Solvent-Dependent Triboelectric Output Performance of Poly(vinylidene fluoride–trifluoroethylene–chlorofluoroethylene) Terpolymer
Abstract
:1. Introduction
2. Experimental Details
2.1. Reagents
2.2. Film Fabrication
2.3. Characterization
2.4. Triboelectric Power Generation Measurement
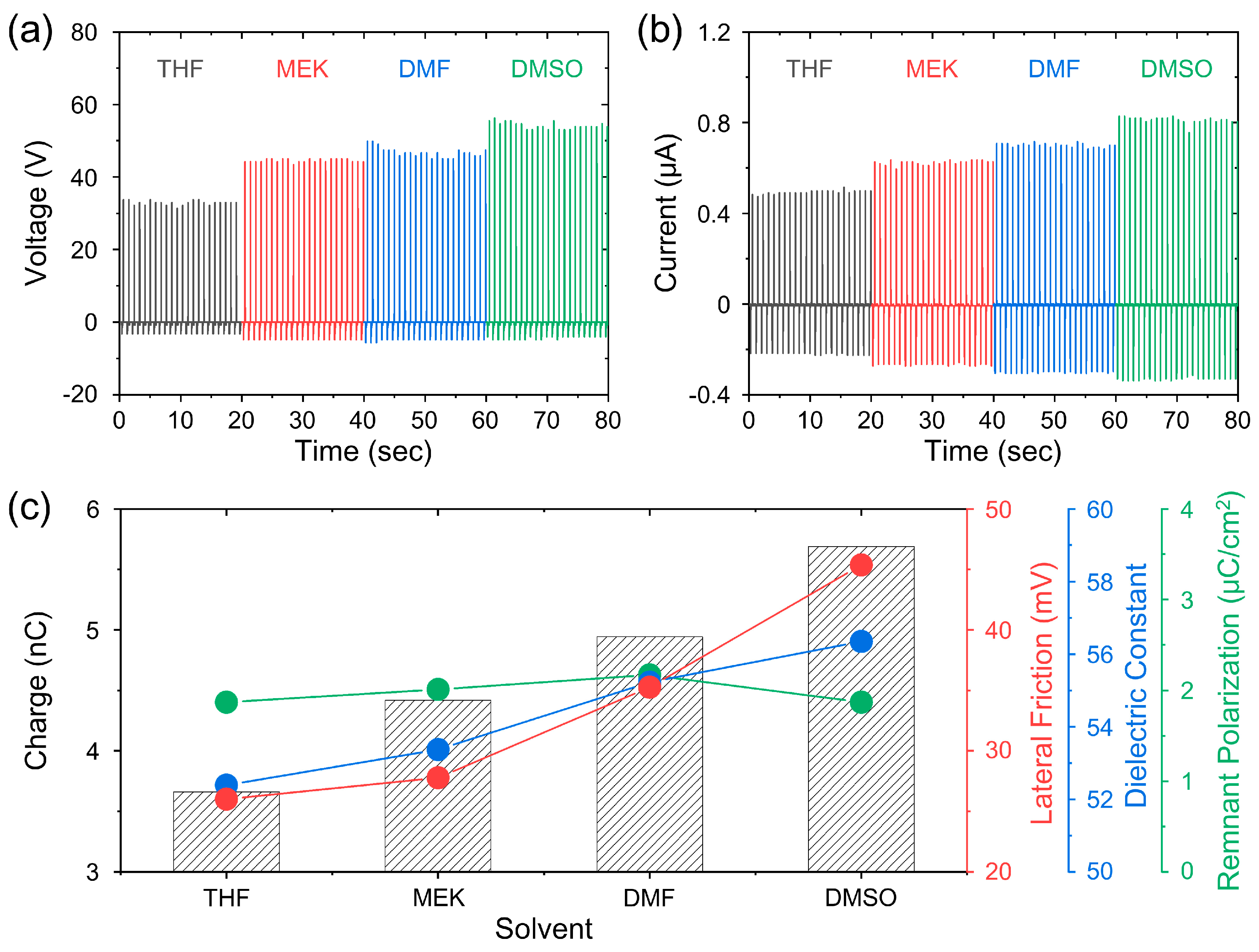
3. Results and Discussion
4. Summary
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, F.-R.; Tian, Z.-Q.; Wang, Z.L. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric nanogenerators as new energy technology for self-powered systems and as active mechanical and chemical sensors. ACS Nano 2013, 7, 9533–9557. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Hur, S.; Lee, D.-G.; Shin, J.; Qiao, H.; Mun, S.; Lee, H.; Moon, W.; Kim, Y.; Baik, J.M. Ferroelectrically augmented contact electrification enables efficient acoustic energy transfer through liquid and solid media. Energy Environ. Sci. 2022, 15, 1243–1255. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, J.H.; Kim, J.K.; Jeong, U. Material aspects of triboelectric energy generation and sensors. NPG Asia Mater. 2020, 12, 6. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, Y.; Guo, L.; Wang, P.; He, X.; Dai, G.; Zheng, H.; Chen, C.; Wang, A.C.; Xu, C. Quantifying the triboelectric series. Nat. Commun. 2019, 10, 1427. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Kwon, Y.H.; Kim, Y.-H.; Jung, J.