Investigating the Formation of Different (NH4)2[M(H2O)5(NH3CH2CH2COO)]2[V10O28]·nH2O (M = CoII, NiII, ZnII, n = 4; M = CdII, MnII, n = 2) Crystallohydrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis and Crystallisation
2.2.1. Synthesis of (NH4)2[Co(H2O)5(β-HAla)]2[V10O28]·4H2O (1)
2.2.2. Synthesis of (NH4)2[Ni(H2O)5(β-HAla)]2[V10O28]·4H2O (2)
2.2.3. Synthesis of (NH4)2[Cd(H2O)5(β-HAla)]2[V10O28]·2H2O (3)
2.3. X-ray Data Collection and Structure Determination
2.4. Paramagnetic 1H-NMR Measurements
3. Results and Discussion
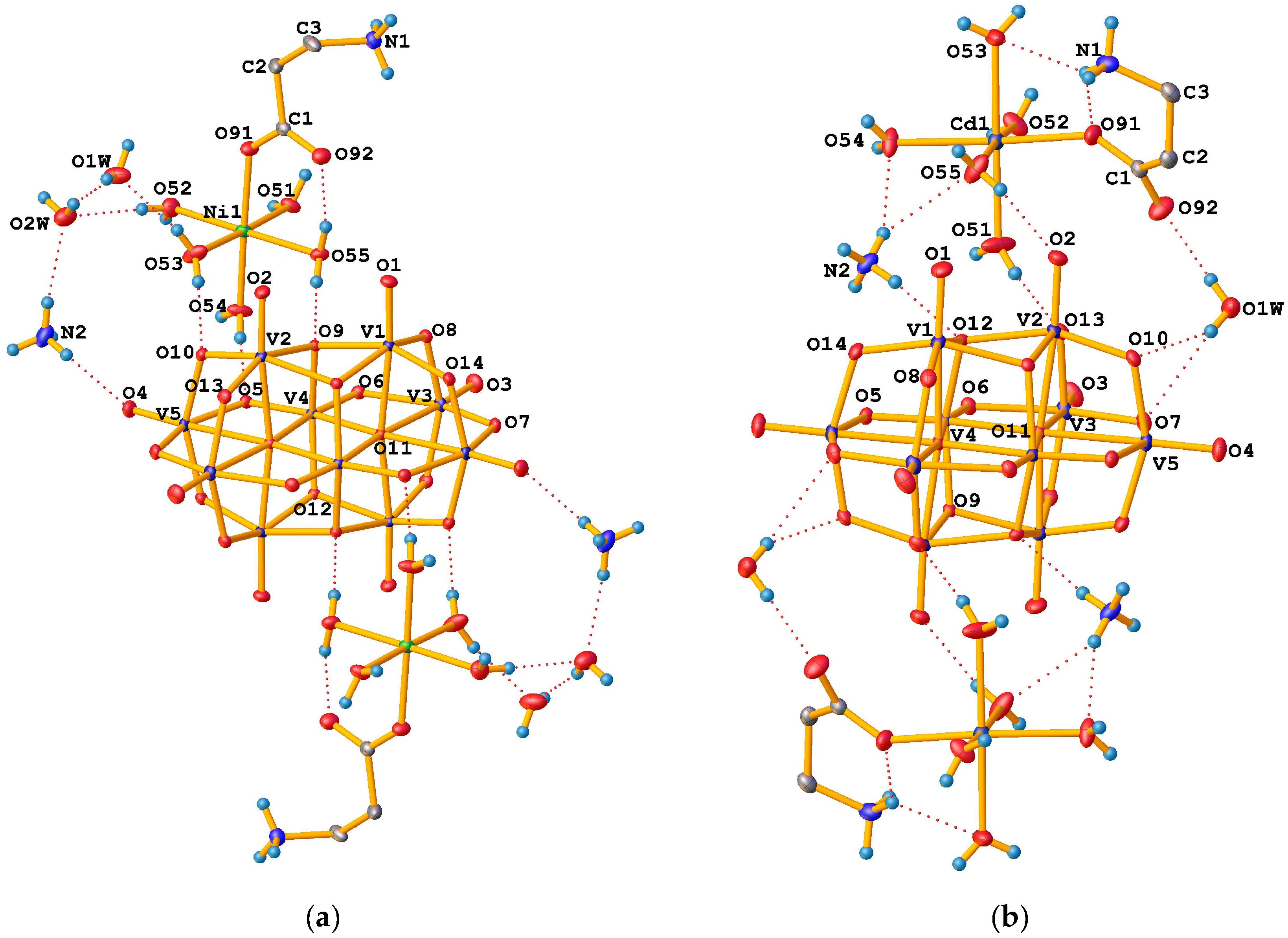
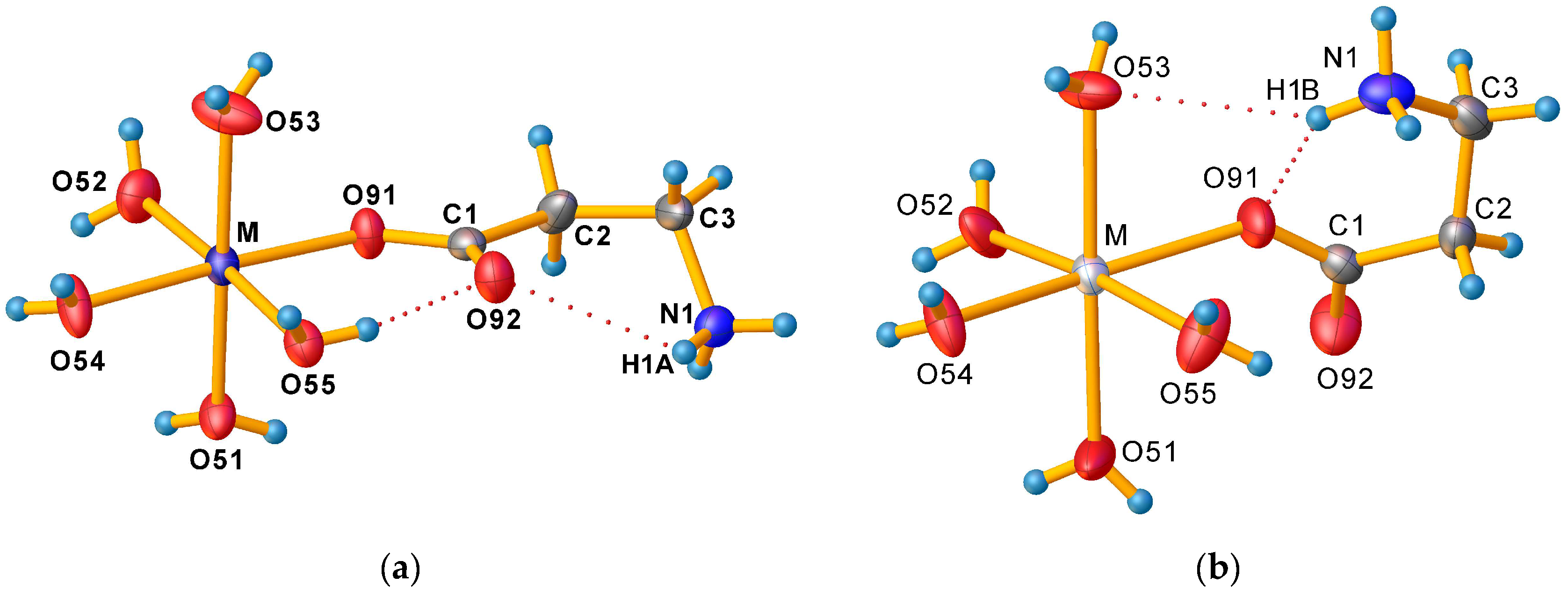
3.1. Crystallographic Characterisation of Prepared Compounds
3.2. Molar Susceptibility Determination of [M(H2O)5(β-HAla)]2+ Ions in Solution by Paramagnetic 1H-NMR
3.3. Vibrational Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayashi, Y. Hetero and Lacunary Polyoxovanadate Chemistry: Synthesis, Reactivity and Structural Aspects. Coord. Chem. Rev. 2011, 255, 2270–2280. [Google Scholar] [CrossRef]
- Kempf, J.Y.; Rohmer, M.M.; Poblet, J.M.; Bo, C.; Benard, M. Relative basicities of the oxygen sites in [V10O28]6–. An analysis of the ab initio determined distributions of the electrostatic potential and of the Laplacian of charge density. J. Am. Chem. Soc. 1992, 114, 1136–1146. [Google Scholar] [CrossRef]
- Day, V.W.; Klemperer, W.G.; Maltbie, D.J. Where are the protons in H3V10O283–? J. Am. Chem. Soc. 1987, 109, 2991–3002. [Google Scholar] [CrossRef]
- Biagioli, M.; Strinna-Erre, L.; Micera, G.; Panzanelli, A.; Zema, M. Tetrahydrogendecavanadate(V) and Its Binding to Glycylglycine. Inorg. Chem. Commun. 1999, 2, 214–217. [Google Scholar] [CrossRef]
- Rakovský, E.; Joniaková, D.; Gyepes, R.; Schwendt, P.; Mička, Z. Synthesis and Crystal Structure of [CuCl(phen)2]3H3V10O28·7H2O. Cryst. Res. Technol. 2005, 40, 719–722. [Google Scholar] [CrossRef]
