Assessment of the Amino Acid L-Histidine as a Corrosion Inhibitor for a 1018 Carbon Steel in Aqueous Sodium Chloride Solution
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Gravimetric and Electrochemical Tests
2.2. Characterization of L-Histidine
2.3. Surface Characterization
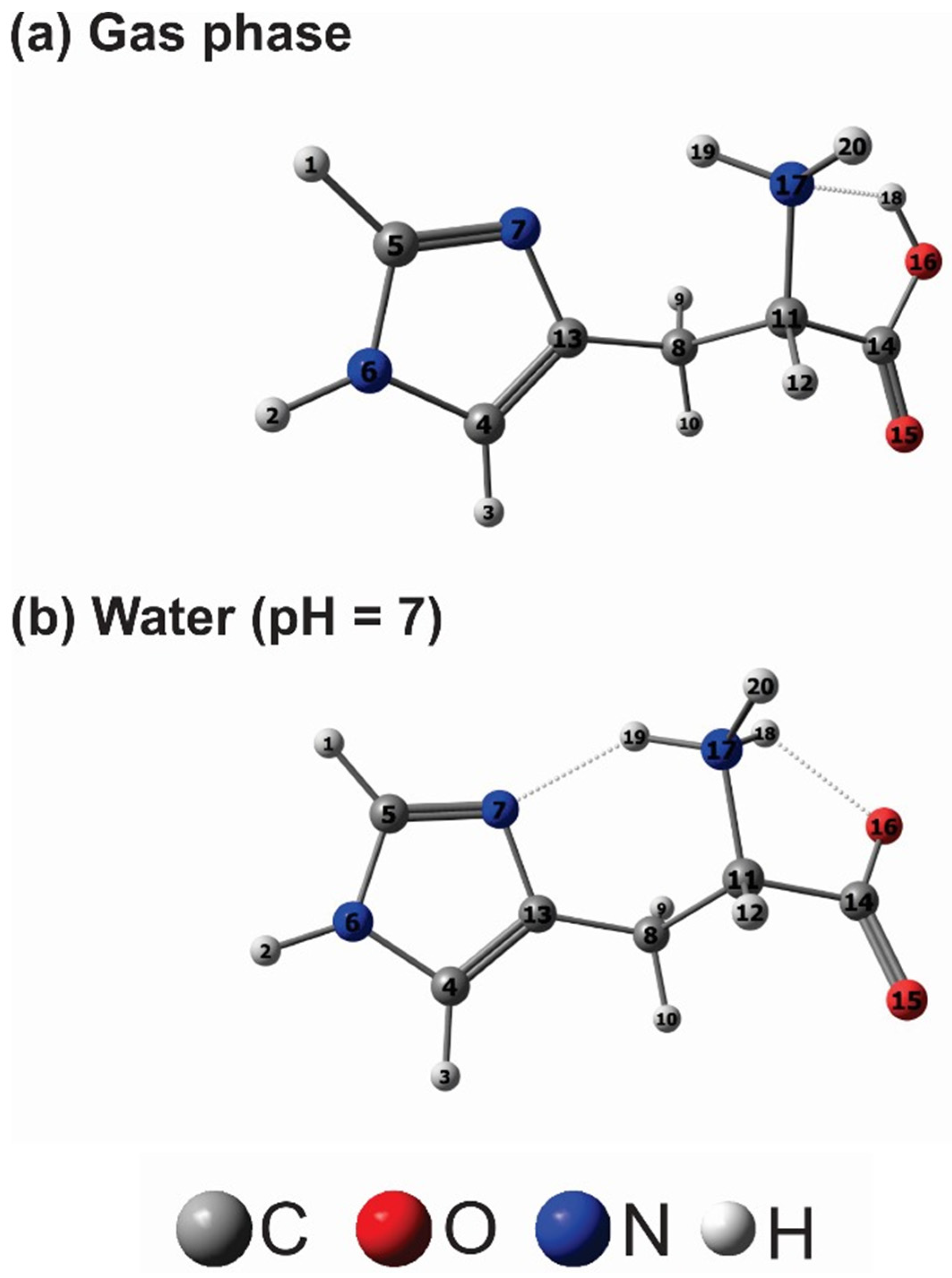
2.4. Quantum Chemical Calculation
3. Results and Discussion
3.1. Amino Acid Characterization: L-Histidine
3.1.1. Fourier Transform Infrared (FTIR) Spectral Analysis
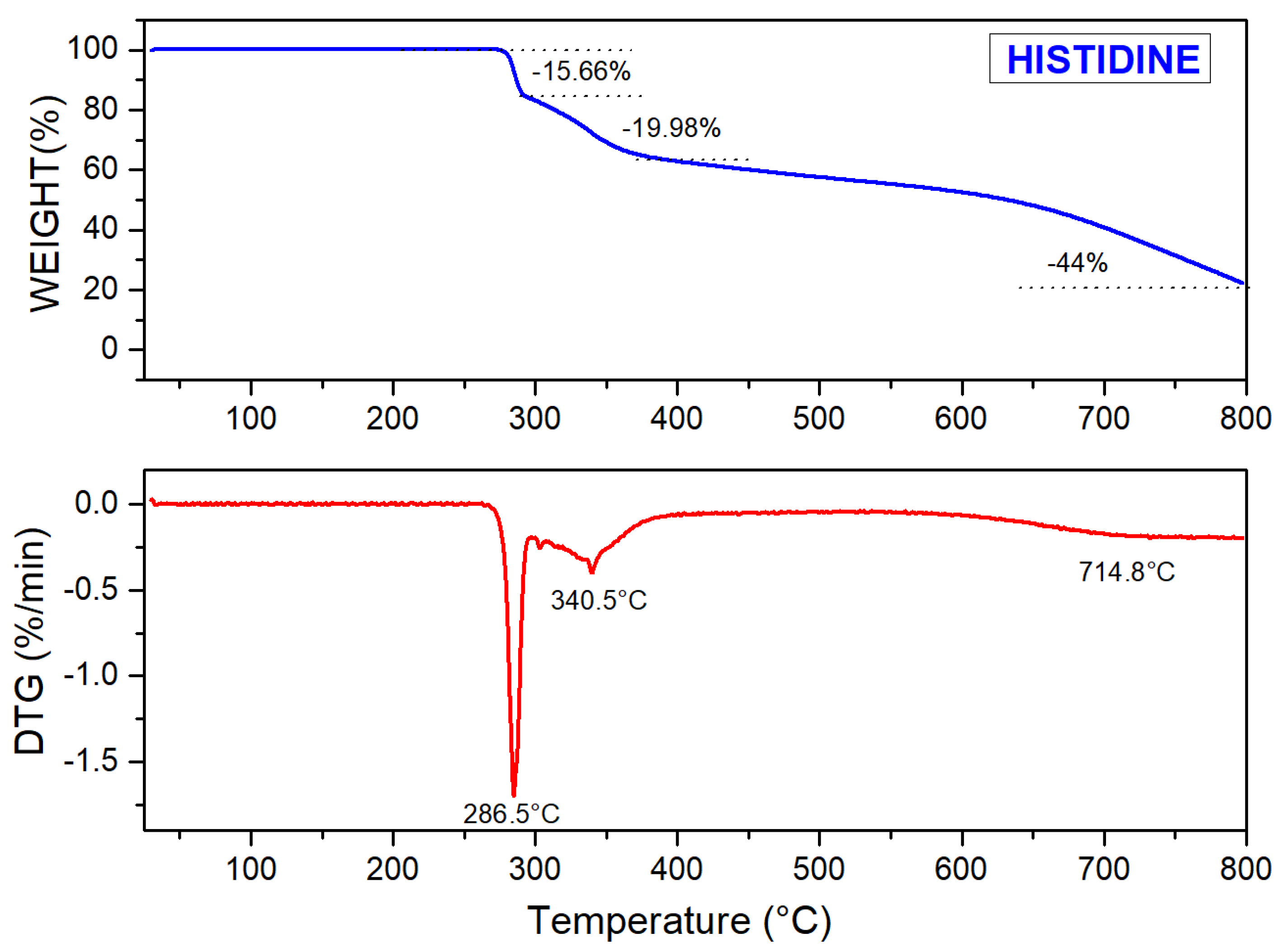
3.1.2. Thermo Gravimetric Analysis (TGA)
3.2. Weight Loss
3.3. Adsorption Isotherms
3.4. Electrochemical Tests
3.4.1. Linear Polarization
3.4.2. Electrochemical Impedance Spectroscopy (EIE)
3.5. UV–Vis Absorption Spectroscopy
3.6. Surface Analyses
3.6.1. Scanning Electron Microscopy (SEM)
3.6.2. Atomic Force Microscopy (AFM)
3.7. Theoretical Calculations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, F.; Zhang, Y.; Wang, H.; Li, W.; Hou, B. Inhibition behavior of chitooligosaccharide derivatives for carbon steel in 3.5% NaCl solution. Int. J. Electrochem. Sci. 2018, 13, 235–249. [Google Scholar] [CrossRef]
- Singh, P.; Bhrara, K.; Singh, G. Adsorption and kinetic studies of L-leucine as an inhibitor on mild steel in acidic media. Appl. Surf. Sci. 2008, 254, 5927–5935. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Majidi, M.R.; Seyyedi, K. Investigation of inhibition effect of some amino acids against steel corrosion in HCl solution. Appl. Surf. Sci. 2004, 225, 176–185. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Ghalebsaz-Jeddi, N. Inhibition effect of polyethylene glycol on the corrosion of carbon steel in sulphuric acid. Mater. Chem. Phys. 2005, 92, 480–486. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, D.; Wan, Y. 2-Mercaptobenzothiazole doped chitosan/11-alkanethiolate acid composite coating: Dual function for copper protection. Appl. Surf. Sci. 2011, 257, 10529–10534. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, S.; Liu, T.; Chang, X.; Yin, Y. Carboxymenthylchitosan as an ecofriendly inhibitor for mild steel in 1 M HCl. Mater. Lett. 2007, 61, 3276–3280. [Google Scholar] [CrossRef]
- Shamsa, A.; Barker, R.; Hua, Y.; Barmatov, E.; Hughes, T.L.; Neville, A. Impact of corrosion products on performance of imidazoline corrosion inhibitor on X65 carbon steel in CO2 environments. Corros. Sci. 2021, 185, 109423. [Google Scholar] [CrossRef]