-Y.; Lee, M.H.; Nah, J. Triboelectric charging sequence induced by surface functionalization as a method to fabricate high performance triboelectric generators. ACS Nano 2015, 9, 4621–4627. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zi, Y.; Zhou, Y.S.; Li, S.; Fan, F.; Lin, L.; Wang, Z.L. Molecular surface functionalization to enhance the power output of triboelectric nanogenerators. J. Mater. Chem. A 2016, 4, 3728–3734. [Google Scholar] [CrossRef]
- Yun, S.-Y.; Kim, M.H.; Yang, G.G.; Choi, H.J.; Kim, D.-W.; Choi, Y.-K.; Kim, S.O. A triboelectric nanogenerator with synergistic complementary nanopatterns fabricated by block copolymer self-assembly. J. Mater. Chem. A 2024, 12, 11302–11309. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, Z.-H.; Jing, Q.; Bai, P.; Pan, C.; Yang, Y.; Zhou, Y.; Wang, Z.L. Toward large-scale energy harvesting by a nanoparticle-enhanced triboelectric nanogenerator. Nano Lett. 2013, 13, 847–853. [Google Scholar] [CrossRef]
- Kim, J.-K.; Han, G.H.; Kim, S.-W.; Kim, H.J.; Purbia, R.; Lee, D.-M.; Kim, J.K.; Hwang, H.J.; Song, H.-C.; Choi, D. Electric-field-driven interfacial trapping of drifting triboelectric charges via contact electrification. Energy Environ. Sci. 2023, 16, 598–609. [Google Scholar] [CrossRef]
- Chung, J.; Lee, S.; Yong, H.; Moon, H.; Choi, D.; Lee, S. Self-packaging elastic bellows-type triboelectric nanogenerator. Nano Energy 2016, 20, 84–93. [Google Scholar] [CrossRef]
