Abstract
Three hybrid compounds based on decavanadates, i.e., (NH4)2[Co(H2O)5(β-HAla)]2[V10O28]·4H2O (1), (NH4)2[Ni(H2O)5(β-HAla)]2[V10O28]·4H2O (2), and (NH4)2[Cd(H2O)5(β-HAla)]2[V10O28]·2H2O (3), (where β-Hala = zwitterionic form of β-alanine) were prepared by reactions in mildly acidic conditions (pH ~ 4) at room temperature. These compounds crystallise in two structure types, both crystallising in monoclinic P21/n space group but with dissimilar cell packing, i.e., as tetrahydrates (1 and 2) and as a dihydrate (3). An influence of crystal radii and spin state of the central atom in [M(H2O)5(β-HAla)]2+ complex cations on the crystal packing leading to the formation of different crystallohydrate forms was investigated together with previously prepared (NH4)2[Zn(H2O)5(β-HAla)]2[V10O28]·4H2O (4) and (NH4)2[Mn(H2O)5(β-HAla)]2[V10O28]·2H2O (5) and spin states of [M(H2O)5(β-HAla)]2+ (M = Co2+, Ni2+, and Mn2+) cations in solution were confirmed by 1H-NMR paramagnetic effects. FT-IR and FT-Raman spectra for 1–5 are in agreement with the X-ray structure analysis results.
1. Introduction
Decavanadate anion is the predominant and most stable V(V) oxo-anion found in the acidic aqueous solutions [1]. It is known in different protonation states, [HnV10O28](6–n)– (n = 0–4) [2,3,4].
The decavanadate anion consists of ten edge-sharing VO6 octahedra. Six of them form a rectangular 2 × 3 arrangement, with two VO6 octahedra joining the arrangement from the top and two from the bottom via edge sharing with the six octahedra lying in the central plane. The ideal symmetry of [V10O28]6– anion is given by the D2h point group symmetry. In most crystal structures, [V10O28]6– anion usually occupies special positions, mostly inversion centres, and its symmetry is changed correspondingly; however, its geometry remains close to the ideal D2h symmetry.
In crystal structures, decavanadates act as acceptors of protons in supramolecular arrangements; protonated decavanadate species can also act as proton donors. Typically, if decavanadate anion is not protonated on mutually centrosymmetrically arranged sites, such decavanadate anions will likely form a centrosymmetric dimer interconnected via anion-anion hydrogen bonds [5] or via hydrogen bonding to counter-cations lying on a centre of symmetry [6]. Considering the sterical effect of the decavanadate anion and the influence of size, shape, and nature of counter-cations, a substantial part of decavanadates include water molecules as a stabilising element of the crystal structure. Formation of different crystallohydrates depends on reaction conditions [7] or on temperature changes, even if the reaction conditions remain the same [8]. As a result, decavanadates are known to form a large variety of supramolecular assemblies [9].
The oxovanadates (V), including decavanadates and peroxovanadium compounds, are of great interest in bioinorganic chemistry and biochemistry because of their antidiabetic, antibacterial, antiprotozoal, antiviral, and anticancer properties [10,11]. Studying of non-covalent interactions based on electrostatic attractive forces between decavanadate anion and appropriate molecules (organic cations, hybrid inorganic–organic complex cations, and biomacromolecules) could provide important information on the cooperative effects of decavanadate ions in biological systems [12,13,14].
Apart from the biological importance of decavanadates, there are other emerging application possibilities. Some decavanadates exhibit water oxidation activity [15] or heterogeneous bifunctional catalytic properties in the selective oxidation of sulphides and Mannich reaction [16]. Decavanadates with superior proton conductivity [17] and photoluminescent sensing properties for the detection of Zn2+ and Co2+ [18] were also prepared. Ammonium decavanadate nanodots—holey reduced graphene oxide nanoribbons [19] and α-Co(OH)2 nanoplates with decavanadate anion [20] can be used as electrodes for supercapacitors.
In this work, we report the synthesis, crystal structure determination, and properties of three decavanadates, i.e., (NH4)2[Co(H2O)5(β-HAla)]2[V10O28]·4H2O (1), (NH4)2[Ni(H2O)5(β-HAla)]2[V10O28]·4H2O (2), and (NH4)2[Cd(H2O)5(β-HAla)]2[V10O28]·2H2O (3), prepared by the same procedure we used previously for the preparation of two decavanadates containing the same [M(H2O)5(β-HAla)]2+ cations in two different crystallohydrate forms, i.e., tetrahydrate (NH4)2[Zn(H2O)5(β-HAla)]2[V10O28]·4H2O (4) and dihydrate (NH4)2[Mn(H2O)5(β-HAla)]2[V10O28]·2H2O (5) [21]. The compounds 1 and 2 are isostructural with 4, while 3 is isostructural with 5. In [22], compound 2 was prepared by using an alternative hydrothermal “Direct Synthesis” approach by the reaction of metal powder in anion-deficient conditions with V2O5 in an aqueous solution of β-alanine and ammonium acetate. Subsequently, compound 2 was used as a precursor for the preparation of V2O5/MnV2O6 mixed oxide, and its ability to act as a water oxidation catalyst for oxygen production was studied. Later, 1 was prepared by the same “Direct Synthesis” method and used as a precursor for the preparation of solid mixed oxides CoV2O6/V2O5 and Co2V2O7/V2O5 by thermal decomposition; both these mixed oxides and 1 can act as catalysts for photoinduced water oxidation [23]. The aim of the present study is to obtain more complete insight into the formation of different crystallohydrates with the general formula (NH4)2[M(H2O)5(β-HAla)]2[V10O28]·nH2O, where M is a divalent metal ion, from the same reaction conditions.