- Okafor, P.C.; Liu, X.; Zheng, Y.G. Corrosion inhibition of mild steel by ethylamino imidazoline derivative in CO2-saturated solution. Corros. Sci. 2009, 51, 761–768. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.; Rai, B. Imidazole derivatives as corrosion inhibitors for copper: A DFT and reactive force field study. Corros. Sci. 2020, 171, 108724. [Google Scholar] [CrossRef]
- Haque, J.; Ansari, K.R.; Srivastava, V.; Quraishi, M.A.; Obot, I.B. Pyrimidine derivatives as novel acidizing corrosion inhibitors for N80 steel useful for petroleum industry: A combined experimental and theoretical approach. J. Ind. Eng. Chem. 2017, 49, 176–188. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Shaabani, B.; Seifzadeh, D. Corrosion inhibition of mild steel by some schiff base compounds in hydrochloric acid. Appl. Surf. Sci. 2005, 239, 154–164. [Google Scholar] [CrossRef]
- Barreto, L.S.; Santos, A.M.; de Almeida, T.F.; Silva, D.R.; Cotting, F.; Capelossi, V.R. Application of a Mix of Vegetables Residues as Inhibitor for Carbon Steel. Mater. Res. 2022, 25, 1–10. [Google Scholar] [CrossRef]
- Furtado, L.B.; Leoni, G.B.; Nascimento, R.C.; Santos, P.H.C.; Brasil, S.L.D.C.; De Tecnologia, C.; De Janeiro, R.; De Tecnologia, C.; De Janeiro, R.; De Janeiro, R. Experimental and Theoretical Studies of Tailor-made Schiff Bases as Corrosion Inhibitors for Carbon Steel in HCl. Mater. Res. 2023, 26, e20220398. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2017, 13, 740–771. [Google Scholar] [CrossRef]
- Ismail, K.M. Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions. Electrochim. Acta 2007, 52, 7811–7819. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Effect of 2-amino-3-mercaptopropanoic acid (cysteine) on the corrosion behaviour of low carbon steel in sulphuric acid. Electrochim. Acta 2007, 53, 909–914. [Google Scholar] [CrossRef]
- Jafari, H.; Ameri, E.; Rezaeivala, M.; Berisha, A. Dimethylpropane-1,3-Diyl)Bis(Azanediyl)Bis(Methylene) Bis(2-Methoxyphenol) as New Reduced Form of Schiff Base for Protecting API 5L Grade B in 1 M HCl. Arab. J. Sci. Eng. 2023, 48, 7359–7372. [Google Scholar] [CrossRef]
- Özcan, M. AC impedance measurement of cystine adsorption at mild steel/sulfuric acid interface as corrosion inhibitor. J. Solid State Electrochem. 2008, 12, 1653–1661. [Google Scholar] [CrossRef]