- Lee, D.W.; Jeong, D.G.; Kim, J.H.; Kim, H.S.; Murillo, G.; Lee, G.-H.; Song, H.-C.; Jung, J.H. Polarization-controlled PVDF-based hybrid nanogenerator for an effective vibrational energy harvesting from human foot. Nano Energy 2020, 76, 105066. [Google Scholar] [CrossRef]
- Rana, S.S.; Rahman, M.T.; Salauddin, M.; Sharma, S.; Maharjan, P.; Bhatta, T.; Cho, H.; Park, C.; Park, J.Y. Electrospun PVDF-TrFE/MXene nanofiber mat-based triboelectric nanogenerator for smart home appliances. ACS Appl. Mater. Interfaces 2021, 13, 4955–4967. [Google Scholar] [CrossRef]
- Bai, P.; Zhu, G.; Zhou, Y.S.; Wang, S.; Ma, J.; Zhang, G.; Wang, Z.L. Dipole-moment-induced effect on contact electrification for triboelectric nanogenerators. Nano Res. 2014, 7, 990–997. [Google Scholar] [CrossRef]
- Seung, W.; Yoon, H.J.; Kim, T.Y.; Ryu, H.; Kim, J.; Lee, J.H.; Lee, J.H.; Kim, S.; Park, Y.K.; Park, Y.J. Boosting power-generating performance of triboelectric nanogenerators via artificial control of ferroelectric polarization and dielectric properties. Adv. Energy Mater. 2017, 7, 1600988. [Google Scholar] [CrossRef]
- Jeong, D.G.; Ko, Y.J.; Kim, J.H.; Kong, D.S.; Hu, Y.C.; Lee, D.W.; Im, S.H.; Lee, J.; Kim, M.S.; Lee, G.-H. On the origin of enhanced power output in ferroelectric polymer-based triboelectric nanogenerators: Role of dipole charge versus piezoelectric charge. Nano Energy 2022, 103, 107806. [Google Scholar] [CrossRef]