- Kaziev, G.Z.; Oreshkina, A.V.; Stepnova, A.F.; Holguin Quinones, S.; Stash, A.I.; Morales Sanchez, L.A. Synthesis and Study of the Physicochemical Properties of Ammonium Hydrogen Hexaaquacobaltate(III) Isopolyvanadate [(NH4)2][Co(H2O)6]·H[V10O28]·8H2O. Russ. J. Coord. Chem. 2011, 37, 766–771. [Google Scholar] [CrossRef]
- Sánchez-Lara, E.; Pérez-Benítez, A.; Treviño, S.; Mendoza, A.; Meléndez, F.J.; Sánchez-Mora, E.; Bernès, S.; González-Vergara, E. Synthesis and 3D Network Architecture of 1- and 16-Hydrated Salts of 4-Dimethylaminopyridinium Decavanadate, (DMAPH)6[V10O28]·nH2O. Crystals 2016, 6, 65. [Google Scholar] [CrossRef]
- Lv, Y.-K.; Jiang, Z.-G.; Gan, L.-H.; Liu, M.-X.; Feng, Y.-L. Three Novel Organic-Inorganic Hybrid Materials Based on Decaoxovanadates Obtained from a New Liquid Phase Reaction. CrystEngComm 2012, 14, 314–322. [Google Scholar] [CrossRef]
- Ferreira da Silva, J.L.; Fátima Minas da Piedade, M.; Teresa Duarte, M. Decavanadates: A Building-Block for Supramolecular Assemblies. Inorganica Chim. Acta 2003, 356, 222–242. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with Emerging Biomedical Activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ Interactions with Proteins: An Overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
- Samart, N.; Saeger, J.; Haller, K.J.; Aureliano, M.; Crans, D.C. Interaction of Decavanadate With Interfaces and Biological Model Membrane Systems: Characterization of Soft Oxometalate Systems. J. Mol. Eng. Mater. 2014, 02, 1440007. [Google Scholar] [CrossRef]
- Aureliano, M.; Gândara, R.M.C. Decavanadate Effects in Biological Systems. J. Inorg. Biochem. 2005, 99, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Buvailo, H.I.; Pavliuk, M.V.; Makhankova, V.G.; Kokozay, V.N.; Bon, V.; Mijangos, E.; Shylin, S.I.; Jezierska, J. Facile One-Pot Synthesis of Hybrid Compounds Based on Decavanadate Showing Water Oxidation Activity. Inorg. Chem. Commun. 2020, 119, 108111. [Google Scholar] [CrossRef]