- Navarrete, J. Electrochemical and XPS studies of decylamides of a-amino acids adsorption on carbon steel in acidic environment. Appl. Surf. Sci. 2006, 252, 2894–2909. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Ghasemi, Z.; Seifzadeh, D. The inhibition effect of some amino acids towards the corrosion of aluminum in 1 M HCl + 1 M H2SO4 solution. Appl. Surf. Sci. 2005, 249, 408–418. [Google Scholar] [CrossRef]
- Li, J.F.S.; Wang, Y. Computational and electrochemical studies of some amino acid compounds as corrosion inhibitors for mild steel in hydrochloric acid solution. J. Mater. Sci. 2010, 45, 6255–6265. [Google Scholar] [CrossRef]
- Bobina, M.; Kellenberger, A.; Millet, J.P.; Muntean, C.; Vaszilcsin, N. Corrosion resistance of carbon steel in weak acid solutions in the presence of l-histidine as corrosion inhibitor. Corros. Sci. 2013, 69, 389–395. [Google Scholar] [CrossRef]
- Vasques, R.B.; Levy, M.M.; Wagner, F.; Almeida, D.Q.; Vaz, G.L.; Paes, L. A theoretical and experimental study of phosphate ester inhibitors for AISI 1018 in carbon dioxide—Saturated 3.5 wt% NaCl solution. Mater. Corros. 2021, 72, 1417–1432. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R. Investigation of Corrosion Inhibition Effect and Adsorption Activities of Achyranthes aspera Extract for Mild Steel in 0.5 M H2SO4. J. Fail. Anal. Prev. 2018, 18, 957–968. [Google Scholar] [CrossRef]
- Haldhar, R.; Raorane, C.J.; Mishra, V.K.; Periyasamy, T.; Berisha, A.; Kim, S.C. Development of different chain lengths ionic liquids as green corrosion inhibitors for oil and gas industries: Experimental and theoretical investigations. J. Mol. Liq. 2023, 372, 121168. [Google Scholar] [CrossRef]
- Solmaz, R.; Altunba, E.; Karda, G. Adsorption and corrosion inhibition effect of 2-((5-mercapto-1,3,4-thiadiazol-2-ylimino)methyl)phenol Schiff base on mild steel. Mater. Chem. Phys. 2011, 125, 796–801. [Google Scholar] [CrossRef]
- Kokalj, A. On the Use of the Langmuir and Other Adsorption Isotherms in Corrosion Inhibition. SSRN Electron. J. 2022, 217, 111112. [Google Scholar] [CrossRef]
- Niu, H.; Yang, H.; Zhao, R.; Tong, L.; Cristiani, P. Inhibition of carbonaceous matters adsorbing gold using a green and efficient chlorinating agent TCCA: Inhibition behavior, kinetics and isotherms, inhibition mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 131836. [Google Scholar] [CrossRef]