- Šutka, A.; Mālnieks, K.; Linarts, A.; Timusk, M.; Jurķāns, V.; Gorņevs, I.; Blūms, J.; Bērziņa, A.; Joost, U.; Knite, M. Inversely polarised ferroelectric polymer contact electrodes for triboelectric-like generators from identical materials. Energy Environ. Sci. 2018, 11, 1437–1443. [Google Scholar] [CrossRef]
- Ong, D.T.K.; Koay, J.S.C.; Sim, M.T.; Aw, K.C.; Nakajima, T.; Chen, B.; Tan, S.T.; Gan, W.C. High performance composition-tailored PVDF triboelectric nanogenerator enabled by low temperature-induced phase transition. Nano Energy 2023, 113, 108555. [Google Scholar] [CrossRef]
- Shvartsman, V.V.; Lupascu, D.C. Lead-free relaxor ferroelectrics. J. Am. Ceram. Soc. 2012, 95, 1–26. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.H.; Ryu, H.; Lee, J.H.; Khan, U.; Kim, H.; Kwak, S.S.; Kim, S.W. High-performance piezoelectric, pyroelectric, and triboelectric nanogenerators based on P (VDF-TrFE) with controlled crystallinity and dipole alignment. Adv. Funct. Mater. 2017, 27, 1700702. [Google Scholar] [CrossRef]
- Available online: https://piezotech.arkema.com/en/Products/high-k-terpolymers/ (accessed on 16 July 2024).
- Patterson, A. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Bao, H.-M.; Song, J.-F.; Zhang, J.; Shen, Q.-D.; Yang, C.-Z.; Zhang, Q. Phase transitions and ferroelectric relaxor behavior in P (VDF− TrFE− CFE) terpolymers. Macromolecules 2007, 40, 2371–2379. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, J.; Wang, X.; Wang, S. Effect of PMMA on crystallization behavior and hydrophilicity of poly (vinylidene fluoride)/poly (methyl methacrylate) blend prepared in semi-dilute solutions. Appl. Surf. Sci. 2007, 253, 8377–8388. [Google Scholar] [CrossRef]