2. Materials and Methods
2.1. General
All chemicals were of reagent or better grade, obtained from commercial sources (Mikrochem (Pezinok, Slovakia), Sigma-Aldrich (Saint Louis, MI, USA), Slavus (Donja Stubica, Croatia), Lachema (Brno, Czech Republic)). Infrared spectra were obtained from KBr pellets on a Thermo Scientific Nicolet 6700 FTIR spectrometer in the 400–4000 cm−1 range (Waltham, MA, USA). The Raman spectra were registered with a Raman micro-spectrometer Senterra (Bruker Optik, Ettlingen, Germany), using a laser with the wavelength 532 nm, with a maximum power of 2 mW, in the range 64–4467 cm−1, with 10× objective lenses, and 2 scans for 10 s each. 1H-NMR spectra were recorded on a Bruker AVANCE Neo 400 MHz (operating at 9.37 T, 400 MHz) using D2O as a reference. Chemical shifts are reported in Hz. Vanadium (V) was determined volumetrically by titration with FeSO4 (c = 0.1 m) using diphenylamine as the indicator.
2.2. Synthesis and Crystallisation
2.2.1. Synthesis of (NH4)2[Co(H2O)5(β-HAla)]2[V10O28]·4H2O (1)
CoSO4.7H2O (1.4055 g; 5 mmol) was added to a solution of β-alanine (0.89 g; 10 mmol) in water (20 mL). After stirring for 15 min, a solution of NH4VO3 (1.170 g; 10 mmol) in water (40 mL) was added under the immediate formation of a fine precipitate. The solution with precipitate was stirred for 30 min and filtered. The pH of the filtrate was adjusted to 4.0 with 2M H2SO4. To the reddish-brown solution obtained, ethanol (10 mL) was added. Reddish brown crystals were isolated after standing for 22 days at 4 °C in the refrigerator.
Yield: 0.8 g/52.1% (calc. for vanadium). Anal. Calc. for C6H50N4O46Co2V10 (MW = 1541.76 g/mol) V, 34.10%. Found: V, 33.96%.
2.2.2. Synthesis of (NH4)2[Ni(H2O)5(β-HAla)]2[V10O28]·4H2O (2)
NiCl2.6H2O (1.188 g; 5 mmol) was added to a solution of β-alanine (0.89 g; 10 mmol) in water (20 mL). After stirring for 15 min, a solution of NH4VO3 (1.170 g; 10 mmol) in water (60 mL) was added, followed by the immediate formation of a fine precipitate. The solution with precipitate was stirred for 30 min and filtered. The pH of the filtrate was adjusted to 4.0 with 4M HCl. To the yellow solution obtained, ethanol (10 mL) was added. Dark orange crystals with an intense green tone were isolated after standing for 20 days at 4 °C in the refrigerator.
Yield: 1.343 g/87.5% (calc. for vanadium). Anal. Calc. for C6H50N4O46Ni2V10 (MW = 1541.32 g/mol) V, 34.11%. Found: V, 33.69%.
2.2.3. Synthesis of (NH4)2[Cd(H2O)5(β-HAla)]2[V10O28]·2H2O (3)
Cd(NO3)2.4H2O (1.542 g; 5 mmol) was added to a solution of β-alanine (0.89 g; 10 mmol) in water (20 mL). After stirring for 15 min, a solution of NH4VO3 (1.170 g; 10 mmol) in water (40 mL) was added, followed by the immediate formation of a fine precipitate. The solution with precipitate was stirred for 30 min and filtered. The pH of the filtrate was adjusted to 4.25 with 4M HNO3. To the yellow solution obtained, ethanol (10 mL) was added. Orange crystals were isolated after standing for 15 days at 4 °C in the refrigerator.
Yield: 0.702 g/43.7% (calc. for vanadium). Anal. Calc. for C6H46N4O44Cd2V10 (MW = 1612.67 g/mol) V, 31.59%. Found: V, 31.44%.
(NH4)2[Zn(H2O)5(β-HAla)]2[V10O28]·4H2O (4) and (NH4)2[Mn(H2O)5(β-HAla)]2[V10O28]·2H2O (5) samples were prepared for physical measurements according to [22].
2.3. X-ray Data Collection and Structure Determination
Intensity data for the compounds 1–3 were collected on a Kuma KM–4 CCD diffractometer using graphite monochromated MoKα radiation (0.71073 Å) by the ω- and φ-scan techniques at room temperature. Data collection, data reduction, and finalisation were carried out using CrysAlis Pro-Version 1.171.43.128a software [24]. Intensity data for 4 and 5 were reprocessed from original images collected under the conditions in [21] by using the above-mentioned up-to-date version of CrysAlis Pro software to take advantage of improved data processing, especially regarding twin data reduction handling.
The structures were solved by SHELXT [25] and refined by the full matrix least-squares method on all F2 data using SHELXL-2018/3 [26]. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms of methylene groups were refined using a riding model, and the ammonium group was refined as a free rotor, with C–H and N–H distances free to refine. Hydrogen atoms of water molecules were refined with all O–H and all H…H distances restrained to be equal; similarly, the tetrahedral shape of ammonium cation was retained by restraining all N–H and all H…H distances to be equal. Thermal parameters of the H atoms were constrained to Uiso(H) = 1.2Ueq(C) and Uiso(H) = 1.5Ueq(N, O).
Both compounds 3 and 5 crystallise as non-merohedral twins. Two domains related to the twofold rotation about the c-axis were found using Ewald Explorer in the CrysAlis Pro software, and data were refinalised using internal programme features. The twin domain volume ratios were refined to 0.6515(7):0.3485(7) for 3 and 0.8436(4):0.1564(4) for 5.