- Chebabe, D.; Chikh, Z.A.; Hajjaji, N.; Srhiri, A.; Zucchi, F. Corrosion inhibition of Armco iron in 1 M, HCl solution by alkyltriazoles. Corros. Sci. 2003, 45, 309–320. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdallah, M.; Awad, M.K.; Rezk, M. Three novel di-quaternary ammonium salts as corrosion inhibitors for API X65 steel pipeline in acidic solution. Part I: Experimental results. Corros. Sci. 2014, 81, 54–64. [Google Scholar] [CrossRef]
- Fawzy, A.; Abdallah, M.; Zaafarany, I.A.; Ahmed, S.A.; Althagafi, I.I. Thermodynamic, kinetic and mechanistic approach to the corrosion inhibition of carbon steel by new synthesized amino acids-based surfactants as green inhibitors in neutral and alkaline aqueous media. J. Mol. Liq. 2018, 265, 276–291. [Google Scholar] [CrossRef]
- Fontana, I.; Lauria, A.; Spinolo, G. Optical absorption spectra of Fe2+ and Fe3+ in aqueous solutions and hydrated crystals. Phys. Status Solidi Basic Res. 2007, 244, 4669–4677. [Google Scholar] [CrossRef]
- Wang, L.K.; Li, L.; Li, X.M.; Shi, Y.H.; Hu, L.; Le, G.W. Microwave assisted solid state reaction synthesis of methionine complexes of iron (II). Food Chem. 2008, 106, 315–323. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Tizpar, A. The inhibition effect of some amino acids towards Pb-Sb-Se-As alloy corrosion in sulfuric acid solution. Appl. Surf. Sci. 2006, 252, 3667–3672. [Google Scholar] [CrossRef]
- Şahin, M.; Bilgiç, S.; Yilmaz, H. The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Appl. Surf. Sci. 2002, 195, 1–7. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, D.; Wu, Y.; Lai, Q.; Xue, L.; Feng, S. Perylene-containing polysiloxane: An effective candidate for corrosion protection of iron surface. Appl. Surf. Sci. 2011, 257, 10576–10580. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Barsoukov, E. Theory, Experiment, and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Dhas, S.A.M.B.; Natarajan, S. Growth and characterization of two new NLO materials from the amino acid family: L-Histidine nitrate and l-Cysteine tartrate monohydrate. Opt. Commun. 2008, 281, 457–462. [Google Scholar] [CrossRef]