- Huang, X.; Gu, X.; Qi, Y.; Zhang, Y.; Shen, G.; Yang, B.; Duan, W.; Gong, S.; Xue, Z.; Chen, Y. Decavanadate-Based Transition Metal Hybrids as Bifunctional Catalysts for Sulfide Oxidation and C—C Bond Construction. Chin. J. Chem. 2021, 39, 2495–2503. [Google Scholar] [CrossRef]
- Cao, J.-P.; Shen, F.-C.; Luo, X.-M.; Cui, C.-H.; Lan, Y.-Q.; Xu, Y. Proton Conductivity Resulting from Different Triazole-Based Ligands in Two New Bifunctional Decavanadates. RSC Adv. 2018, 8, 18560–18566. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Cao, J.; Han, Y.; Hong, Y.; Yang, M.; Xu, Y. Three New Ln-Decavanadates Materials: Synthesis, Structure, and Photoluminescent Sensing for Detection of Zn2+ and Co2+. Zeitschrift Anorg. Allg. Chem. 2020, 646, 1315–1323. [Google Scholar] [CrossRef]
- Kumar, D.; Tomar, A.K.; Singal, S.; Singh, G.; Sharma, R.K. Ammonium Decavanadate Nanodots/Reduced Graphene Oxide Nanoribbon as “Inorganic-Organic” Hybrid Electrode for High Potential Aqueous Symmetric Supercapacitors. J. Power Sources 2020, 462, 228173. [Google Scholar] [CrossRef]
- Kumar, D.; Tomar, A.K.; Singh, G.; Sharma, R.K. Interlayer Gap Widened 2D α-Co(OH)2 Nanoplates with Decavanadate Anion for High Potential Aqueous Supercapacitor. Electrochim. Acta 2020, 363, 137238. [Google Scholar] [CrossRef]
- Klištincová, L.; Rakovský, E.; Schwendt, P. Decavanadates with Complex Cations: Synthesis and Structure of (NH4)2[M(H2O)5(NH3CH2CH2COO)]2V10O28·nH2O (M. = ZnII, n = 4; M. = MnII, n = 2). Transit. Met. Chem. 2010, 35, 229–236. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Makhankova, V.G.; Kokozay, V.N.; Omelchenko, I.V.; Jezierska, J.; Thapper, A.; Styring, S. Structural, Magnetic, Thermal and Visible Light-Driven Water Oxidation Studies of Heterometallic Mn/V Complexes. Polyhedron 2015, 88, 81–89. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Mijangos, E.; Makhankova, V.G.; Kokozay, V.N.; Pullen, S.; Liu, J.; Zhu, J.; Styring, S.; Thapper, A. Homogeneous Cobalt/Vanadium Complexes as Precursors for Functionalized Mixed Oxides in Visible-Light-Driven Water Oxidation. ChemSusChem 2016, 9, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.43.128a; Rigaku Corporation: Wroclaw, Poland, 2024. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The Anatomy of a Comprehensive Constrained, Restrained Refinement Program for the Modern Computing Environment—Olex2 Dissected. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, M. PARST 95—An Update to PARST: A System of Fortran Routines for Calculating Molecular Structure Parameters from the Results of Crystal Structure Analyses. J. Appl. Crystallogr. 1995, 28, 659. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond. Release 3.2k; Crystal Impact GbR: Bonn, Germany, 2014. [Google Scholar]
- Buron-Le Cointe, M.; Hébert, J.; Baldé, C.; Moisan, N.; Toupet, L.; Guionneau, P.; Létard, J.F.; Freysz, E.; Cailleau, H.; Collet, E. Intermolecular Control of Thermoswitching and Photoswitching Phenomena in Two Spin-Crossover Polymorphs. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 85, 064114. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Woodward, P.M. Jahn-Teller Distortions, Cation Ordering and Octahedral Tilting in Perovskites. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 10–20. [Google Scholar] [CrossRef]
- Marchivie, M.; Guionneau, P.; Létard, J.F.; Chasseau, D. Photo-Induced Spin-Transition: The Role of the Iron(II) Environment Distortion. Acta Crystallogr. Sect. B Struct. Sci. 2005, 61, 25–28. [Google Scholar] [CrossRef]
- McCusker, J.K.; Rheingold, A.L.; Hendrickson, D.N. Variable-Temperature Studies of Laser-Initiated 5T2 → 1A1 Intersystem Crossing in Spin-Crossover Complexes: Empirical Correlations between Activation Parameters and Ligand Structure in a Series of Polypyridyl Ferrous Complexes. Inorg. Chem. 1996, 35, 2100–2112. [Google Scholar] [CrossRef]