Geometrical calculations were performed using SHELXL-2018/3, Olex2 1.5 [27], and PARST [28]. Olex2 1.5 and DIAMOND [29] were used for molecular graphics. Octahedral distortion parameters ζ [30], Δ [31], Σ [32], and Θ [33] were calculated using OctaDist 3.1.0 [34]. Hydrogen bonding geometries were normalised to neutron distances following a literature procedure [35,36].
Crystal data, conditions of data collection, and refinement results for the compounds 1–5 are reported in Table S1.
2.4. Paramagnetic 1H-NMR Measurements
For the Evans method [37,38,39], approx. 2 mM solutions of 1, 2, 4, and 5 using 3% (v/v) t-BuOH solution in H2O were prepared. The inner n.m.r. tube (o.d. 2 mm) was loaded with 3% (v/v) t-BuOH solution in D2O as a reference. The outer n.m.r. tube (o.d. 5 mm) was filled with 500 μL of the sample solution.
The ∆f value, defined as the difference between the chemical shift of the 1H-NMR t-Bu signal in the sample solution and that of the t-BuOH reference solution, was used to calculate the molar susceptibility of the complex using the following equation:
where c is the concentration of the paramagnetic complex in the solution in mmol.L−1 and f is the spectrometer frequency (400 MHz). Considering there are 2 [M(H2O)5(β-HAla)]2+ cations per formula unit, the value of c is twice the concentration of the compound in the solution.
The χp values [cm3mol−1] are molar susceptibilities corrected for diamagnetic contribution to the susceptibility in the sample.
where χd represents diamagnetic contributions from the ligands, ions, inner-core electrons, etc. [40]. The following values were used to calculate the effective magnetic moment:
where k is the Boltzmann constant, T is temperature [K], NA is Avogadro’s number, and μB is the Bohr magneton. Effective magnetic moment is compared to the calculated value for the central atom in question as follows [41]:
3. Results and Discussion
Decavanadates with complex cations, i.e., (NH4)2[Co(H2O)5(β-HAla)]2[V10O28] ·4H2O (1), (NH4)2[Ni(H2O)5(β-HAla)]2[V10O28]·4H2O (2), and (NH4)2[Cd(H2O)5(β-HAla)]2[V10O28]·2H2O (3), were obtained by crystallisation from the β-alanine—CoSO4—NH4VO3—H2SO4—H2O—ethanol (1), β-alanine—NiCl2—NH4VO3—HCl—H2O—ethanol (2), and β-alanine—Cd(NO3)2—NH4VO3—HNO3—H2O—ethanol (3) reaction systems in mildly acidic (pH~4) conditions. Attempts to prepare compounds of Mg2+, Sr2+, Ba2+, Pb2+, and Hg2+ from the same reaction system were unsuccessful, obtaining ammonium decavanadate as a product.
The preparation of compound 1 by the hydrothermal reaction of cobalt powder with V2O5 in an aqueous solution containing β-alanine and ammonium acetate was reported earlier [23]. However, from the described green colour of the solution and products obtained by hydrothermal synthesis when compared with the orange product we obtained, it can be inferred that a partial reduction of V(V) to V(IV) has taken place. This partial reduction can be avoided by using our preparation method. Although the synthesis was targeted towards using the prepared compound as a precursor for the preparation of mixed Co/V oxides by thermal decomposition, to avoid the presence of lower oxidation states of vanadium in the products, not only during the reactions in solutions but even after thermal decomposition in the air atmosphere, it is a good practise to use purified V(V) precursors [42].
3.1. Crystallographic Characterisation of Prepared Compounds
All prepared compounds belong to one of two monoclinic (space group P21/n) structure types with substantially different cell parameters and cell packing—tetrahydrates (1, 2, and 4) (Figure 1a) and dihydrates (3 and 5) (Figure 1b).
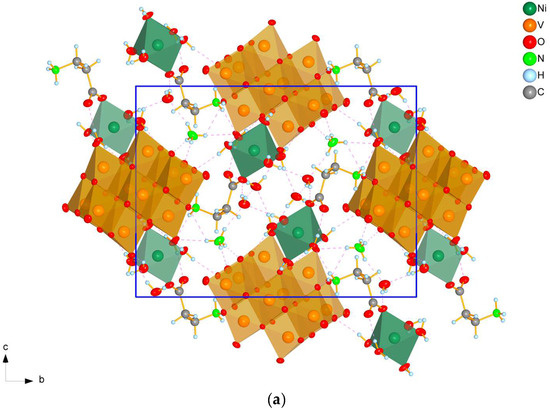
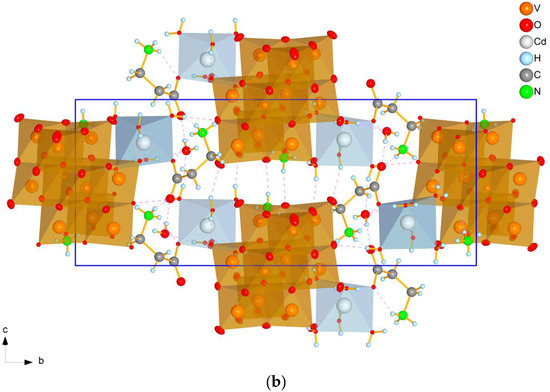
Figure 1.
A view of the cell packing of tetrahydrates (2 as an example) (a) and dihydrates (3 as an example) (b) along the a-axis. Dashed lines indicate hydrogen bonds, blue rectangle denote unit cell boundaries.