- Térmica, A.; L-histidina, T.A.D.C.; Medeiros, J.E. Pereira Completion of Course Work: Análise Térmica e Espectroscopia Raman à Temperatura Ambiente de Cristais L-Histidina Departamento de Física Centro de Ciências Universidade Federal do Ceará, (2012). Available online: http://repositorio.ufc.br/handle/riufc/31441 (accessed on 27 May 2024).
- Fouda, A.S.; Ismail, M.A.; Elewady, G.Y.; Abousalem, A.S. Evaluation of 4-amidinophenyl-2,2′-bithiophene and its aza-analogue as novel corrosion inhibitors for CS in acidic media: Experimental and theoretical study. J. Mol. Liq. 2017, 240, 372–388. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional thermochemistry. I. The effect of the exchange only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to Isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Vinayagamoorthy, R.; Irudayaraj, A.A.; Raj, A.D.; Karthick, S.; Jayakumar, G. Comparative Study of Properties of L-Histidine and L-Histidine Nickel Nitrate Hexahydrate Crystals Grown by Slow Evaporation. Mech. Mater. Sci. Eng. J. 2017, 9, hal-01503639. [Google Scholar] [CrossRef]
- Wu, J.; Wu, J.; Lu, L.; Mei, P. Design, characteristics, and theoretical analyses of 8-hydroxyquinoline derivatives with different heteroatoms as effective corrosion inhibitors. Mater. Chem. Phys. 2023, 304, 127929. [Google Scholar] [CrossRef]
- Alnajjar, A.O.; El-lateef, H.M.A.; Khalaf, M.M.; Mohamed, I.M.A. Steel protection in acidified 3.5% NaCl by novel hybrid composite of CoCrO3/polyaniline: Chemical fabrication, physicochemical properties, and corrosion inhibition performance. Constr. Build. Mater. 2022, 317, 125918. [Google Scholar] [CrossRef]
- Kalantar, M.A.A.M. Thermal Analysis on the Milled AlB2O3 + Si + WO3 System to Synthesize Al2O3-WxSiy-WxBy Powders. Mater. Res. 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Soltani, N.; Salavati-Niasari, M.; Hamadanian, M.; Gandomi, A. Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corros. Sci. 2008, 50, 2172–2181. [Google Scholar] [CrossRef]
- da Silva Hernandes, I.; da Cunha, J.N.; Santana, C.A.; Rodrigues, J.G.A.; D’Elia, E. Application of an aqueous extract of cotton seed as a corrosion inhibitor for mild steel in HCl media. Mater. Res. 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Bentiss, F.; Traisnel, M.; Gengembre, L.; Lagrenée, M. New triazole derivative as inhibitor of the acid corrosion of mild steel: Electrochemical studies, weight loss determination, SEM and XPS. Appl. Surf. Sci. 1999, 152, 237–249. [Google Scholar] [CrossRef]
