion cracking susceptibility of armour layers in CO2 annulus environments—SSRT experimental simulation. Eng. Fail. Anal. 2022, 139, 106451. [Google Scholar] [CrossRef]
- Abe, S.; Kojima, M.; Hosoi, Y. Stress corrosion cracking susceptibility index, ISCC, of austenitic stainless steels in constant strain-rate test. In Stress Corrosion Cracking; The Slow Strain-Rate Technique; Ugiansky, G.M., Payer, J.H., Eds.; ASTM International: West Conshohocken, PA, USA, 1979; pp. 294–304. ISBN 978-0-8031-5548-0. [Google Scholar]
- Sohail, M.G.; Laurens, S.; Deby, F.; Balayssac, J.P.; Al Nuaimi, N. Electrochemical corrosion parameters for active and passive reinforcing steel in carbonated and sound concrete. Mater. Corros. 2021, 72, 1854–1871. [Google Scholar] [CrossRef]
- Fan, C.; Bao, Y.; Wang, Z.; Guo, L.; Song, Q.; Xu, N.; Yang, K.; Jiang, Y. Effect of Mn on microstructure and corrosion resistance of duplex stainless steel surfacing layer. J. Mater. Eng. Perform. 2024, 33, 529–538. [Google Scholar] [CrossRef]
- Martin Diaz, U.; Birbilis, N.; Macdonald, D.D.; Bastidas, D.M. Passivity breakdown and crack propagation mechanisms of lean duplex (UNS S32001) stainless steel reinforcement in high alkaline solution under stress corrosion cracking. Corrosion 2023, 79, 4229. [Google Scholar] [CrossRef] [PubMed]


| Alloy | C | Cr | Mn | Ni | Mo | N | Si |
|---|---|---|---|---|---|---|---|
| UNS S32205 | 0.017 | 22.76 | 1.57 | 4.64 | 3.21 | 0.171 | 0.34 |
| UNS S32304 | 0.019 | 22.75 | 0.81 | 4.32 | 0.29 | 0.138 | 0.35 |
| UNS S32001 | 0.028 | 20.07 | 4.19 | 1.78 | 0.22 | 0.129 | 0.65 |
| [Cl−] wt.% | σy MPa | σUTS MPa | εUTS % | εf % |
|---|---|---|---|---|
| UNS S32205 | ||||
| 0 | 510 | 715 | 15.91 | 21.07 |
| 4 | 463 | 560 | 13.05 | 17.37 |
| 8 | 407 | 479 | 9.95 | 12.02 |
| UNS S32304 | ||||
| 0 | 565 | 759 | 23.92 | 27.77 |
| 4 | 631 | 652 | 20.65 | 24.94 |
| 8 | 483 | 575 | 17.52 | 21.11 |
| UNS S32001 | ||||
| 0 | 466 | 609 | 29.33 | 51.66 |
| 4 | 428 | 532 | 25.09 | 42.65 |
| 8 | 388 | 507 | 23.88 | 38.21 |
| [Cl−] wt.% | Ecorr mVSCE | icorr A/cm2 | βa mV/dec | βc mV/dec | B |
|---|---|---|---|---|---|
| UNS S32205 | |||||
| 0 | −58 | 5.14 × 10−8 | 287 | 249 | 57 |
| 4 | −189 | 3.58 × 10−8 | 285 | 225 | 54 |
| 8 | −255 | 5.44 × 10−7 | 197 | 176 | 40 |
| UNS S32304 | |||||
| 0 | −264 | 2.38 × 10−7 | 186 | 216 | 43 |
| 4 | −329 | 2.14 × 10−6 | 172 | 205 | 40 |
| 8 | −455 | 1.61 × 10−6 | 166 | 197 | 39 |
| UNS S32001 | |||||
| 0 | −248 | 5.13 × 10−8 | 277 | 285 | 60 |
| 4 | −254 | 6.34 × 10−8 | 202 | 265 | 49 |
| 8 | −268 | 1.82 × 10−7 | 154 | 245 | 41 |
| [Cl−] wt.% | ISCC % | Iδ % | REL % |
|---|---|---|---|
| UNS S32205 | |||
| 4 | 33.31 | 17.56 | 21.68 |
| 8 | 76.09 | 42.95 | 33.01 |
| UNS S32304 | |||
| 4 | 7.55 | 10.19 | 14.10 |
| 8 | 22.23 | 23.98 | 24.24 |
| UNS S32001 | |||
| 4 | 22.30 | 17.44 | 12.64 |
| 8 | 27.88 | 26.04 | 16.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, U.; Bastidas, D.M. Environmentally Assisted Cracking of Duplex and Lean Duplex Stainless Steel Reinforcements in Alkaline Medium Contaminated with Chlorides. Crystals 2024, 14, 651. https://doi.org/10.3390/cryst14070651
Martin U, Bastidas DM. Environmentally Assisted Cracking of Duplex and Lean Duplex Stainless Steel Reinforcements in Alkaline Medium Contaminated with Chlorides. Crystals. 2024; 14(7):651. https://doi.org/10.3390/cryst14070651
Chicago/Turabian StyleMartin, Ulises, and David M. Bastidas. 2024. "Environmentally Assisted Cracking of Duplex and Lean Duplex Stainless Steel Reinforcements in Alkaline Medium Contaminated with Chlorides" Crystals 14, no. 7: 651. https://doi.org/10.3390/cryst14070651