The asymmetric unit of all prepared dihydrates consists of one half of the [V10O28]6– anion with Ci symmetry lying on an inversion centre, one [M(H2O)5(β-HAla)]2+ cation, one NH4+ cation, and two water molecules in general positions (Figure 2a).
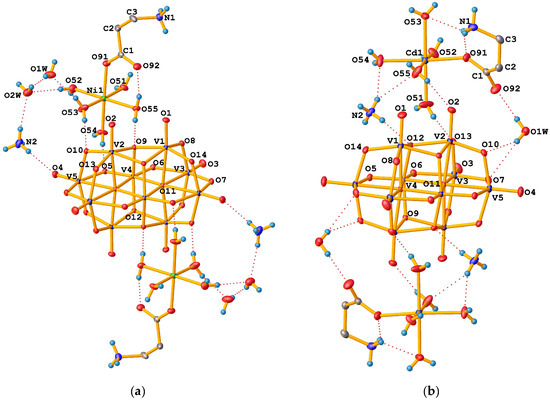
Figure 2.
ADP representations of the crystal structure of (a) tetrahydrates (2 as an example) and (b) dihydrates (3 as an example) with numbering scheme (methylene H atoms are excluded). Labelled atoms are related to the unlabelled ones by the centre of symmetry. Displacement ellipsoids are drawn at 30% probability level. Dashed lines indicate hydrogen bonds.
The asymmetric unit of tetrahydrates consists of one half of the [V10O28]6– anion with Ci symmetry lying on an inversion centre, one [M(H2O)5(β-HAla)]2+ cation, one NH4+ cation, and one water molecule in general positions (Figure 2b).
The quality of X-ray diffraction data allowed us to find all hydrogen atoms from the ifference electron density map and refine them semi-freely using a set of suitable restraints [43]. As a result, the accurate orientations of water molecules, –NH3+ groups, and NH4+ cations were successfully determined.
The following four types of oxygen atoms can be distinguished in the decavanadate anions: terminal OT bonded to only one vanadium atom, and O(μ2), O(μ3), and O(μ6) bridging atoms, connecting 2, 3, and 6 vanadium atoms, respectively. Table S2 contains V–O and M–O bond lengths and bond valences/bond valence sums (BVS) calculated using the following equation [44,45]:
where R is bond length and R0 and B are empirical parameters for given bond type, to confirm protonation state of the decavanadate anion, oxidation number of V, and central atoms of [M(H2O)5(β-HAla)]2+ complex cations. For VV–O bonds, the values R0 = 1.803 Å and B = 0.37 were used [46]. The BVS values obtained are in the range of 1.61–1.76 for V = OT bonds, 1.764–1.893 for V–O(μ2) bonds, 1.88–1.92 for V–O(μ3) bonds, and 1.975–1.998 for V–O(μ6) bonds. A comparison of BVS values obtained for particular bonds also confirms the rigidity of the [V10O28]6– anion, only marginally influenced by adjacent molecules. For protonated O atoms, expected BVS values are Σs < 1.5; thus, in all prepared compounds, the presence of an unprotonated [V10O28]6– anion was confirmed. BVS values for all V atoms are in the range of 5.02–5.11, confirming oxidation state V(V).
For the BVS calculations for central atoms M in [M(H2O)5(β-HAla)]2+ cations, the following values of the bond parameters were used: R0 = 1.685 Å and B = 0.37 for Co2+ in 1 [47], R0 = 1.675 Å and B = 0.37 for Ni2+ in 2 [45], R0 = 1.904 Å and B = 0.37 for Cd2+ in 3 [46], R0 = 1.704 Å and B = 0.37 for Zn2+ in 4 [46], and R0 = 1.762 Å and B = 0.4 for Mn2+ in 5 [48]. In all [M(H2O)5(β-HAla)]2+ cations in the compounds 1–5, oxidation state II for the central atom was confirmed.
All the molecules present in both dihydrates and tetrahydrates are involved in the creation of an extensive hydrogen bonding network (Table S3). Most of these interactions are medium hydrogen bonds with bond distances d(H…A) between 1.5 to 2.2 Å and bond angles belonging to the range 130–180° after normalisation to neutron distances [49].
The values of average M–O distances (M–O) in [M(H2O)5(β-HAla)]2+ complex cations of the compounds 1–5 (Table 1) are in good agreement with the values of the crystal radii for r(Co2+ hs) = 85.5 pm, r(Ni2+) = 83 pm, r(Cd2+) = 109 pm, r(Zn2+) = 88 pm, r(Mn2+ hs) = 97 pm, and r(O2–) = 121 pm, respectively; crystal radii corresponding with low spin states are significantly smaller (r(Co2+ ls) = 79 pm, r(Mn2+ ls) = 81 pm) [50]. The M–Owater distances are in the range 2.070(2)–2.1195(19) Å for 1, 2.038(2)–2.0729(19) Å for 2, 2.232(3)–2.327(3) Å for 3, 2.0665(11)–2.1152(10) Å for 4, and 2.1369(19)–2.2197(18) for 5. The M–Oβ-HAla distances d(M–O91) (Table S2) are in the range 2.0614(18)–2.200(3) Å in the order of increasing crystal radii. Octahedral distortion parameters in [M(H2O)5(β-HAla)]2+ complex cations (Table 1) also show significant differences between particular values for tetrahydrates and for dihydrates.

Table 1.
Average M–O distances, octahedral distortion parameters, and octahedron volumes in [M(H2O)5(β-HAla)]2+ complex cations of the compounds 1–5.
