Plasma-Enhanced Atomic Layer Deposition of Hematite for Photoelectrochemical Water Splitting Applications
Abstract
1. Introduction
2. Results
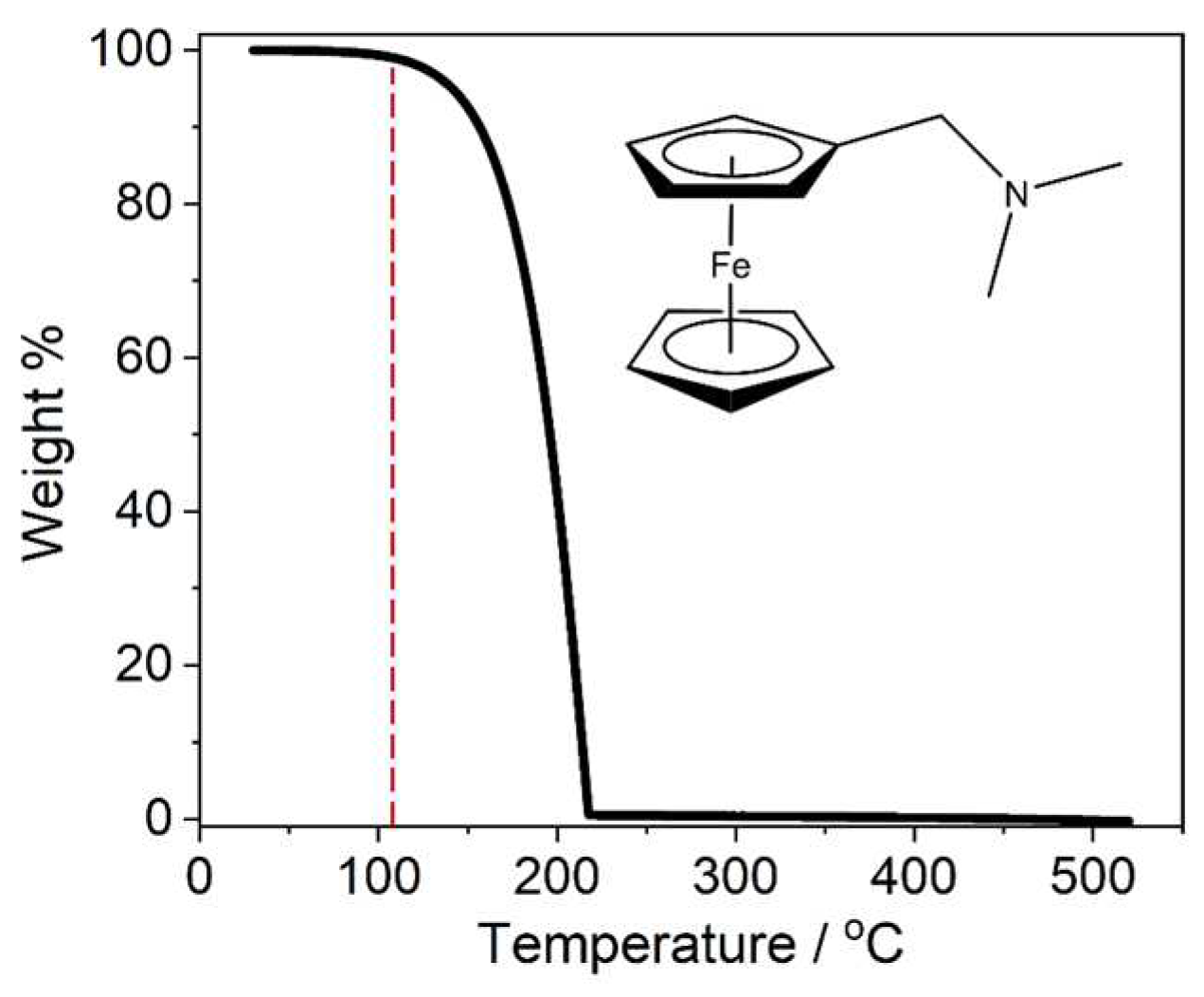
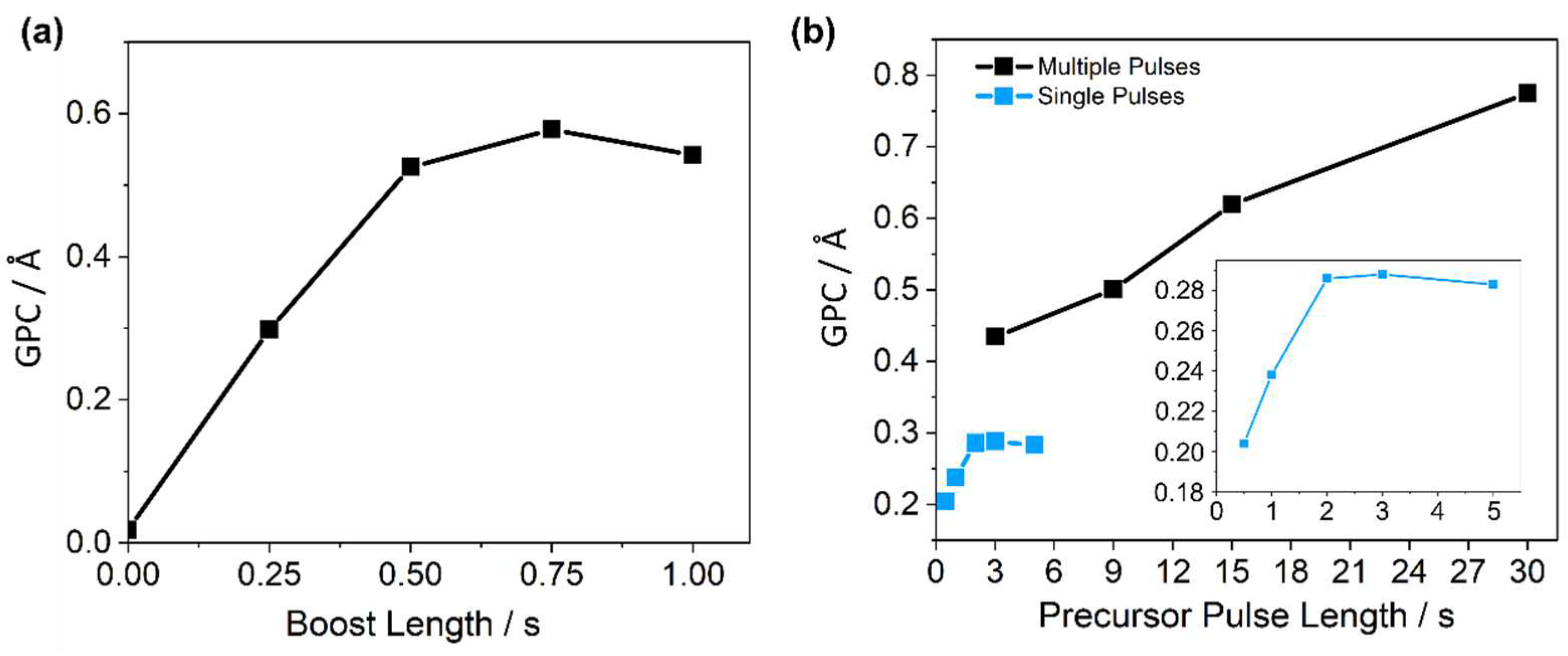
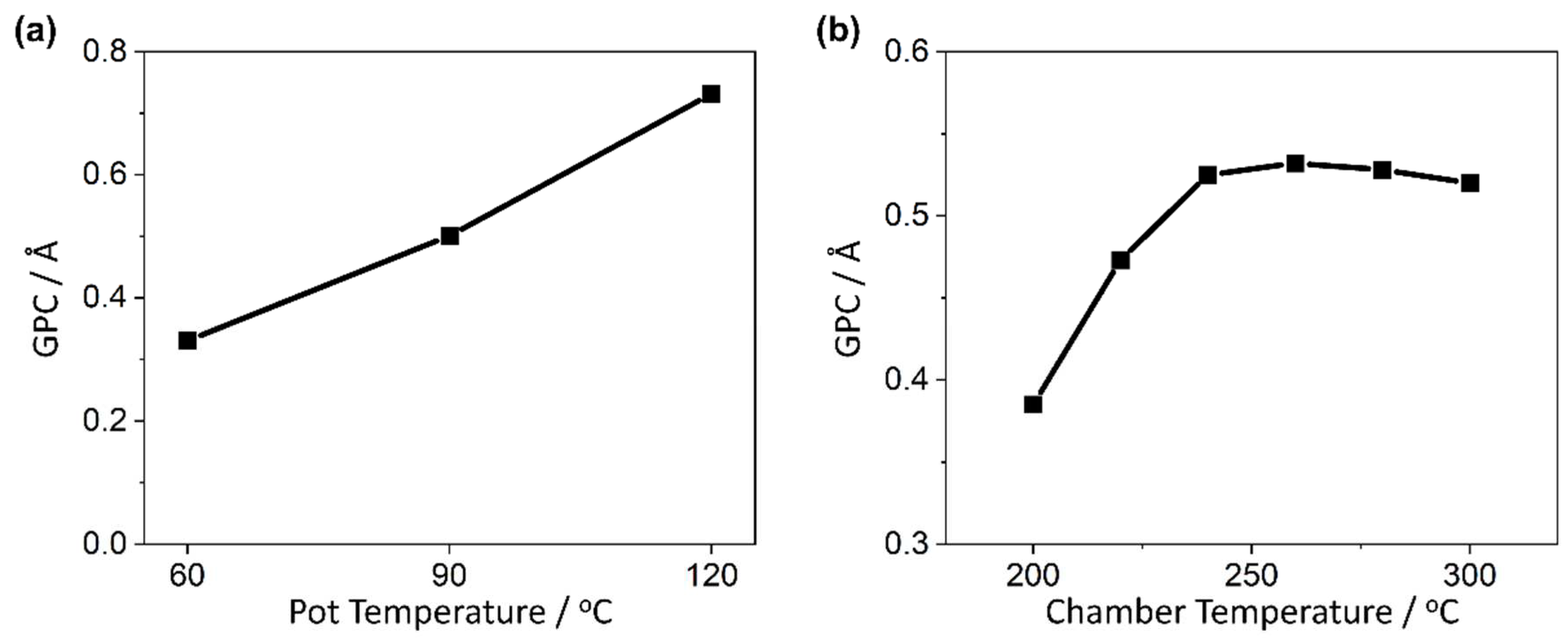
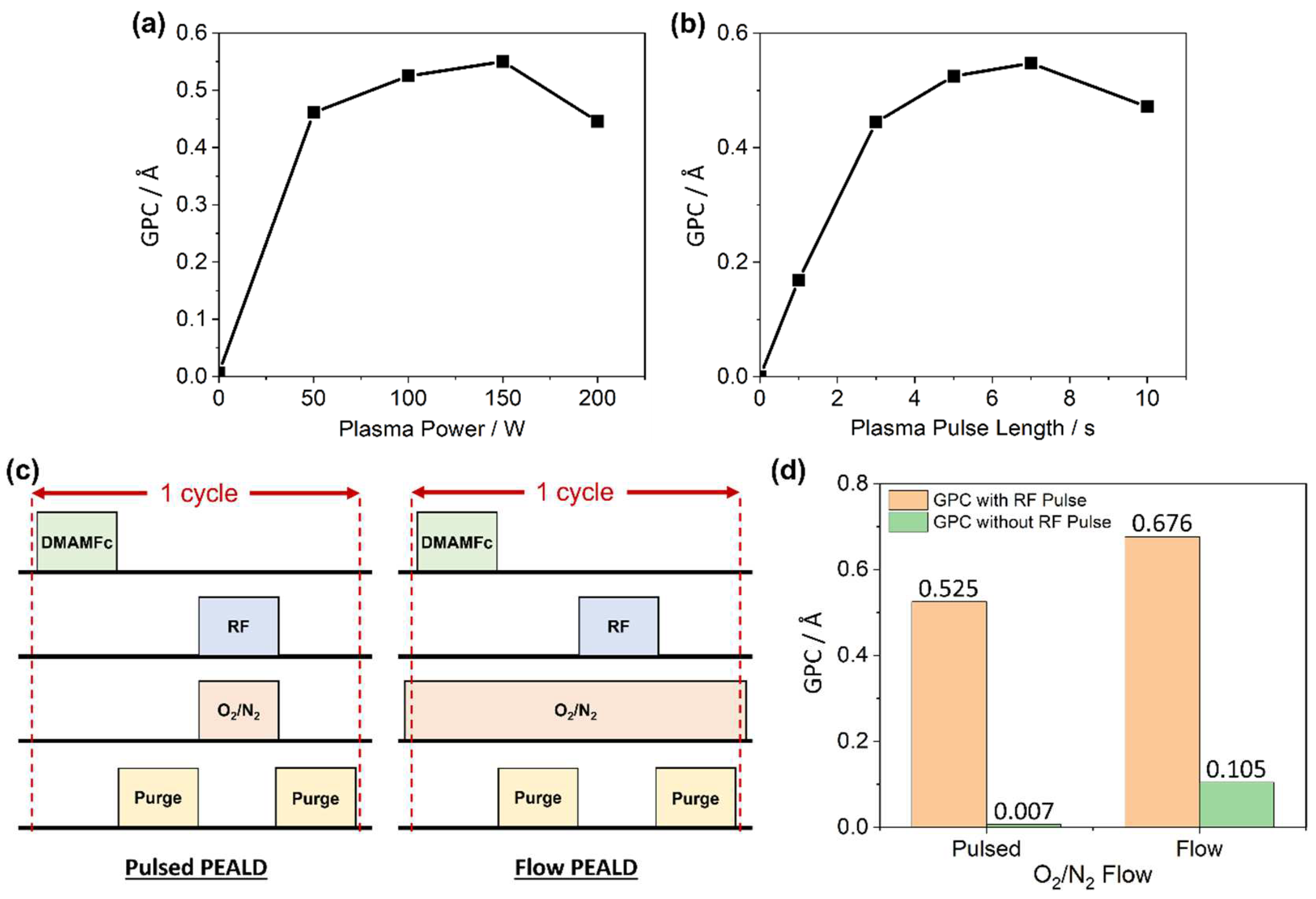
2.1. PEALD Process Development
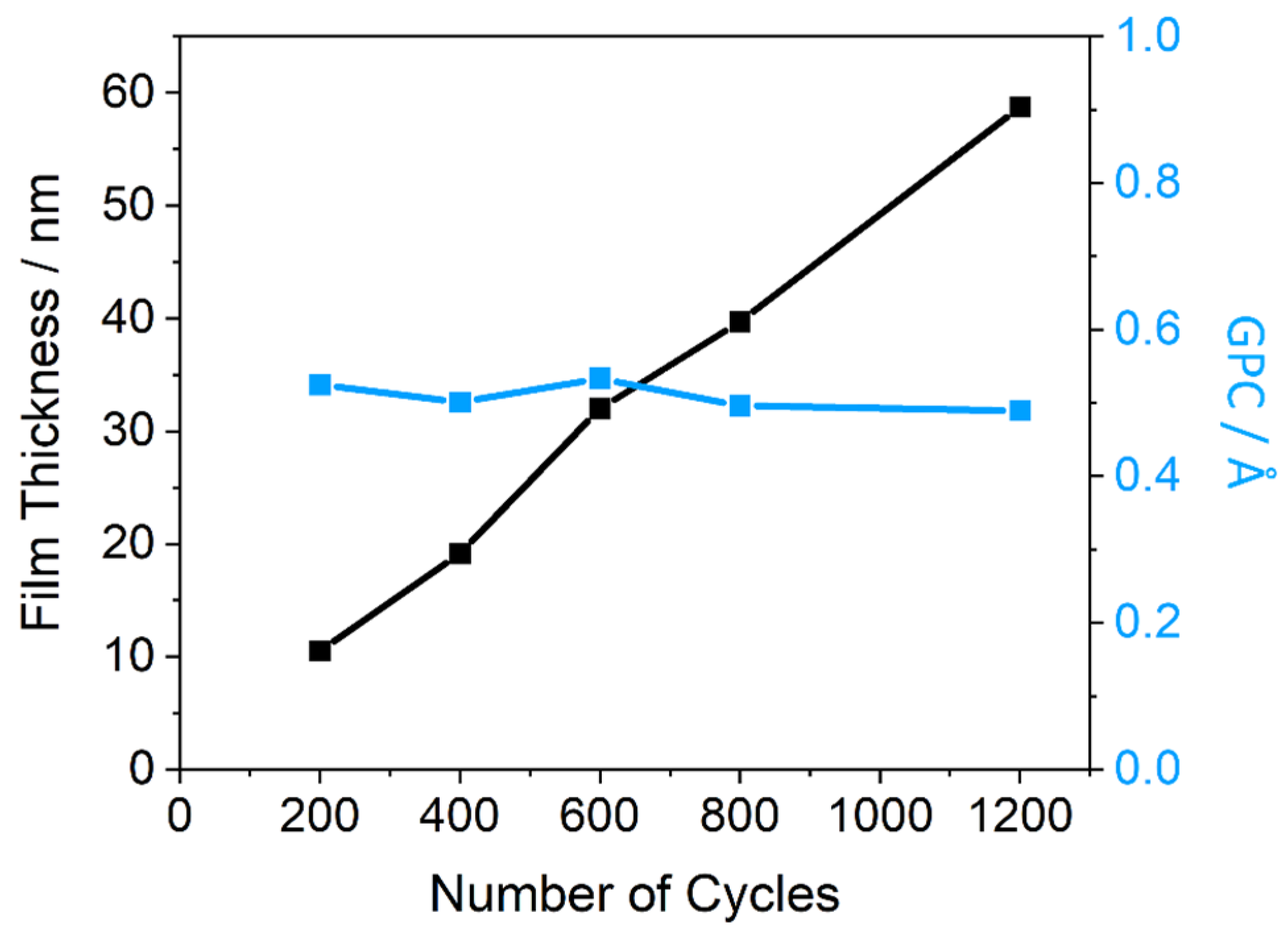
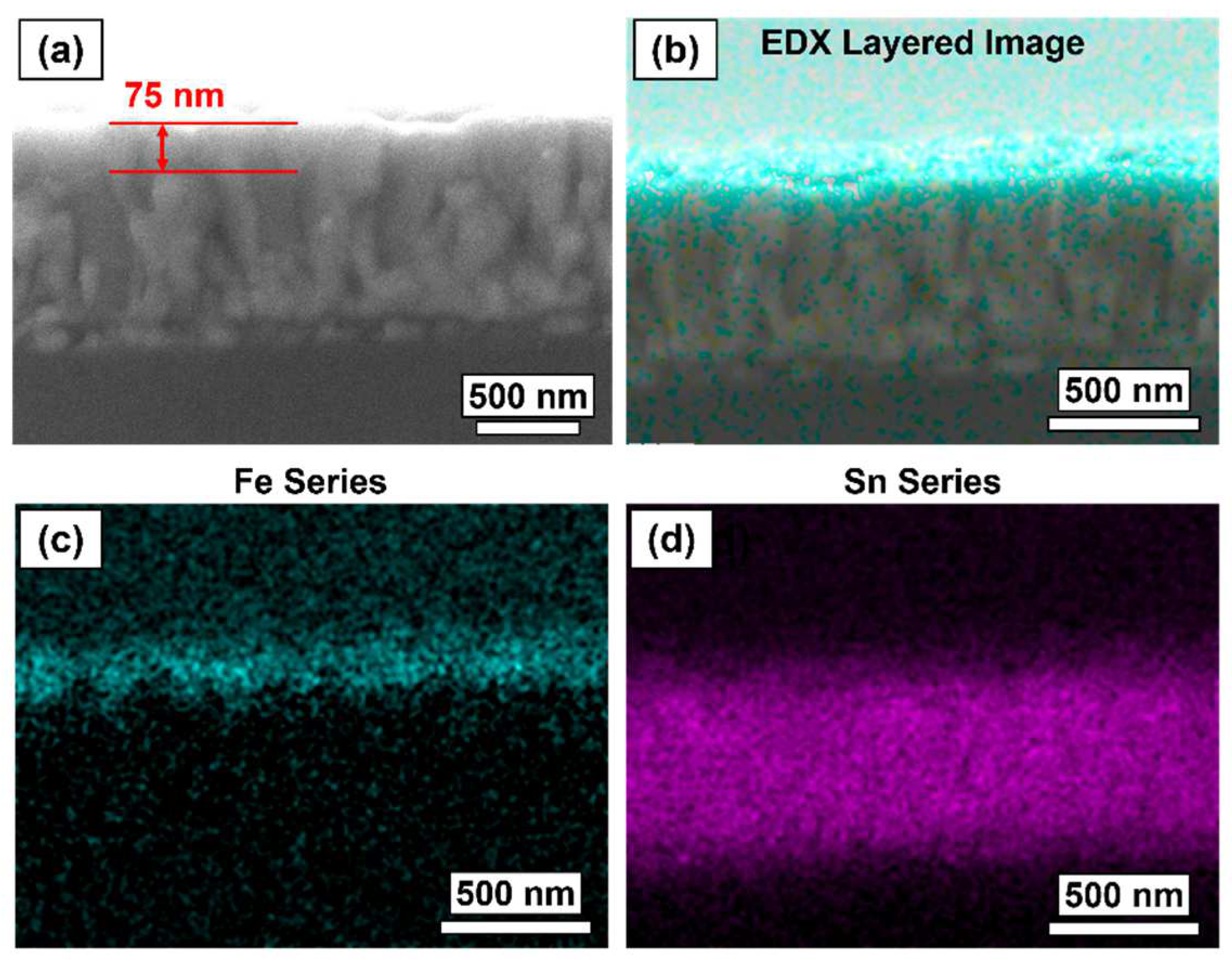
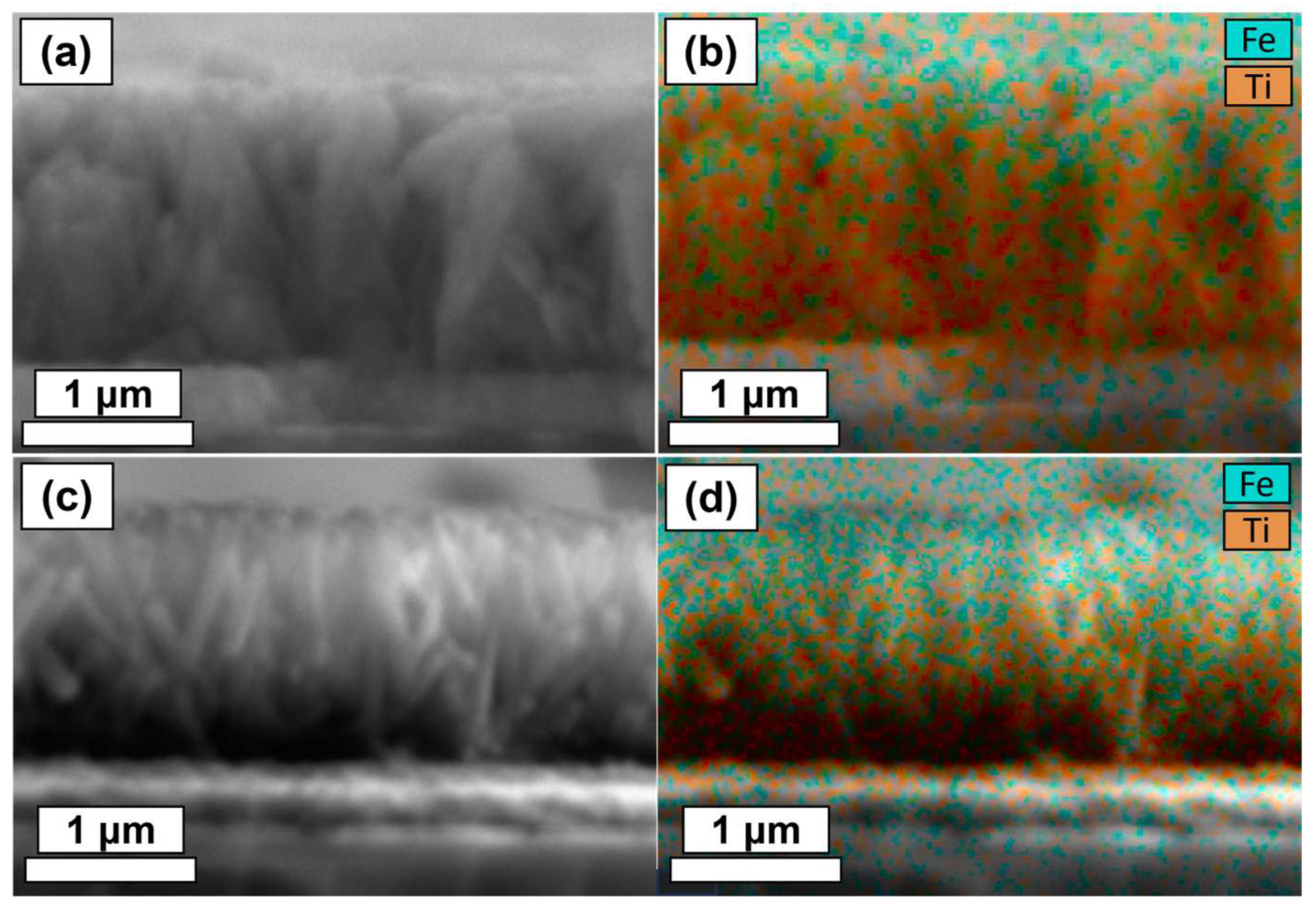