- Ketkaew, R.; Tantirungrotechai, Y.; Harding, P.; Chastanet, G.; Guionneau, P.; Marchivie, M.; Harding, D.J. OctaDist: A Tool for Calculating Distortion Parameters in Spin Crossover and Coordination Complexes. J. Chem. Soc. Dalt. Trans. 2021, 50, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, G.A.; Lewis, L. Cooperative Aspects of Hydrogen Bonding in Carbohydrates. Carbohydr. Res. 1978, 60, 179–182. [Google Scholar] [CrossRef]
- Taylor, R.; Kennard, O. Comparison of X-ray and Neutron Diffraction Results for the N-H ⋯O=C Hydrogen Bond. Acta Crystallogr. Sect. B 1983, 39, 133–138. [Google Scholar] [CrossRef]
- Evans, D.F. The Determination of the Paramagnetic Susceptibility of Substances in Solution by Nuclear Magnetic Resonance. J. Chem. Soc. 1959, 2003–2005. [Google Scholar] [CrossRef]
- Evans, D.F.; Fazakerley, G.V.; Phillips, R.F. Organometallic Compounds of Bivalent Europium, Ytterbium, and Samarium. J. Chem. Soc. A Inorg. Phys. Theor. Chem. 1971, 1931–1934. [Google Scholar] [CrossRef]
- Schubert, E.M. Utilizing the Evans Method with a Superconducting NMR Spectrometer in the Undergraduate Laboratory. J. Chem. Educ. 1992, 69, 62. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Mugiraneza, S.; Hallas, A.M. Tutorial: A Beginner’s Guide to Interpreting Magnetic Susceptibility Data with the Curie-Weiss Law. Commun. Phys. 2022, 5, 95. [Google Scholar] [CrossRef]
- Rakovský, E.; Krivosudský, L. Tetrakis(2,6-Dimethylpyridinium) Dihydrogen Decavanadate Dihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, m225–m226. [Google Scholar] [CrossRef]
- Cooper, R.I.; Thompson, A.L.; Watkin, D.J. CRYSTALS Enhancements: Dealing with Hydrogen Atoms in Refinement. J. Appl. Crystallogr. 2010, 43, 1100–1107. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical bond in Inorganic Chemistry; IUCr Monographs on Crystallography; Oxford University Press: New York, NY, USA, 2002; Volume 12. [Google Scholar]
- (IUCr) Bond Valence Parameters. Available online: https://www.iucr.org/__data/assets/file/0011/150779/bvparm2020.cif (accessed on 23 July 2024).