- Agarwal, V.K. Langmuir-Blodgett Films. Phys. Today 1988, 41, 40–46. [Google Scholar] [CrossRef]
- Silbey, R.J.; Alberty, R.A.; Bawendi, M.G. Physical Chemistry, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Paramasivam, S.; Kulanthai, K.; Sadhasivam, G.; Subramani, R. Corrosion Inhibition of Mild Steel in Hydrochloric Acid using4- (pyridin-2yl)-N-p-tolylpiperazine-1-carboxamide. Int. J. Electrochem. Sci. 2016, 11, 3393–3414. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Zhou, Y.; Pan, R.; Du, H.; Liu, F.; Yang, Y. Expired Glucosamine Drugs as Green Corrosion Inhibitors for Carbon Steel in H2SO4 Solution and Synergistic Effect of Glucosamine Molecules with Iodide Ions: Combined experimental and theoretical investigations. Crystals 2023, 13, 205. [Google Scholar] [CrossRef]
- da Rocha, J.C. Obtenção de Inibidores de Corrosão a Partir de Extratos de Produtos Naturais. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2016; 106p. [Google Scholar]
- Zhang, Q.H.; Li, Y.Y.; Lei, Y.; Wang, X.; Liu, H.F.; Zhang, G.A. Comparison of the synergistic inhibition mechanism of two eco-friendly amino acids combined corrosion inhibitors for carbon steel pipelines in oil and gas production. Appl. Surf. Sci. 2022, 583, 152559. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy Theory, Experiment, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Zhao, P.; Liang, Q.; Li, Y. Electrochemical, SEM/EDS and quantum chemical study of phthalocyanines as corrosion inhibitors for mild steel in 1 mol/L HCl. Appl. Surf. Sci. 2005, 252, 1596–1607. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; He, Y.; Yang, R.; Ma, L.; Xia, Y.; He, Z. Histidine and histidine dimer as green inhibitors for carbon steel in 3wt% sodium chloride solution; Electrochemical, XPS and quantum chemical calculation studies. Int. J. Electrochem. Sci. 2018, 13, 2136–2153. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Ahmed, A.H. Alleviation of iron corrosion in chloride solution by n,n′-bis[2-methoxynaphthylidene]amino]oxamide as a corrosion inhibitor. Crystals 2021, 11, 1516. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Ansari, K.R.; Sorour, A.A.; Quraishi, M.A.; Lgaz, H.; Salghi, R. Thiosemicarbazide and thiocarbohydrazide functionalized chitosan as ecofriendly corrosion inhibitors for carbon steel in hydrochloric acid solution. Int. J. Biol. Macromol. 