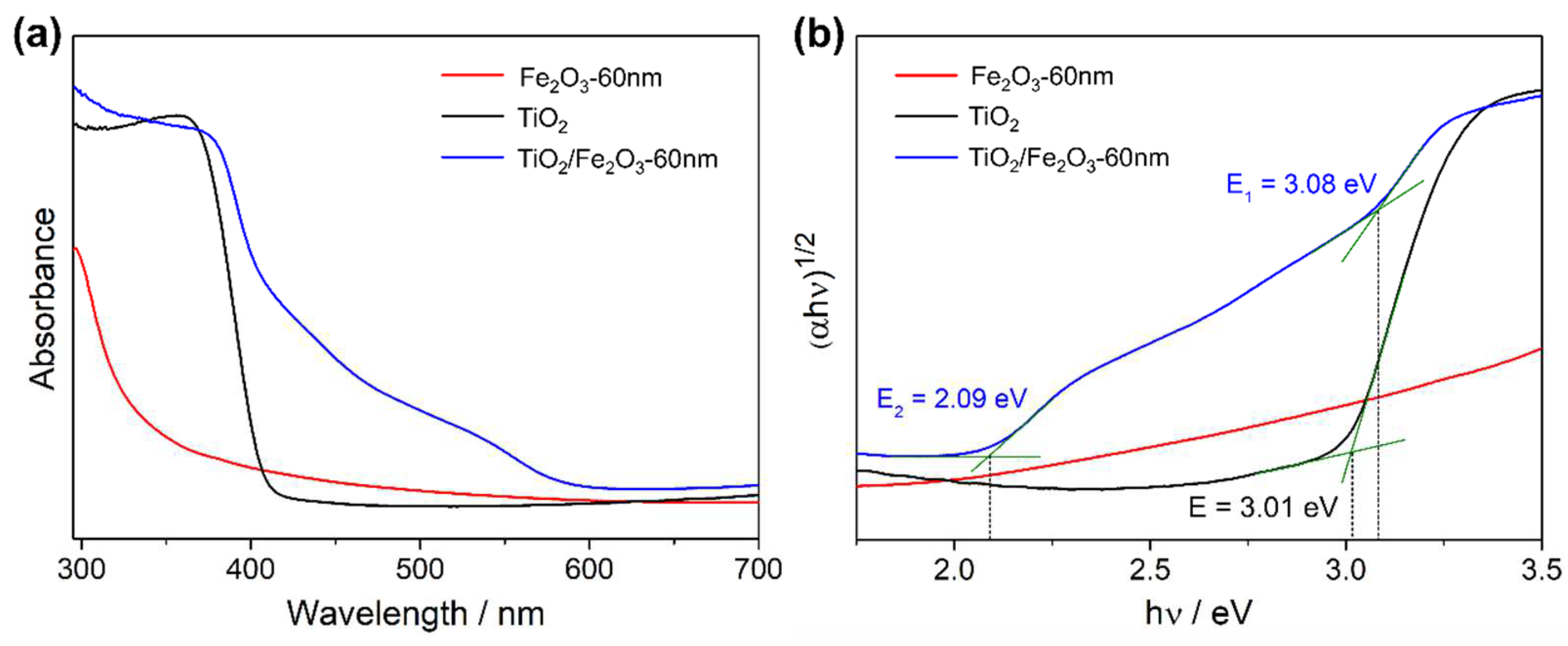
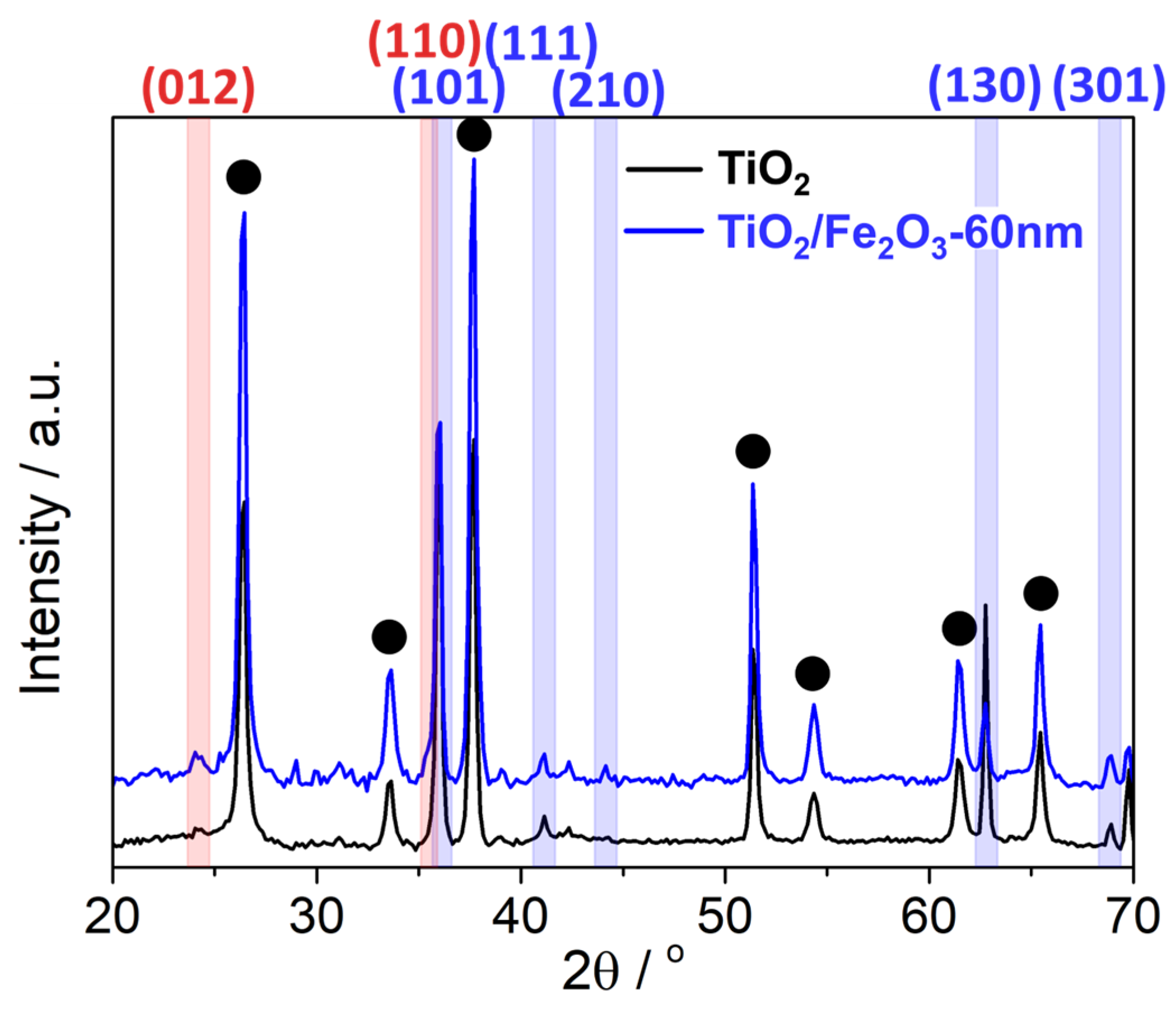
2.2. Thin Film Physical Characterisation
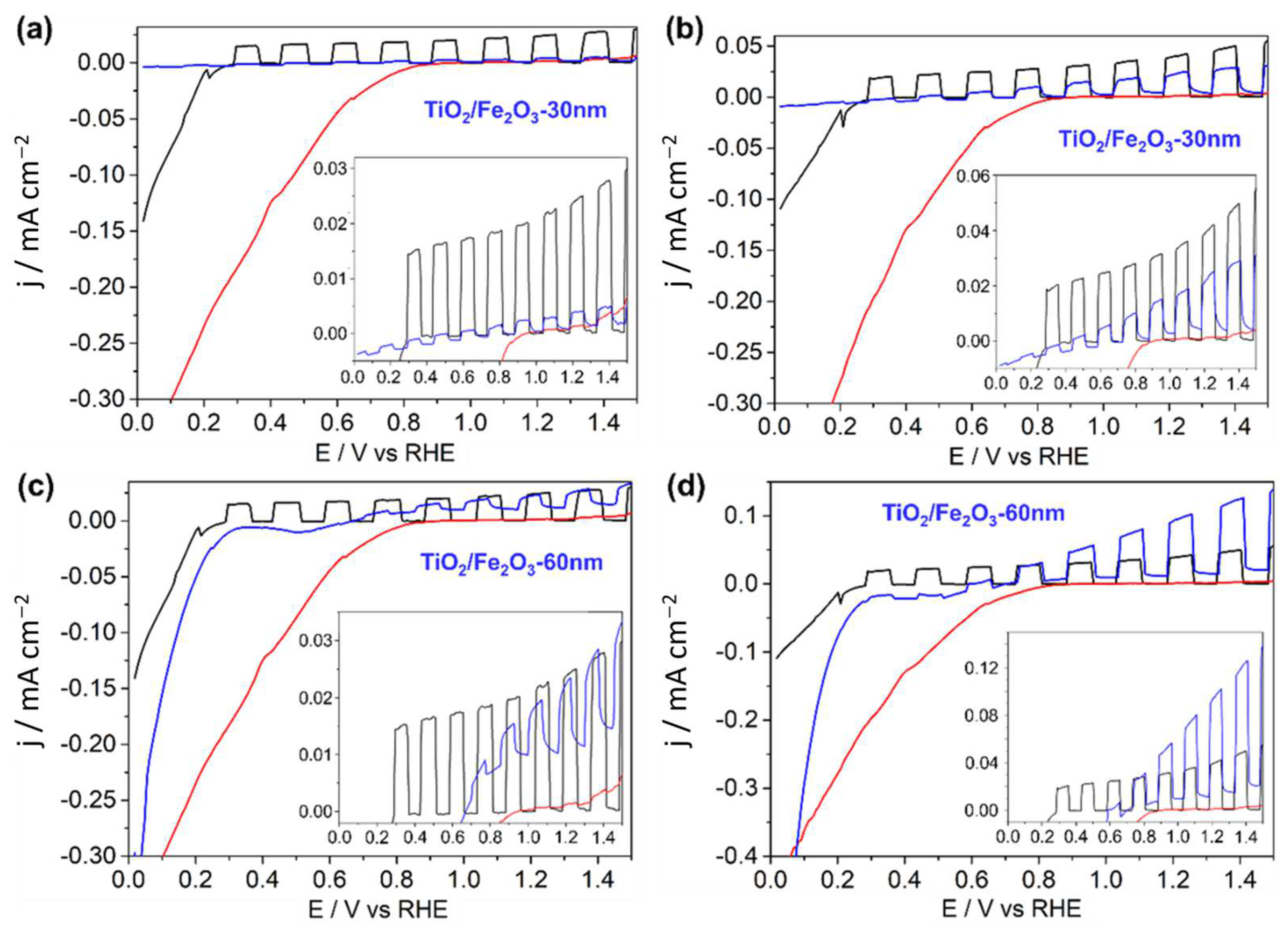
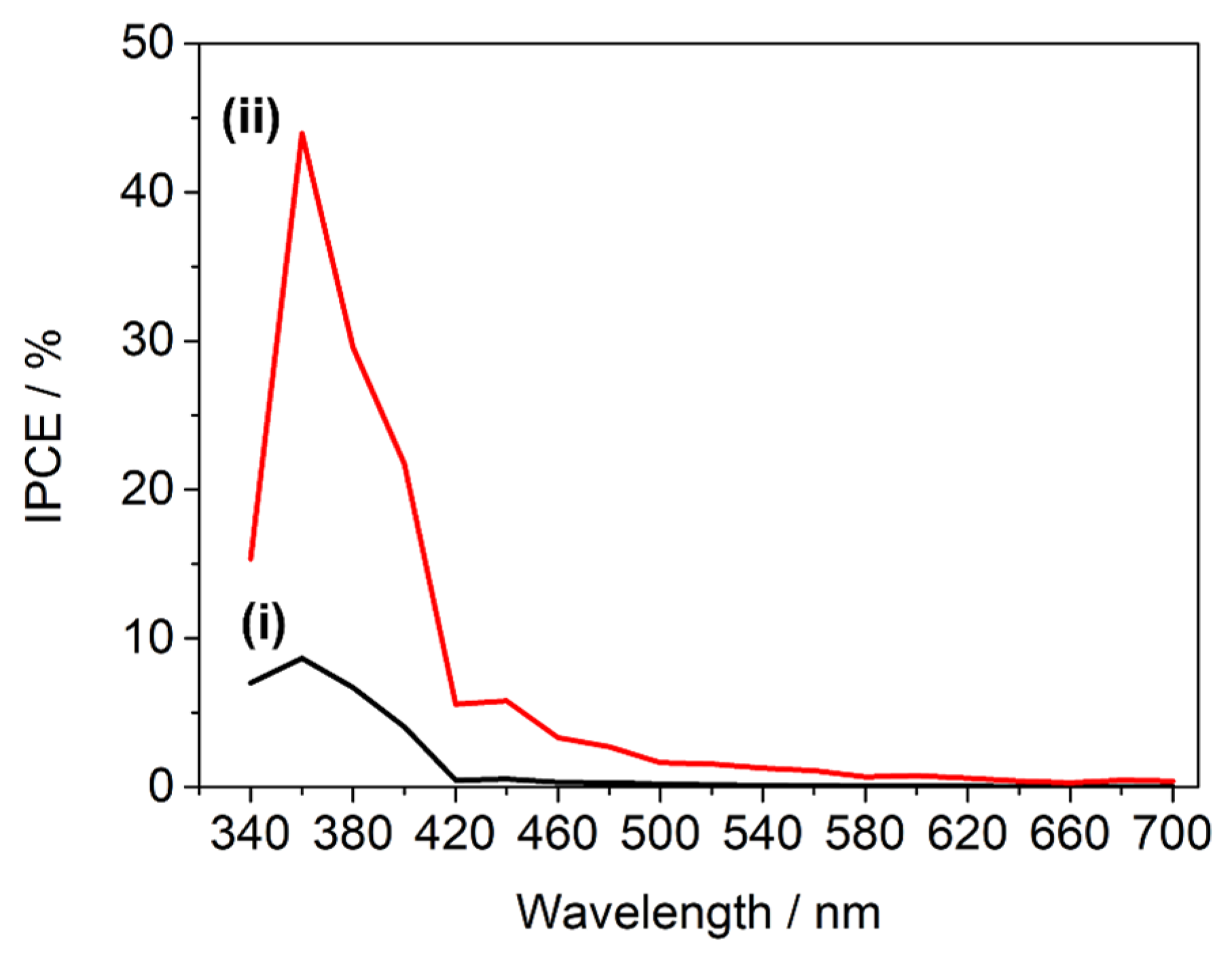
2.3. Thin Film Photoelectrochemical Characterisation
3. Conclusions
4. Experimental
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2009, 110, 111–131. [Google Scholar] [CrossRef]
- Malygin, A.A.; Drozd, V.E.; Malkov, A.A.; Smirnov, V.M.; From, V.B. Aleskovskii’s “Framework” Hypothesis