- Della Schiava, N.; Pedroli, F.; Thetpraphi, K.; Flocchini, A.; Le, M.-Q.; Lermusiaux, P.; Capsal, J.-F.; Cottinet, P.-J. Effect of beta-based sterilization on P (VDF-TrFE-CFE) terpolymer for medical applications. Sci. Rep. 2020, 10, 8805. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.J.; Runt, J.; Zhang, Q. Influence of Crystallization Conditions on the Microstructure and Electromechanical Properties of Poly (vinylidene fluoride− trifluoroethylene− chlorofluoroethylene) Terpolymers. Macromolecules 2003, 36, 7220–7226. [Google Scholar] [CrossRef]
- Tung, K.-L.; Lu, K.-T.; Ruaan, R.-C.; Lai, J.-Y. Molecular dynamics study of the effect of solvent types on the dynamic properties of polymer chains in solution. Desalination 2006, 192, 380–390. [Google Scholar] [CrossRef]
- Šutka, A.; Mālnieks, K.; Lapčinskis, L.; Kaufelde, P.; Linarts, A.; Bērziņa, A.; Zābels, R.; Jurķāns, V.; Gorņevs, I.; Blūms, J. The role of intermolecular forces in contact electrification on polymer surfaces and triboelectric nanogenerators. Energy Environ. Sci. 2019, 12, 2417–2421. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Seol, M.L.; Woo, J.H.; Lee, D.I.; Im, H.; Hur, J.; Choi, Y.K. Nature-replicated nano-in-micro structures for triboelectric energy harvesting. Small 2014, 10, 3887–3894. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kobayashi, K.; Yamada, H.; Horiuchi, T.; Ishida, K.; Matsushige, K. Study on orientation mechanisms of poly (vinylidenefluoride-trifluoroethylene) molecules aligned by atomic force microscopy. Appl. Surf. Sci. 2006, 252, 5489–5494. [Google Scholar] [CrossRef]