2018, 107, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Mao, J.; Ma, Q.; Tang, Y. Corrosion inhibition of perimidine derivatives for mild steel in acidic media: Electrochemical and computational studies. J. Mol. Liq. 2018, 269, 260–268. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Asl, S.K. Casein as a natural protein to inhibit the corrosion of mild steel in HCl solution. J. Mol. Liq. 2019, 276, 694–704. [Google Scholar] [CrossRef]
- Kaya, S.; Tüzün, B.; Kaya, C.; Obot, I.B. Determination of corrosion inhibition effects of amino acids: Quantum chemical and molecular dynamic simulation study. J. Taiwan Inst. Chem. Eng. 2016, 58, 528–535. [Google Scholar] [CrossRef]

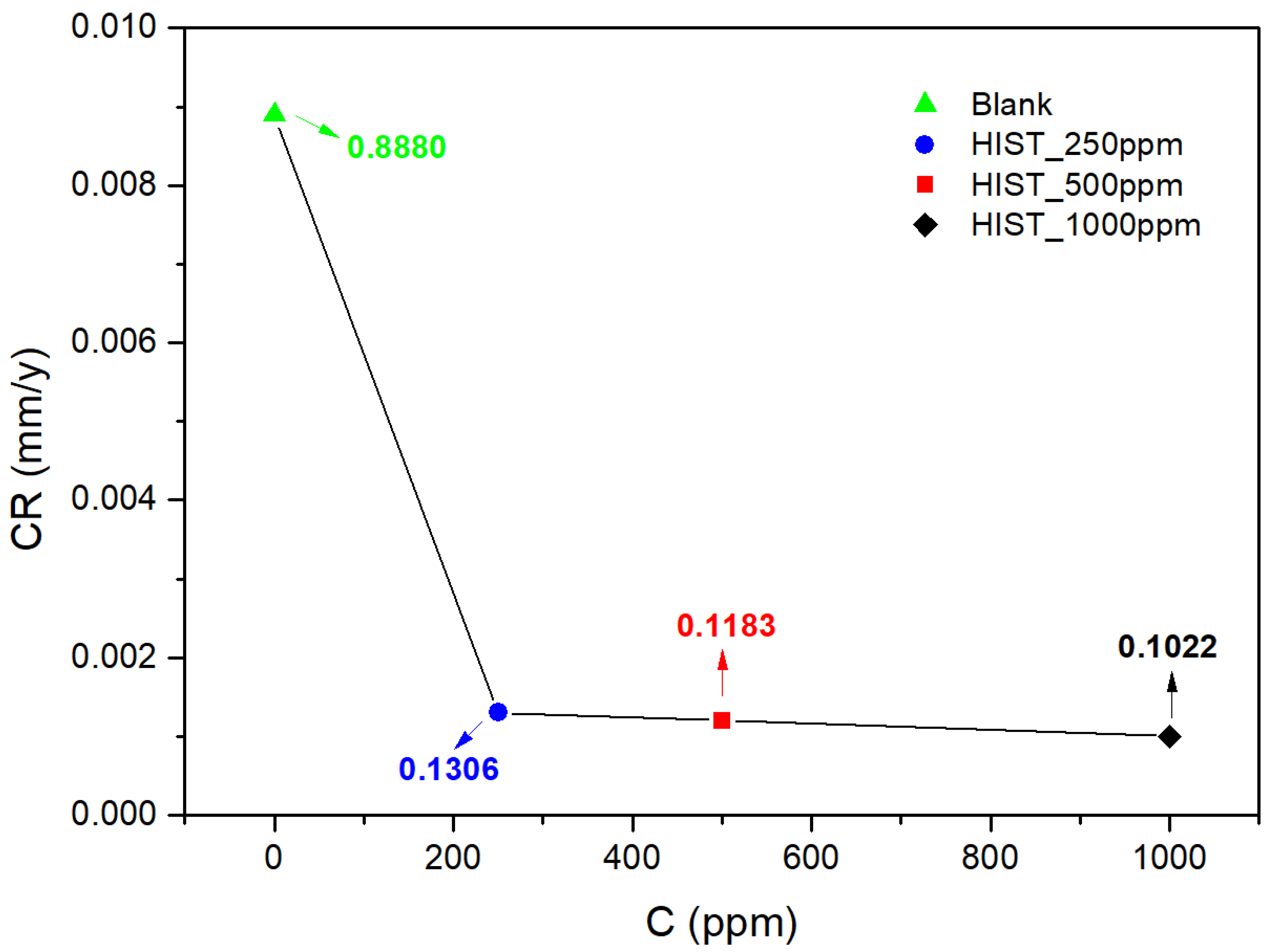
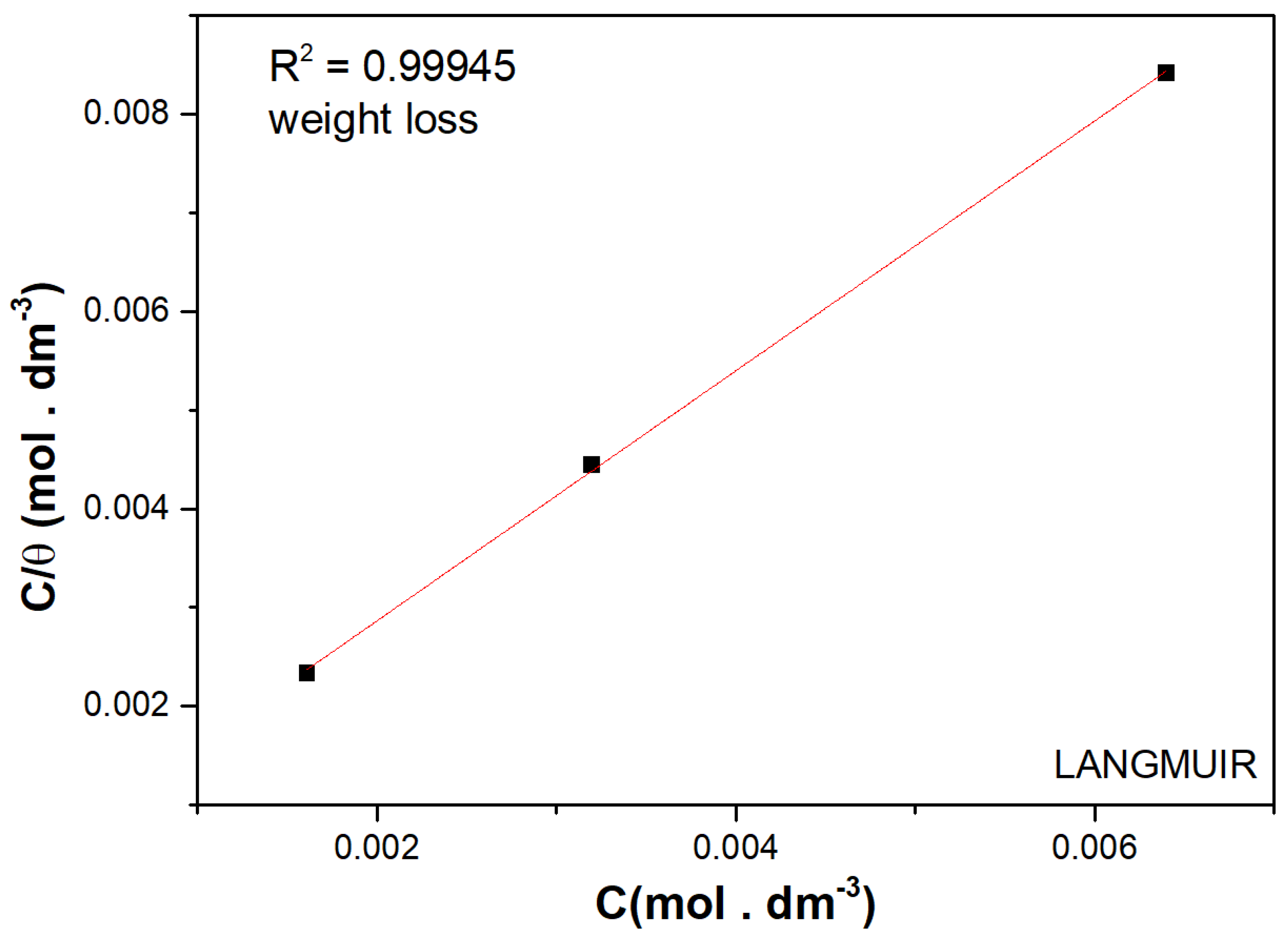
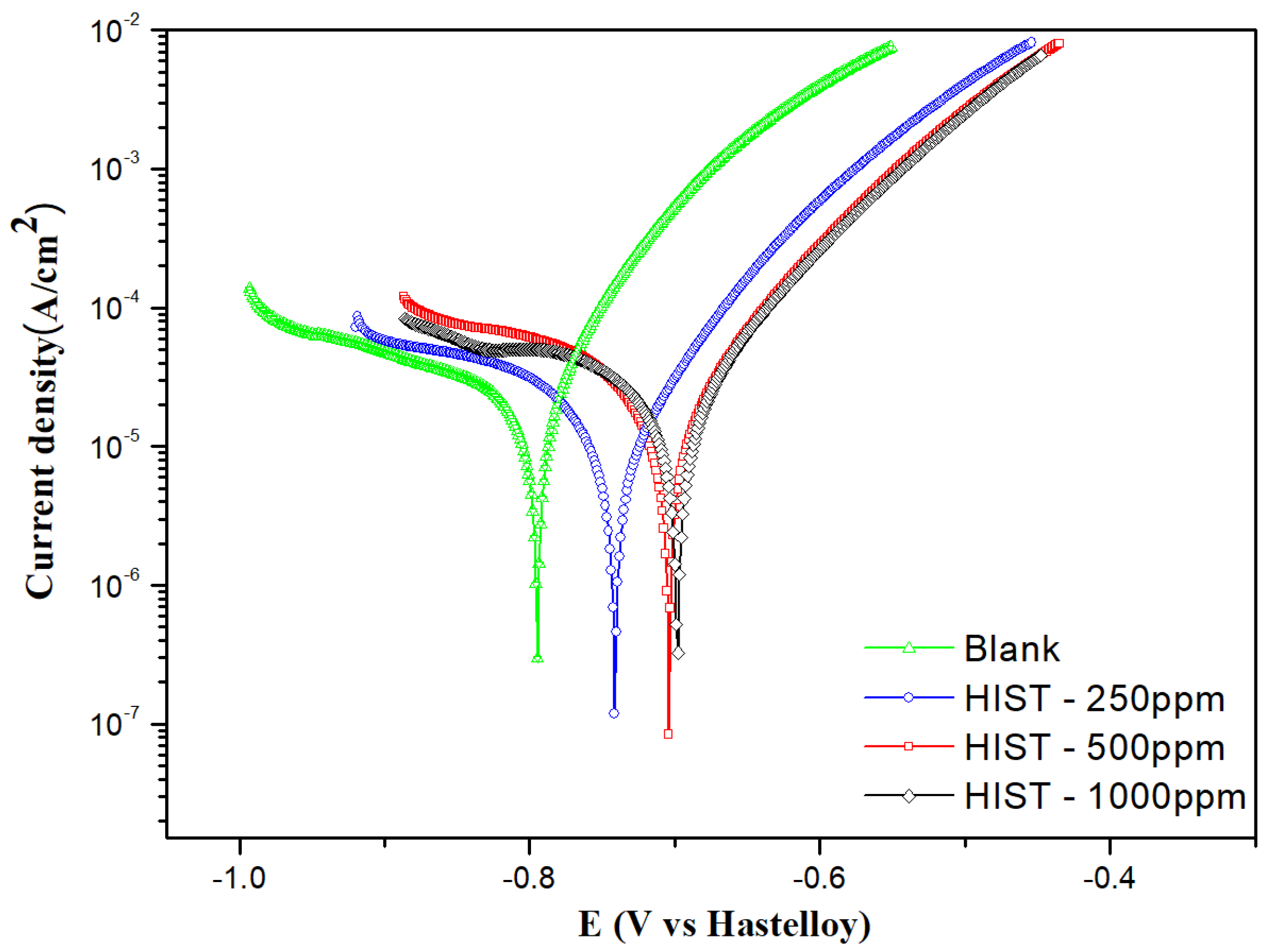
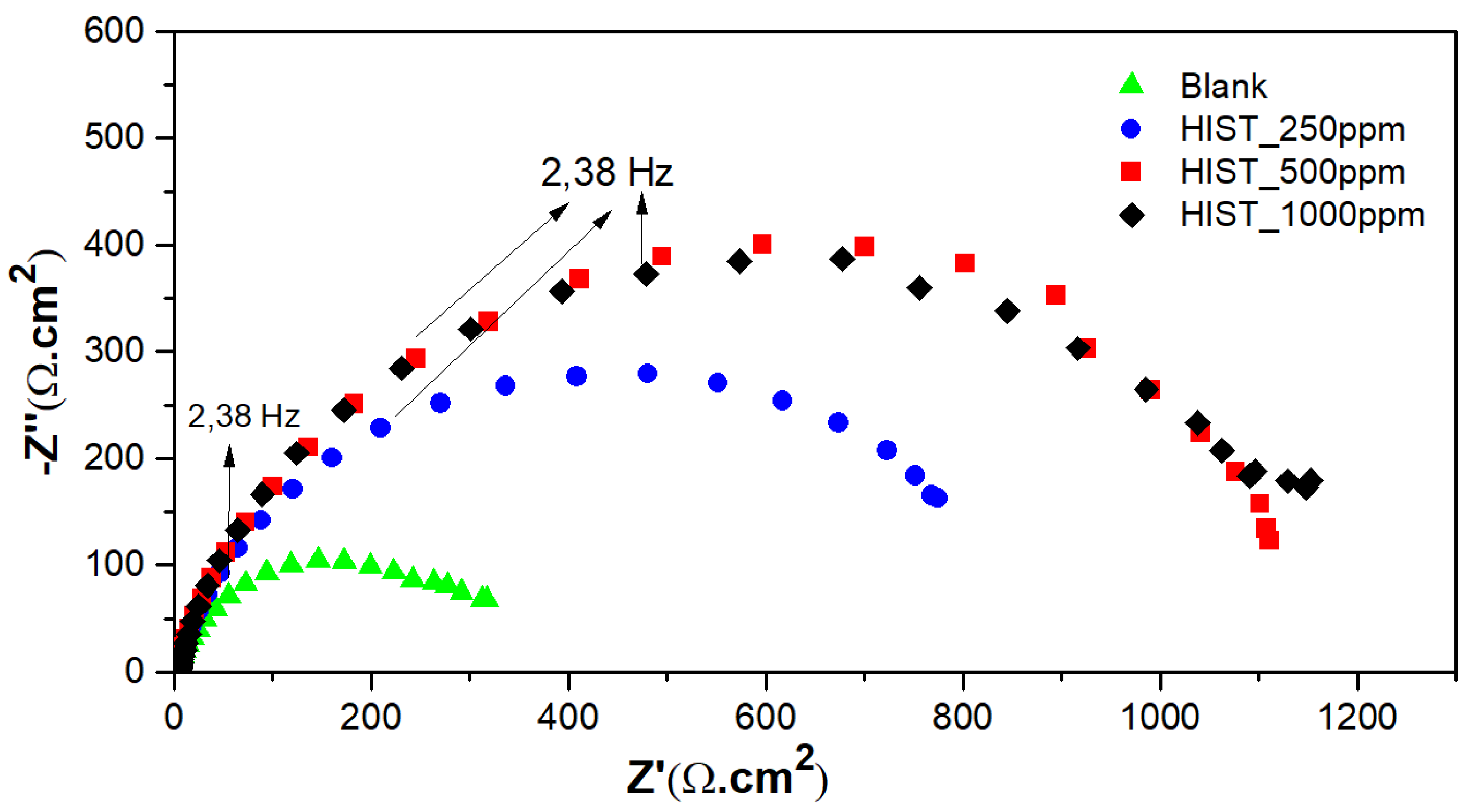




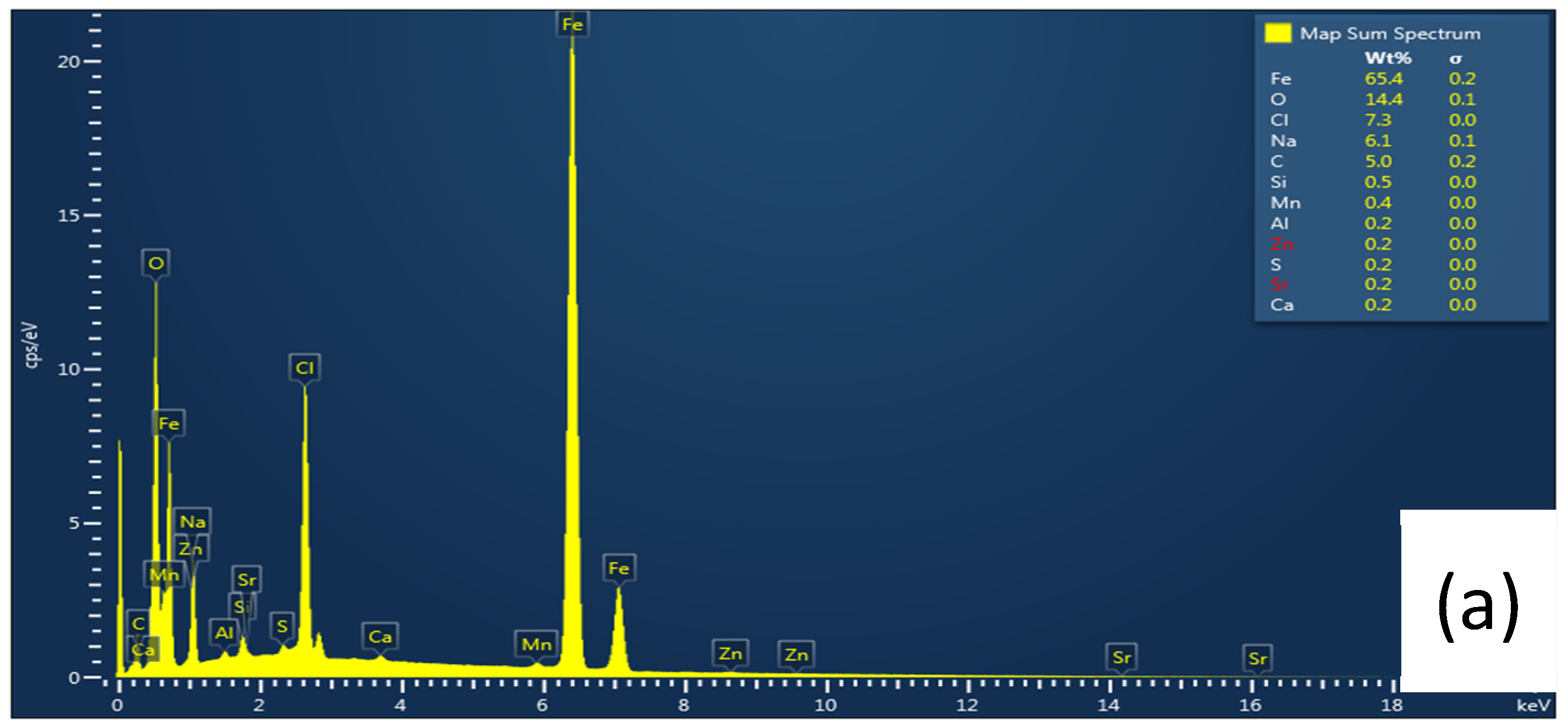
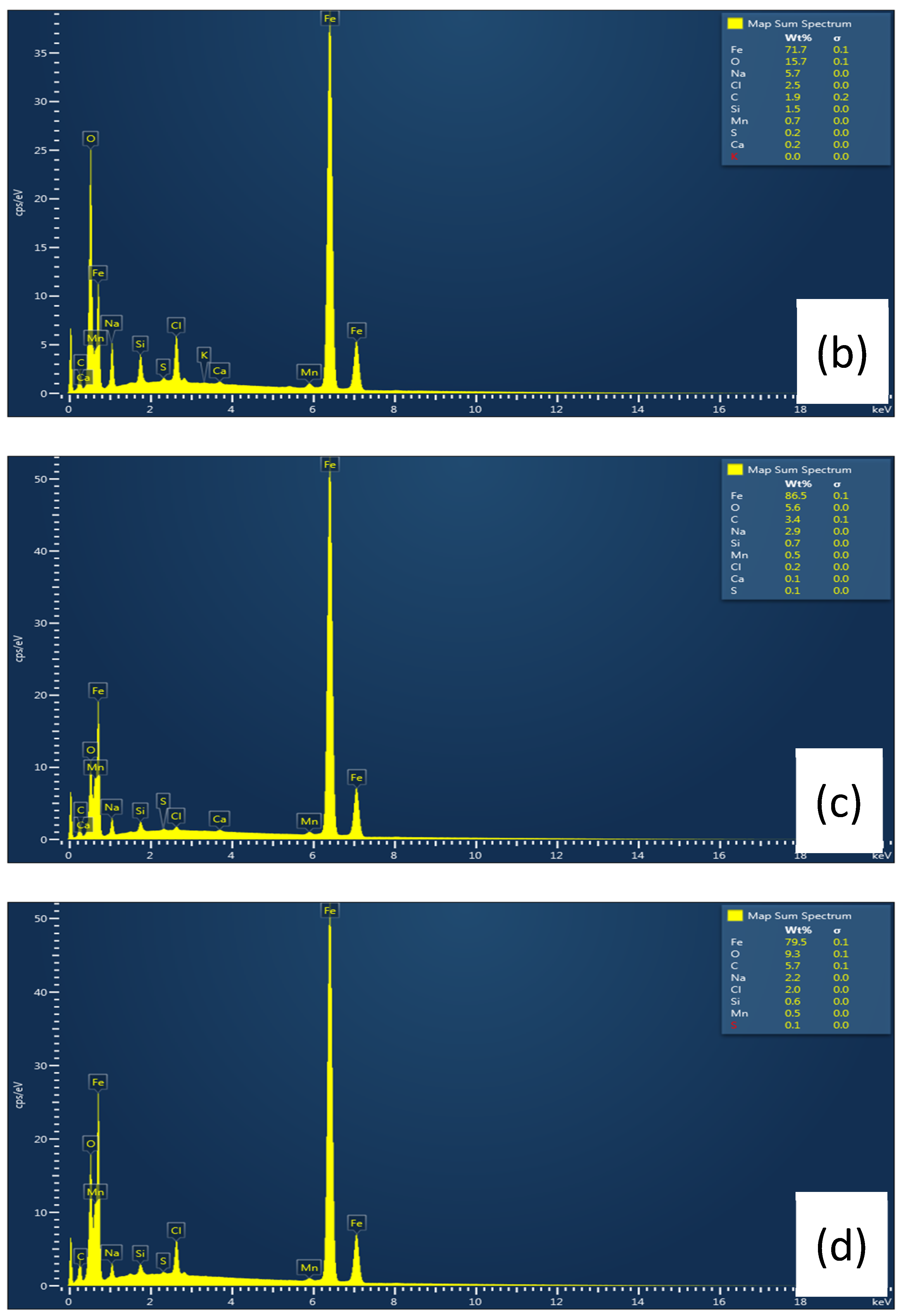
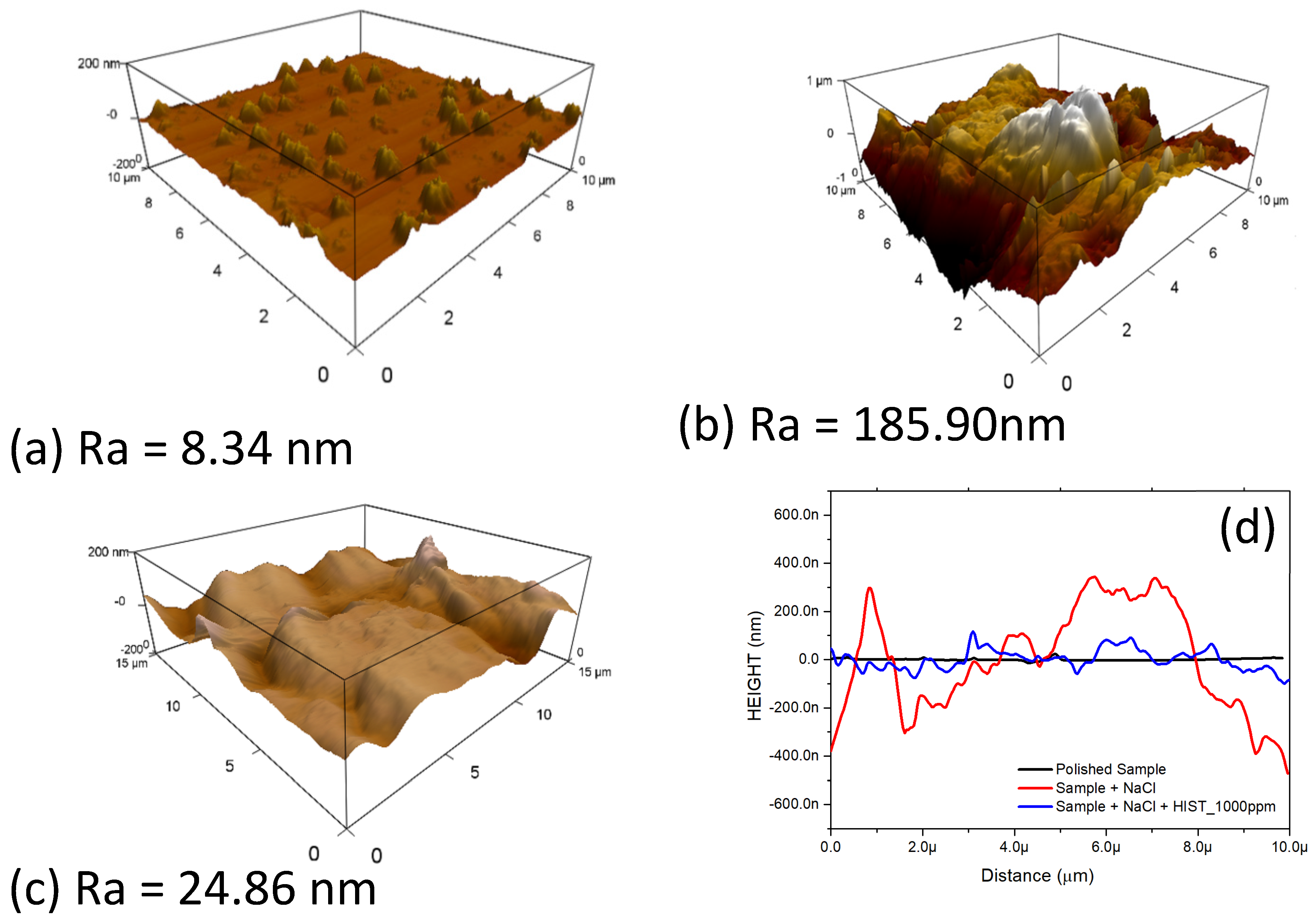

| L-Histidine (ppm) | η (%) | θ (Surface Coverage) | CR (mm/y) |
| 0 | --- | --- | 0.8888 ± 0.091 |
| 250 | 83 | 0.83 ± 0.09 | 0.1306 ± 0.076 |
| 500 | 87 | 0.87 ± 0.07 | 0.1183 ± 0.084 |
| 1000 | 88 | 0.88 ± 0.04 | 0.1022 ± 0.096 |
| L-Histidine conc. (ppm) | (V) | (A/cm2) | (mV/dec) | (mV/dec) | Rct (Ωcm2) | θ | (%) |
|---|---|---|---|---|---|---|---|
| 0 | −0.794 ± 0.04 | (3.39 ± 0.83) × 10−5 | 68 | 6239 | --- | ---- | |
| 250 | −0.741 ± 0.06 | (3.99 ± 0.36) × 10−6 | 42 | 52 | 10,185 | 0.8822 | 88.22 |
| 500 | −0.704 ± 0.10 | (3.41 ± 0.19) × 10−6 | 47 | 49 | 9963 | 0.8995 | 89.95 |
| 1000 | −0.698 ± 0.07 | (3.16 ± 0.18) × 10−6 | 24 | 32 | 9668 | 0.9068 | 90.68 |
| L-Histidine (ppm) | Rct (Ωcm2) | η% |
|---|---|---|
| 0 | 336 ± 53 | … |
| 250 | 895 ± 51 | 62 |
| 500 | 1119 ± 38 | 69 |
| 1000 | 1181 ± 45 | 72 |
| Quantum Reactivity Descriptor | Gas Phase | Water | Histidine [21] | Histidine [63] | Histidine [63] | Histidine [58] |
|---|---|---|---|---|---|---|
| /eV) | −6.8268 | −6.7014 | −6.367 | −6.72725 | −6.71337 | −6.700 |
| /eV) | −0.7606 | −0.2952 | −0.272 | −0.88955 | −0.31538 | −0.094 |
| /eV) | 6.0663 | 6.4062 | 6.095 | 5.83770 | 6.39799 | 6.600 |
| Ionization Potential (I/eV) | 6.8268 | 6.7014 | 6.3670 | 6.72725 | 6.71337 | 6.700 |