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B Struct. Sci. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Wood, R.M.; Palenik, G.J. Bond Valence Sums in Coordination Chemistry. A Simple Method for Calculating the Oxidation State of Cobalt in Complexes Containing Only Co−O Bonds. Inorg. Chem. 1998, 37, 4149–4151. [Google Scholar] [CrossRef] [PubMed]
- Urusov, V.S. Problem of Optimization of Bond Valence Model Parameters (as Exemplified by Managanese in Different Oxidation States). Dokl. Phys. Chem. 2006, 408, 152–155. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding, 1st ed.; Oxford University Press: New York, NY, USA, 1997; p. 12. [Google Scholar]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-Set Analysis of Hydrogen-Bond Patterns in Organic Crystals. Acta Crystallogr. Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chemie Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Berezhinsky, L.I.; Dovbeshko, G.I.; Lisitsa, M.P.; Litvinov, G.S. Vibrational Spectra of Crystalline β-Alanine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 349–358. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]




| Compound | (M–O) [Å] | ζ | Δ | Σ | Θ | V [Å3] |
|---|---|---|---|---|---|---|
| 1 | 2.090(15) | 0.065267 | 5.1 × 10−5 | 21.9313 | 51.4217 | 12.14 |
| 2 | 2.055(11) | 0.050418 | 2.7 × 10−5 | 23.2468 | 50.2841 | 11.54 |
| 3 | 2.26(4) | 0.195609 | 3.10 × 10−4 | 53.3874 | 144.5566 | 15.30 |
| 4 | 2.090(15) | 0.064040 | 4.8 × 10−5 | 28.7336 | 66.9865 | 12.14 |
| 5 | 2.17(4) | 0.198905 | 3.38 × 10−4 | 43.3304 | 113.7109 | 13.47 |
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| M–O91–C1–O92 | 3.8(4) | 0.8(4) | 51.7(7) | 4.3(2) | 59.8(4) |
| M–O91–C1–C2 | −176.41(18) | −179.03(17) | −128.4(4) | −175.70(9) | −119.9(3) |
| O91–C1–C2–C3 | 174.9(2) | 173.3(2) | −36.8(5) | 174.61(12) | −39.3(3) |
| O92–C1–C2–C3 | −5.3(4) | −6.5(4) | 143.1(4) | −5.41(18) | 141.0(2) |
| C1–C2–C3–N1 | 70.5(3) | 71.4(3) | 59.0(5) | 70.03(16) | 60.9(3) |
| Compound | Δf [Hz] | c [mmol.L−1] | T [K] | χm [cm3mol−1] | μeff [μB] |
|---|---|---|---|---|---|
| 1 | 67.576 | 4.011 | 302.35 | 0.010052 | 4.95 |
| 2 | 30.2619 | 4.004 | 302.25 | 0.004509 | 3.32 |
| 5 | 95.534 | 4.000 | 300.85 | 0.014235 | 5.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrappová, J.; Pateda, Y.R.; Bartošová, L.; Rakovský, E. Investigating the Formation of Different (NH4)2[M(H2O)5(NH3CH2CH2COO)]2[V10O28]·nH2O (M = CoII, NiII, ZnII, n = 4; M = CdII, MnII, n = 2) Crystallohydrates. Crystals 2024, 14, 685. https://doi.org/10.3390/cryst14080685
Chrappová J, Pateda YR, Bartošová L, Rakovský E. Investigating the Formation of Different (NH4)2[M(H2O)5(NH3CH2CH2COO)]2[V10O28]·nH2O (M = CoII, NiII, ZnII, n = 4; M = CdII, MnII, n = 2) Crystallohydrates. Crystals. 2024; 14(8):685. https://doi.org/10.3390/cryst14080685
Chicago/Turabian StyleChrappová, Jana, Yogeswara Rao Pateda, Lenka Bartošová, and Erik Rakovský. 2024. "Investigating the Formation of Different (NH4)2[M(H2O)5(NH3CH2CH2COO)]2[V10O28]·nH2O (M = CoII, NiII, ZnII, n = 4; M = CdII, MnII, n = 2) Crystallohydrates" Crystals 14, no. 8: 685. https://doi.org/10.3390/cryst14080685