- Monroy, F.; Arriaga, L.; Langevin, D. Langmuir polymer films: Recent results and new perspectives. Phys. Chem. Chem. Phys. 2012, 14, 14450–14459. [Google Scholar] [CrossRef]
- Kunwar, D.; Vazquez, I.R.; Jackson, N. Effects of solvents on synthesis of piezoelectric polyvinylidene fluoride trifluoroethylene thin films. Thin Solid Films 2022, 757, 139414. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Luo, B.; Wang, X.; Wang, Y.; Li, L. Fabrication, characterization, properties and theoretical analysis of ceramic/PVDF composite flexible films with high dielectric constant and low dielectric loss. J. Mater. Chem. A 2014, 2, 510–519. [Google Scholar] [CrossRef]
- Wang, J.-W.; Shen, Q.-D.; Yang, C.-Z.; Zhang, Q.-M. High dielectric constant composite of P (VDF− TrFE) with grafted copper phthalocyanine oligomer. Macromolecules 2004, 37, 2294–2298. [Google Scholar] [CrossRef]
- Pirc, R.; Blinc, R. Vogel-Fulcher freezing in relaxor ferroelectrics. Phys. Rev. B 2007, 76, 020101. [Google Scholar] [CrossRef]
- Viehland, D.; Jang, S.; Cross, L.E.; Wuttig, M. Freezing of the polarization fluctuations in lead magnesium niobate relaxors. J. Appl. Phys. 1990, 68, 2916–2921. [Google Scholar] [CrossRef]