The zwitterionic form of β-alanine is characterised by the formation of an intramolecular salt bridge between the ammonium group and the carboxylic group, forming a six-membered S(6) ring [51,52]. In tetrahydrates (Figure 3a), smaller Co2+, Ni2+, and Zn2+ ions prefer the coordination of the less bulky oxygen atom of the carboxylate group not involved in the intramolecular hydrogen bond, while larger Mn2+ and Cd2+ cations in dihydrates (Figure 3b) prefer the coordination of the oxygen atom involved in the formation of the intramolecular hydrogen bond. This different behaviour influences not only the strength of the intermolecular hydrogen bond in β-alanine but also the entire hydrogen bond network. As a result, the [M(H2O)5(β-HAla)]2+ cation in tetrahydrates contains two intramolecular hydrogen bonds, i.e., the above-mentioned salt bridge N1–H1…O92 between –NH3+ hydrogen and non-coordinated carboxylate oxygen, and O55–H55B…O92 hydrogen bond between coordinated water molecules and the same non-coordinated carboxylate oxygen, thus forming another S(6) supramolecular ring. In dihydrates, the [M(H2O)5(β-HAla)]2+ cation contains one three-centred chelated intramolecular hydrogen bond, i.e., N1–H1B…O53 and N1–H1B…O91, where H1B belongs to the –NH3+ group, O53 to the coordinated water molecule, and O91 carboxylate oxygen coordinated to the metal atom. Alongside the intramolecular S(6) ring formed by β-alanine, the S(4) ring involving the coordinated oxygen atom of the carboxylate group, the hydrogen atom of the ammonium group, and the oxygen atom of the coordinated water molecule is formed. These changes are also reflected as a change in corresponding torsion angles (Table 2) and in the presence of different numbers of cocrystallised water molecules in both crystal forms.
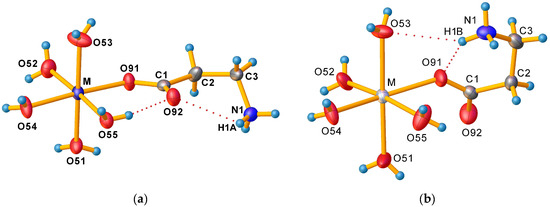
Figure 3.
A detailed view of [M(H2O)5(β-HAla)]2+ geometry and intramolecular hydrogen bonds (a) in tetrahydrates and (b) in dihydrates. Displacement ellipsoids are drawn at 50% probability level.

Table 2.
Selected torsion angles in [M(H2O)5(β-HAla)]2+ complex cations of the compounds 1–5.
3.2. Molar Susceptibility Determination of [M(H2O)5(β-HAla)]2+ Ions in Solution by Paramagnetic 1H-NMR
To determine the spin state of paramagnetic central atoms in the [M(H2O)5(β-HAla)]2+ (M = CoII, NiII, MnII) complex cations, molar susceptibilities of the complex cations in the solutions of 1, 2, and 5 were determined based on the paramagnetic shift of the 1H-NMR signal of the t-Bu group in the paramagnetic solution against the diamagnetic reference (3% v/v t-BuOH solution in D2O). For the assessment of diamagnetic contribution, the 1H-NMR chemical shifts of the signal belonging to the t-Bu group in the solution of 4 and in a blank sample containing t-BuOH in D2O were also recorded (Figure 4).
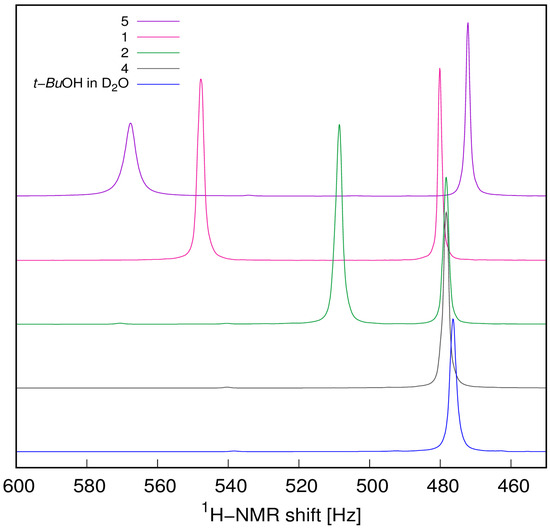
Figure 4.
Paramagnetic shifts of the t-Bu 1H-NMR signal in the solutions of the compounds 1, 2, 4, and 5 and a blank sample against the reference solution of t-BuOH in D2O.
At the concentrations given, there was no significant diamagnetic contribution χd from the ligands and other ions in the solution based on a comparison of 4 and blank t-BuOH 1H-NMR spectra, thus μeff values were calculated directly from χm values (Table 3).

Table 3.
Experimental data, molar magnetic susceptibilities, and effective magnetic moments of the [M(H2O)5(β-HAla)]2+ complex cations in the compounds 1, 2, and 5.
The results are in good agreement with the calculated μcal values by using Equation (4) for three unpaired electrons of Co2+(hs) in 1, two unpaired electrons of Ni2+ in 2, and five unpaired electrons of Mn2+(hs) in 5. These findings agree with the assignment of spin states based on average (M–O) distances.
3.3. Vibrational Spectroscopy
The FT-IR (Figure 5a) and FT-Raman (Figure 5b) spectra of crystalline 1–5 are quite similar because of the same chemical components present. The FT-IR and FT-Raman spectra of the individual compounds are mutually compared on Figure S1a–e. The assignments of the bands are summarised in Table S4 [7,21,53].
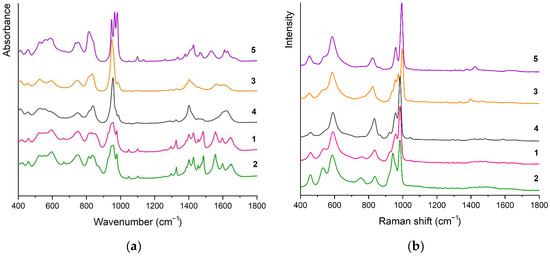
Figure 5.