to the Method of Molecular Layering/Atomic Layer Deposition. Chem. Vap. Depos. 2015, 21, 216–240. [Google Scholar] [CrossRef]
- Mallick, B.C.; Hsieh, C.-T.; Yin, K.-M.; Gandomi, Y.A.; Huang, K.-T. Review—On Atomic Layer Deposition: Current Progress and Future Challenges. ECS J. Solid State Sci. Technol. 2019, 8, N55–N78. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- Innocent, J.W.F.; Napari, M.; Johnson, A.L.; Harris-Lee, T.R.; Regue, M.; Sajavaara, T.; MacManus-Driscoll, J.L.; Marken, F.; Alkhalil, F. Atomic scale surface modification of TiO2 3D nano-arrays: Plasma enhanced atomic layer deposition of NiO for photocatalysis. Mater. Adv. 2021, 2, 273–279. [Google Scholar] [CrossRef]
- O’Donnell, S.; Jose, F.; Shiel, K.; Snelgrove, M.; McFeely, C.; McGill, E.; O’Connor, R. Thermal and plasma enhanced atomic layer deposition of ultrathin TiO2 on silicon from amide and alkoxide precursors: Growth chemistry and photoelectrochemical performance. J. Phys. D Appl. Phys. 2022, 55, 085105. [Google Scholar] [CrossRef]
- Hu, H.; Dong, B.; Hu, H.; Chen, F.; Kong, M.; Zhang, Q.; Luo, T.; Zhao, L.; Guo, Z.; Li, J.; et al. Atomic Layer Deposition of TiO2 for a High-Efficiency Hole-Blocking Layer in Hole-Conductor-Free Perovskite Solar Cells Processed in Ambient Air. ACS Appl. Mater. Interfaces 2016, 8, 17999–18007. [Google Scholar] [CrossRef] [PubMed]