| Electron Affinity (A/eV) | 0.7606 | 0.2952 | 0.2720 | 0.88955 | 0.31538 | 0.094 |
| Electronegativity (χ/eV) | 3.7937 | 3.4983 | 3.3195 | 3.80840 | 3.51438 | 3.397 |
| Global Hardness (η/eV) | 3.0331 | 3.2031 | 3.0475 | 2.91885 | 3.19900 | 5.200 |
| Global Softness (σ/eV−1) | 0.3297 | 0.3122 | 0.3281 | 0.34260 | 0.31260 | 0.303 |
| Electrophilicity index (ε/eV) | 2.3725 | 1.9104 | 1.8079 | 2.4845 | 1.9304 | 1.747 |
| Nucleophilicity index (ω/eV−1) | 0.4215 | 0.5235 | 0.5531 | 0.4025 | 0.5180 | 0.573 |
| Fraction of electrons transferred (∆N) | 0.528544 | 0.546611 | 0.5782 | 0.546722 | 0.544800 | 0.540 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, M.J.d.S.; Vasques, R.B.; Magalhães, S.J.d.m.; Almeida Neto, F.W.d.Q.; de Lima Neto, P.; dos Santos, L.P.M.; Florez, M.A.C.; Ribas, G.F.; Medeiros, S.L.S.; Salomão, F.C.C.S.; et al. Assessment of the Amino Acid L-Histidine as a Corrosion Inhibitor for a 1018 Carbon Steel in Aqueous Sodium Chloride Solution. Crystals 2024, 14, 703. https://doi.org/10.3390/cryst14080703
Moura MJdS, Vasques RB, Magalhães SJdm, Almeida Neto FWdQ, de Lima Neto P, dos Santos LPM, Florez MAC, Ribas GF, Medeiros SLS, Salomão FCCS, et al. Assessment of the Amino Acid L-Histidine as a Corrosion Inhibitor for a 1018 Carbon Steel in Aqueous Sodium Chloride Solution. Crystals. 2024; 14(8):703. https://doi.org/10.3390/cryst14080703
Chicago/Turabian StyleMoura, Milena Jacinto da Silva, Roberta Bastos Vasques, Saulo Jose de melo Magalhães, Francisco Wagner de Queiroz Almeida Neto, Pedro de Lima Neto, Luís Paulo Mourão dos Santos, Mauro Andres Cerra Florez, Gemma Fargas Ribas, Samuel Lucas Santos Medeiros, Francisco Carlos Carneiro Soares Salomão, and et al. 2024. "Assessment of the Amino Acid L-Histidine as a Corrosion Inhibitor for a 1018 Carbon Steel in Aqueous Sodium Chloride Solution" Crystals 14, no. 8: 703. https://doi.org/10.3390/cryst14080703
APA StyleMoura, M. J. d. S., Vasques, R. B., Magalhães, S. J. d. m., Almeida Neto, F. W. d. Q., de Lima Neto, P., dos Santos, L. P. M., Florez, M. A. C., Ribas, G. F., Medeiros, S. L. S., Salomão, F. C. C. S., Barros, E. B., & Araújo, W. S. (2024). Assessment of the Amino Acid L-Histidine as a Corrosion Inhibitor for a 1018 Carbon Steel in Aqueous Sodium Chloride Solution. Crystals, 14(8), 703. https://doi.org/10.3390/cryst14080703