- Cross, L. Relaxor ferroelectrics. In Piezoelectricity; Heywang, W., Lubits, K., Wersing, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 114, pp. 131–155. [Google Scholar]
- Hambal, Y.; Shvartsman, V.V.; Lewin, D.; Huat, C.H.; Chen, X.; Michiels, I.; Zhang, Q.; Lupascu, D.C. Effect of composition on polarization hysteresis and energy storage ability of P (VDF-TrFE-CFE) relaxor terpolymers. Polymers 2021, 13, 1343. [Google Scholar] [CrossRef]
- Tsutsumi, N.; Okumachi, K.; Kinashi, K.; Sakai, W. Re-evaluation of the origin of relaxor ferroelectricity in vinylidene fluoride terpolymers: An approach using switching current measurements. Sci. Rep. 2017, 7, 15871. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, J.H.; Jung, Y.; Shin, J.C.; Lee, Y.; Kim, K.; Kim, N.; van der Zande, A.M.; Son, J.; Lee, G.-H. Evolution of defect formation during atomically precise desulfurization of monolayer MoS2. Commun. Mater. 2021, 2, 80. [Google Scholar] [CrossRef]
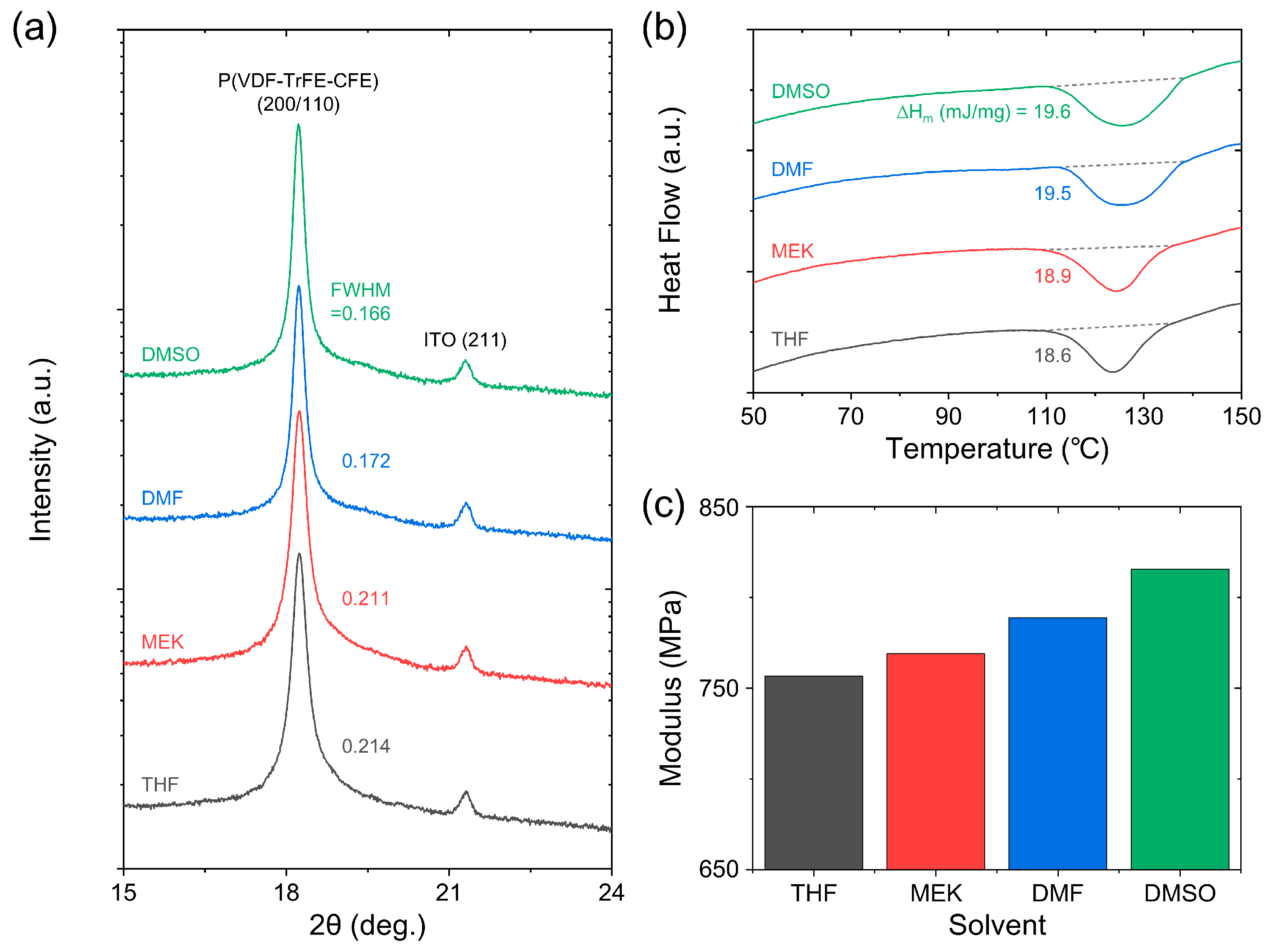
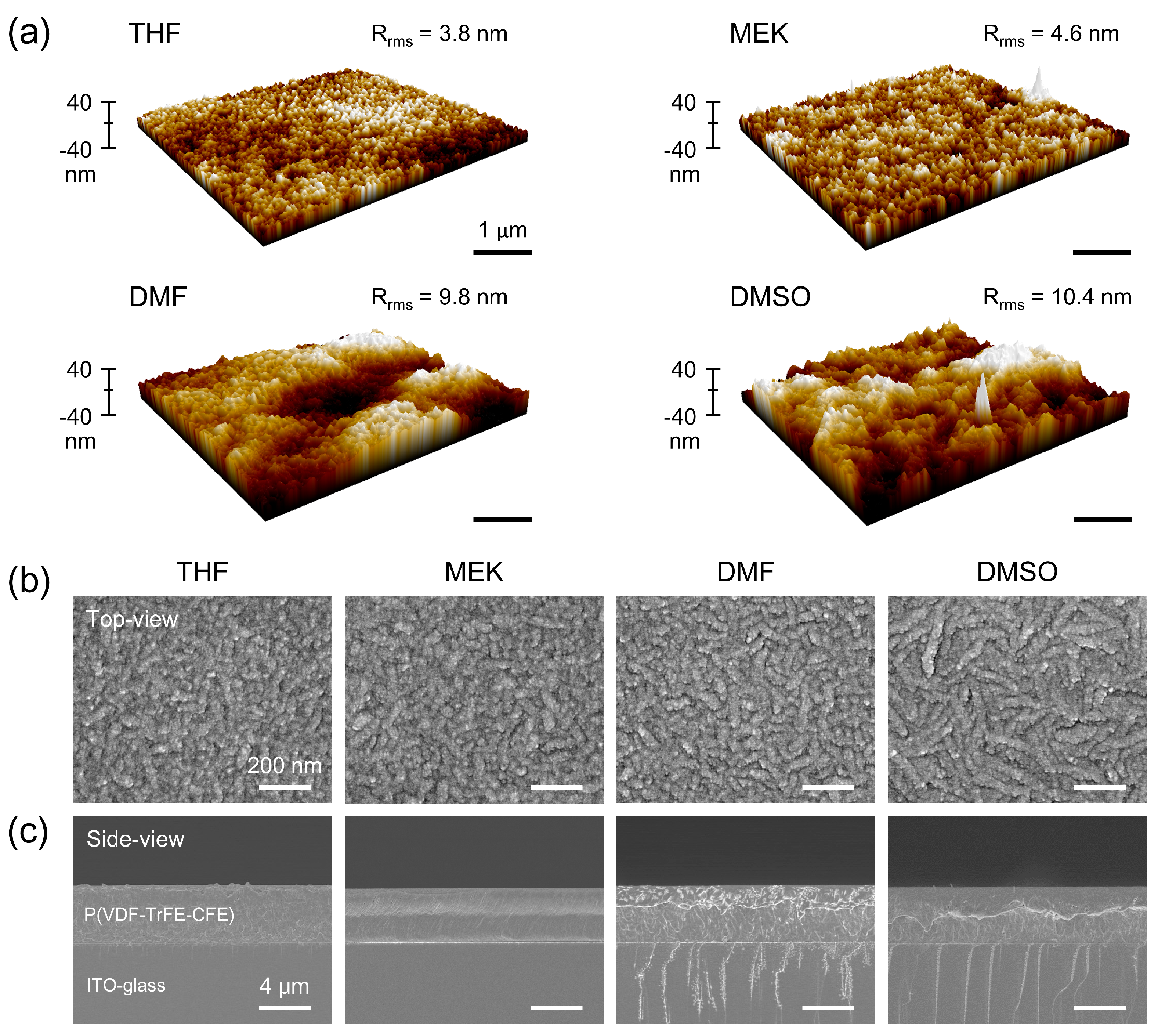
| Solvent | Crystallinity (%) | Grain Length (nm) | Grain Width (nm) | Thickness (μm) | Roughness (nm) |
|---|---|---|---|---|---|
| THF | 44.4 | 119.8 | 39.7 | 4.4 | 3.8 |
| MEK | 45.1 | 155.3 | 40.1 | 4.2 | 4.6 |
| DMF | 46.4 | 164.5 | 43.5 | 4.4 | 9.8 |
| DSMO | 46.7 | 213.6 | 48.2 | 4.4 | 10.4 |
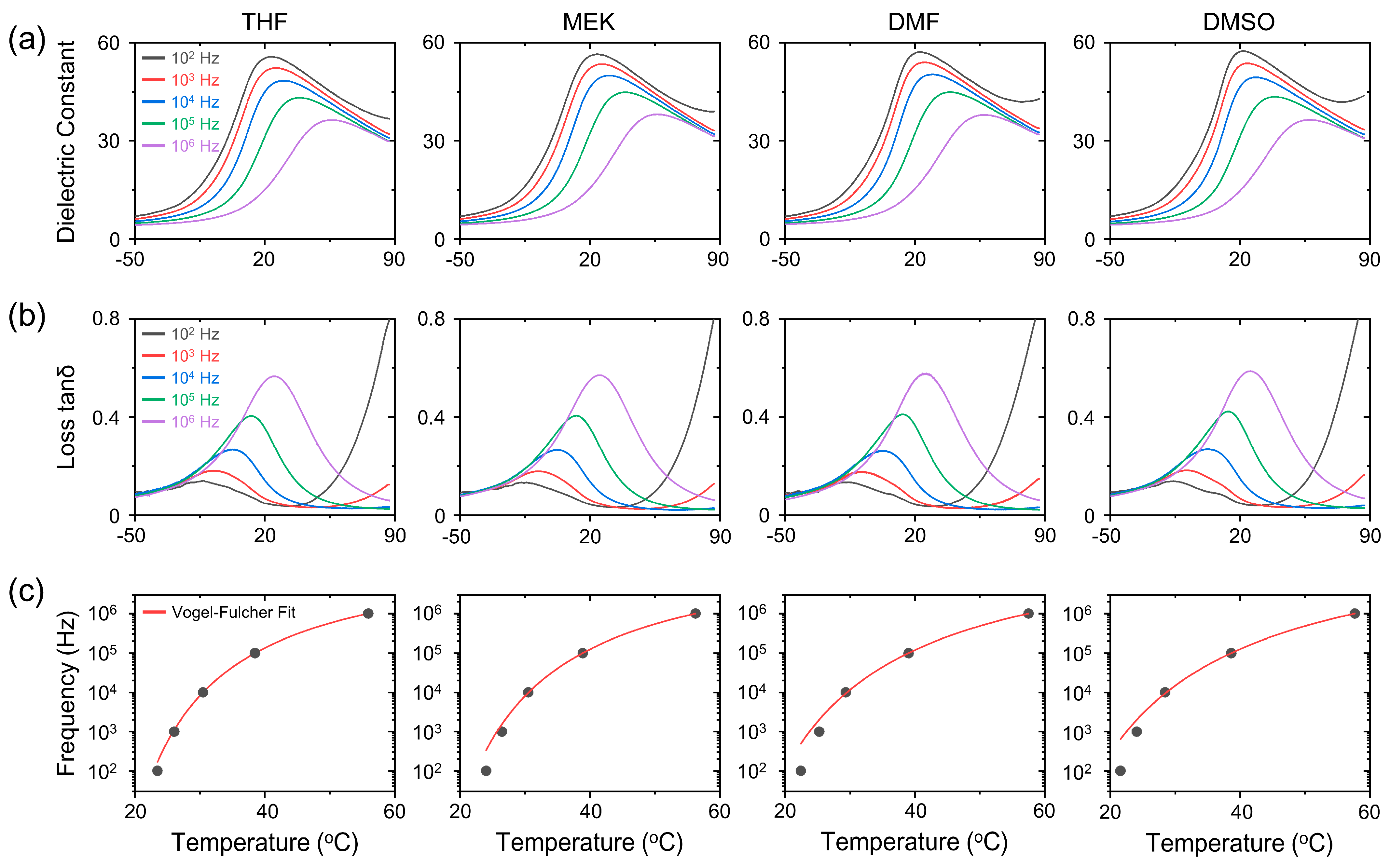
| Solvent | Activation Energy (meV) | Freezing Temperature (°C) |
|---|---|---|
| THF | 15.9 | 8.8 |
| MEK | 17.9 | 6.9 |