Stacked FT-IR (a) and FT-Raman (b) spectra of the compounds 1–5.
The common features of decavanadate IR spectra involve the bands with highest intensity in the range 920–1000 cm−1 corresponding to valence vibrations of terminal V=O bonds and two broad series of bands related to asymmetric and symmetric bridging O–V2 vibration modes in the range of 843–748 cm−1 (νas, IR)/866–823 cm−1 (νas, Raman) and 596–524 cm−1 (νs, IR)/591–533 cm−1 (νs, Raman), respectively.
The assignment of the bands corresponding to δd(NH3+), δd(NH4+), and both νs and νas(COO−), is based on a comparison of partially deuterated samples [21].
To distinguish between binding modes of carboxylate group in complexes, it is possible to use the differences between frequencies of asymmetric and symmetric stretching vibrations, ∆ = νas(COO−)—νs(COO−). Carboxylato complexes exhibiting ∆ values that are significantly greater than ionic values, typically with ∆ ≥ 200 cm−1 (cf. 164 and 184 cm−1 for zwitterionic β-HAla [53]), have usually unidentate coordination [54]. The ∆ values of studied compounds (∆ = 230 for 1, 2, and 3; ∆ = 233 for 4; and ∆ = 199 for 5) are typical for monodentate mode of carboxylate group bonding, thus confirming the results of X-ray structure analysis.
The presence of water molecules is confirmed by the bands in the region 3484–3380 cm−1. These bands usually appear in the 3600–3400 cm−1 range, and their shift towards lower wavenumbers is caused by the occurrence of O–H…O hydrogen bonds.
4. Conclusions
We prepared (NH4)2[Co(H2O)5(β-HAla)]2[V10O28]·4H2O, (NH4)2[Ni(H2O)5(β-HAla)]2[V10O28]·4H2O, and (NH4)2[Cd(H2O)5(β-HAla)]2[V10O28]·2H2O by synthesising them in a mildly acidic aqueous solution, which, in comparison with hydrothermal direct synthesis from metallic powder and V2O5, prevented partial reduction of V(V) to V(IV) during the preparation of (NH4)2[Co(H2O)5(β-HAla)]2[V10O28]·4H2O. The prepared compounds were characterised by X-ray structure analysis and vibration spectroscopy. The FT-IR and FT-Raman spectra confirmed the presence of the [V10O28]6– anion and monodentate coordination mode of β-HAla in complex cation in accordance with crystallographic findings. To confirm the spin state of central atoms in [M(H2O)5(β-HAla)]2+ cations, including previously prepared (NH4)2[Mn(H2O)5(β-HAla)]2[V10O28]·2H2O, the Evans method was used to confirm the assignment of spin states based on crystallographic data. The increasing crystal radius of central atoms and their different preferences for donor atoms resulting in rebuilding of the hydrogen bonding network is the main cause of the existence of two different crystallohydrate forms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst14080685/s1. Table S1: Crystallographic data for the compounds 1–5; Table S2: Selected bond lengths, bond valences and bond valence sums in the compounds 1–5; Table S3: Hydrogen bonds in the structures of 1–5; Figure S1a: A comparison of IR and Raman spectra of 1; Figure S1b: A comparison of IR and Raman spectra of 2; Figure S1c: A comparison of IR and Raman spectra of 3; Figure S1d: A comparison of IR and Raman spectra of 4; Figure S1e: A comparison of IR and Raman spectra of 5. Table S4: Assignments of the IR and Raman absorption bands for compounds 1–5 [7,21,53].
Author Contributions
Conceptualisation, L.B. and E.R.; methodology, J.C. and E.R.; validation, J.C. and E.R.; formal analysis, Y.R.P. and E.R.; investigation, L.B., J.C., Y.R.P. and E.R.; resources, L.B.; data curation, E.R.; writing—original draft preparation, E.R. and Y.R.P.; writing—review and editing, E.R. and J.C.; visualisation, E.R. and J.C.; project administration, E.R. and Y.R.P.; funding acquisition, Y.R.P. and E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Grant of Comenius University no. UK/3049/2024, Scientific Grant Agency of the Ministry of Education of the Slovak Republic and of Slovak Academy of Sciences VEGA 1/0669/22, and Slovak Research and Development Agency under Contract no. APVV-21-0503.
Data Availability Statement
The deposition numbers CCDC 2366040—2366044 contain the supplementary crystallographic data for this article, including structure factors. These data can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures/ (accessed date 23 July 2024). Crystallographic data can also be obtained from the Crystallography Open Database (COD) under COD ID 3000552—3000556.
Acknowledgments
The authors thank Aleksandra Cyganiuk from Nicolaus Copernicus University in Toruń (Poland) for the Raman spectra measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hayashi, Y. Hetero and Lacunary Polyoxovanadate Chemistry: Synthesis, Reactivity and Structural Aspects. Coord. Chem. Rev. 2011, 255, 2270–2280. [Google Scholar] [CrossRef]
- Kempf, J.Y.; Rohmer, M.M.; Poblet, J.M.; Bo, C.; Benard, M. Relative basicities of the oxygen sites in [V10O28]6–. An analysis of the ab initio determined distributions of the electrostatic potential and of the Laplacian of charge density. J. Am. Chem. Soc. 1992, 114, 1136–1146. [Google Scholar] [CrossRef]
- Day, V.W.; Klemperer, W.G.; Maltbie, D.J. Where are the protons in H3V10O283–? J. Am. Chem. Soc. 1987, 109, 2991–3002. [Google Scholar] [CrossRef]
- Biagioli, M.; Strinna-Erre, L.; Micera, G.; Panzanelli, A.; Zema, M. Tetrahydrogendecavanadate(V) and Its Binding to Glycylglycine. Inorg. Chem. Commun. 1999, 2, 214–217. [Google Scholar] [CrossRef]
- Rakovský, E.; Joniaková, D.; Gyepes, R.; Schwendt, P.; Mička, Z. Synthesis and Crystal Structure of [CuCl(phen)2]3H3V10O28·7H2O. Cryst. Res. Technol. 2005, 40, 719–722. [Google Scholar] [CrossRef]
- Kaziev, G.Z.; Oreshkina, A.V.; Stepnova, A.F.; Holguin Quinones, S.; Stash, A.I.; Morales Sanchez, L.A. Synthesis and Study of the Physicochemical Properties of Ammonium Hydrogen Hexaaquacobaltate(III) Isopolyvanadate [(NH4)2][Co(H2O)6]·H[V10O28]·8H2O. Russ. J. Coord. Chem. 2011, 37, 766–771. [Google Scholar] [CrossRef]
- Sánchez-Lara, E.; Pérez-Benítez, A.; Treviño, S.; Mendoza, A.; Meléndez, F.J.; Sánchez-Mora, E.; Bernès, S.; González-Vergara, E. Synthesis and 3D Network Architecture of 1- and 16-Hydrated Salts of 4-Dimethylaminopyridinium Decavanadate, (DMAPH)6[V10O28]·nH2O. Crystals 2016, 6, 65. [Google Scholar] [CrossRef]
- Lv, Y.-K.; Jiang, Z.-G.; Gan, L.-H.; Liu, M.-X.; Feng, Y.-L. Three Novel Organic-Inorganic Hybrid Materials Based on Decaoxovanadates Obtained from a New Liquid Phase Reaction. CrystEngComm 2012, 14, 314–322. [Google Scholar] [CrossRef]
- Ferreira da Silva, J.L.; Fátima Minas da Piedade, M.; Teresa Duarte, M. Decavanadates: A Building-Block for Supramolecular Assemblies. Inorganica Chim. Acta 2003, 356, 222–242. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with Emerging Biomedical Activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ Interactions with Proteins: An Overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
- Samart, N.; Saeger, J.; Haller, K.J.; Aureliano, M.; Crans, D.C. Interaction of Decavanadate With Interfaces and Biological Model Membrane Systems: Characterization of Soft Oxometalate Systems. J. Mol. Eng. Mater. 2014, 02, 1440007. [Google Scholar] [CrossRef]
- Aureliano, M.; Gândara, R.M.C. Decavanadate Effects in Biological Systems. J. Inorg. Biochem. 2005, 99, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Buvailo, H.I.; Pavliuk, M.V.; Makhankova, V.G.; Kokozay, V.N.; Bon, V.; Mijangos, E.; Shylin, S.I.; Jezierska, J. Facile One-Pot Synthesis of Hybrid Compounds Based on Decavanadate Showing Water Oxidation Activity. Inorg. Chem. Commun. 2020, 119, 108111. [Google Scholar] [CrossRef]
- Huang, X.; Gu, X.; Qi, Y.; Zhang, Y.; Shen, G.; Yang, B.; Duan, W.; Gong, S.; Xue, Z.; Chen, Y. Decavanadate-Based Transition Metal Hybrids as Bifunctional Catalysts for Sulfide Oxidation and C—C Bond Construction. Chin. J. Chem. 2021, 39, 2495–2503. [Google Scholar] [CrossRef]
- Cao, J.-P.; Shen, F.-C.; Luo, X.-M.; Cui, C.-H.; Lan, Y.-Q.; Xu, Y. Proton Conductivity Resulting from Different Triazole-Based Ligands in Two New Bifunctional Decavanadates. RSC Adv. 2018, 8, 18560–18566. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Cao, J.; Han, Y.; Hong, Y.; Yang, M.; Xu, Y. Three New Ln-Decavanadates Materials: Synthesis, Structure, and Photoluminescent Sensing for Detection of Zn2+ and Co2+. Zeitschrift Anorg. Allg. Chem. 2020, 646, 1315–1323. [Google Scholar] [CrossRef]
- Kumar, D.; Tomar, A.K.; Singal, S.; Singh, G.; Sharma, R.K. Ammonium Decavanadate Nanodots/Reduced Graphene Oxide Nanoribbon as “Inorganic-Organic” Hybrid Electrode for High Potential Aqueous Symmetric Supercapacitors. J. Power Sources 2020, 462, 228173. [Google Scholar] [CrossRef]
- Kumar, D.; Tomar, A.K.; Singh, G.; Sharma, R.K. Interlayer Gap Widened 2D α-Co(OH)2 Nanoplates with Decavanadate Anion for High Potential Aqueous Supercapacitor. Electrochim. Acta 2020, 363, 137238. [Google Scholar] [CrossRef]