| DMF | 24.0 | 0 |
| DMSO | 26.7 | −3.4 |
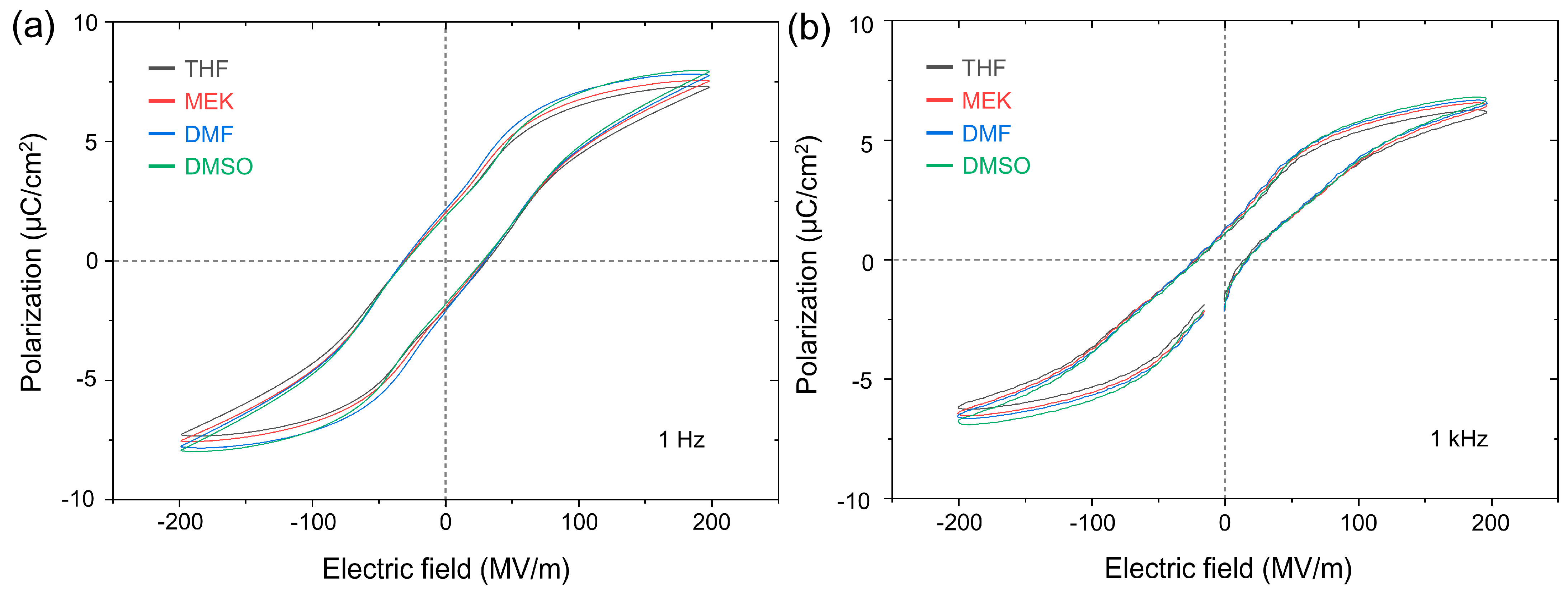
| Solvent | Saturated Polarization (μC/cm2) | Remnant Polarization (μC/cm2) | Coercive Field (MV/m) | |||
|---|---|---|---|---|---|---|
| 1 Hz | 1 kHz | 1 Hz | 1 kHz | 1 Hz | 1 kHz | |
| THF | 7.3 | 6.2 | 1.9 | 1.1 | 30.2 | 21.1 |
| MEK | 7.6 | 6.5 | 2.0 | 1.3 | 30.9 | 22.5 |
| DMF | 7.8 | 6.6 | 2.2 | 1.3 | 31.9 | 23.8 |
| DMSO | 8.0 | 6.8 | 1.9 | 1.1 | 30.0 | 20.2 |
| Solvent | Peak-to-Peak Voltage (V) | Peak-to-Peak Current (μA) |
|---|---|---|
| THF | 35.6 | 0.7 |
| MEK | 49.8 | 0.8 |
| DMF | 51.8 | 1.0 |
| DSMO | 58.6 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.C.; Ahn, H.S.; Lee, J.H.; Kim, K.H.; Kim, J.H.; Jung, J.H. Solvent-Dependent Triboelectric Output Performance of Poly(vinylidene fluoride–trifluoroethylene–chlorofluoroethylene) Terpolymer. Crystals 2024, 14, 664. https://doi.org/10.3390/cryst14070664
Hu YC, Ahn HS, Lee JH, Kim KH, Kim JH, Jung JH. Solvent-Dependent Triboelectric Output Performance of Poly(vinylidene fluoride–trifluoroethylene–chlorofluoroethylene) Terpolymer. Crystals. 2024; 14(7):664. https://doi.org/10.3390/cryst14070664
Chicago/Turabian StyleHu, Ying Chieh, Hyun Soo Ahn, Joo Hyeong Lee, Kyung Hoon Kim, Jong Hun Kim, and Jong Hoon Jung. 2024. "Solvent-Dependent Triboelectric Output Performance of Poly(vinylidene fluoride–trifluoroethylene–chlorofluoroethylene) Terpolymer" Crystals 14, no. 7: 664. https://doi.org/10.3390/cryst14070664