- Klištincová, L.; Rakovský, E.; Schwendt, P. Decavanadates with Complex Cations: Synthesis and Structure of (NH4)2[M(H2O)5(NH3CH2CH2COO)]2V10O28·nH2O (M. = ZnII, n = 4; M. = MnII, n = 2). Transit. Met. Chem. 2010, 35, 229–236. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Makhankova, V.G.; Kokozay, V.N.; Omelchenko, I.V.; Jezierska, J.; Thapper, A.; Styring, S. Structural, Magnetic, Thermal and Visible Light-Driven Water Oxidation Studies of Heterometallic Mn/V Complexes. Polyhedron 2015, 88, 81–89. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Mijangos, E.; Makhankova, V.G.; Kokozay, V.N.; Pullen, S.; Liu, J.; Zhu, J.; Styring, S.; Thapper, A. Homogeneous Cobalt/Vanadium Complexes as Precursors for Functionalized Mixed Oxides in Visible-Light-Driven Water Oxidation. ChemSusChem 2016, 9, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.43.128a; Rigaku Corporation: Wroclaw, Poland, 2024. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The Anatomy of a Comprehensive Constrained, Restrained Refinement Program for the Modern Computing Environment—Olex2 Dissected. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, M. PARST 95—An Update to PARST: A System of Fortran Routines for Calculating Molecular Structure Parameters from the Results of Crystal Structure Analyses. J. Appl. Crystallogr. 1995, 28, 659. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond. Release 3.2k; Crystal Impact GbR: Bonn, Germany, 2014. [Google Scholar]
- Buron-Le Cointe, M.; Hébert, J.; Baldé, C.; Moisan, N.; Toupet, L.; Guionneau, P.; Létard, J.F.; Freysz, E.; Cailleau, H.; Collet, E. Intermolecular Control of Thermoswitching and Photoswitching Phenomena in Two Spin-Crossover Polymorphs. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 85, 064114. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Woodward, P.M. Jahn-Teller Distortions, Cation Ordering and Octahedral Tilting in Perovskites. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 10–20. [Google Scholar] [CrossRef]
- Marchivie, M.; Guionneau, P.; Létard, J.F.; Chasseau, D. Photo-Induced Spin-Transition: The Role of the Iron(II) Environment Distortion. Acta Crystallogr. Sect. B Struct. Sci. 2005, 61, 25–28. [Google Scholar] [CrossRef]
- McCusker, J.K.; Rheingold, A.L.; Hendrickson, D.N. Variable-Temperature Studies of Laser-Initiated 5T2 → 1A1 Intersystem Crossing in Spin-Crossover Complexes: Empirical Correlations between Activation Parameters and Ligand Structure in a Series of Polypyridyl Ferrous Complexes. Inorg. Chem. 1996, 35, 2100–2112. [Google Scholar] [CrossRef]
- Ketkaew, R.; Tantirungrotechai, Y.; Harding, P.; Chastanet, G.; Guionneau, P.; Marchivie, M.; Harding, D.J. OctaDist: A Tool for Calculating Distortion Parameters in Spin Crossover and Coordination Complexes. J. Chem. Soc. Dalt. Trans. 2021, 50, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, G.A.; Lewis, L. Cooperative Aspects of Hydrogen Bonding in Carbohydrates. Carbohydr. Res. 1978, 60, 179–182. [Google Scholar] [CrossRef]
- Taylor, R.; Kennard, O. Comparison of X-ray and Neutron Diffraction Results for the N-H ⋯O=C Hydrogen Bond. Acta Crystallogr. Sect. B 1983, 39, 133–138. [Google Scholar] [CrossRef]
- Evans, D.F. The Determination of the Paramagnetic Susceptibility of Substances in Solution by Nuclear Magnetic Resonance. J. Chem. Soc. 1959, 2003–2005. [Google Scholar] [CrossRef]
- Evans, D.F.; Fazakerley, G.V.; Phillips, R.F. Organometallic Compounds of Bivalent Europium, Ytterbium, and Samarium. J. Chem. Soc. A Inorg. Phys. Theor. Chem. 1971, 1931–1934. [Google Scholar] [CrossRef]
- Schubert, E.M. Utilizing the Evans Method with a Superconducting NMR Spectrometer in the Undergraduate Laboratory. J. Chem. Educ. 1992, 69, 62. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Mugiraneza, S.; Hallas, A.M. Tutorial: A Beginner’s Guide to Interpreting Magnetic Susceptibility Data with the Curie-Weiss Law. Commun. Phys. 2022, 5, 95. [Google Scholar] [CrossRef]
- Rakovský, E.; Krivosudský, L. Tetrakis(2,6-Dimethylpyridinium) Dihydrogen Decavanadate Dihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, m225–m226. [Google Scholar] [CrossRef]
- Cooper, R.I.; Thompson, A.L.; Watkin, D.J. CRYSTALS Enhancements: Dealing with Hydrogen Atoms in Refinement. J. Appl. Crystallogr. 2010, 43, 1100–1107. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical bond in Inorganic Chemistry; IUCr Monographs on Crystallography; Oxford University Press: New York, NY, USA, 2002; Volume 12. [Google Scholar]
- (IUCr) Bond Valence Parameters. Available online: https://www.iucr.org/__data/assets/file/0011/150779/bvparm2020.cif (accessed on 23 July 2024).
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B Struct. Sci. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Wood, R.M.; Palenik, G.J. Bond Valence Sums in Coordination Chemistry. A Simple Method for Calculating the Oxidation State of Cobalt in Complexes Containing Only Co−O Bonds. Inorg. Chem. 1998, 37, 4149–4151. [Google Scholar] [CrossRef] [PubMed]
- Urusov, V.S. Problem of Optimization of Bond Valence Model Parameters (as Exemplified by Managanese in Different Oxidation States). Dokl. Phys. Chem. 2006, 408, 152–155. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding, 1st ed.; Oxford University Press: New York, NY, USA, 1997; p. 12. [Google Scholar]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-Set Analysis of Hydrogen-Bond Patterns in Organic Crystals. Acta Crystallogr. Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chemie Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Berezhinsky, L.I.; Dovbeshko, G.I.; Lisitsa, M.P.; Litvinov, G.S. Vibrational Spectra of Crystalline β-Alanine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 349–358. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).