- Ponraj, J.S.; Attolini, G.; Bosi, M. Review on Atomic Layer Deposition and Applications of Oxide Thin Films. Crit. Rev. Solid State Mater. Sci. 2013, 38, 203–233. [Google Scholar] [CrossRef]
- Profijt, H.B.; Potts, S.E.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-Assisted Atomic Layer Deposition: Basics, Opportunities, and Challenges. J. Vac. Sci. Technol. A Vac. Surf. Film. 2011, 29, 050801. [Google Scholar] [CrossRef]
- Kim, H.; Oh, I.-K. Review of plasma-enhanced atomic layer deposition: Technical enabler of nanoscale device fabrication. Jpn. J. Appl. Phys. 2014, 53, 03DA01. [Google Scholar] [CrossRef]
- Takagi, T. Ion–surface interactions during thin film deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 1984, 2, 382–388. [Google Scholar] [CrossRef]
- Ren, H.; Nishi, Y.; Shohet, J.L. Changes to Charge and Defects in Dielectrics from Ion and Photon Fluences during Plasma Exposure. Electrochem. Solid-State Lett. 2011, 14, H107. [Google Scholar] [CrossRef][Green Version]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.-C. New development of atomic layer deposition: Processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef] [PubMed]
- Lie, M.; Fjellvåg, H.; Kjekshus, A. Growth of Fe2O3 thin films by atomic layer deposition. Thin Solid Film. 2005, 488, 74–81. [Google Scholar] [CrossRef]
- Rooth, M.R.; Johansson, A.; Kukli, K.; Aarik, J.; Boman, M.; Hårsta, A. Atomic Layer Deposition of Iron Oxide Thin Films and Nanotubes using Ferrocene and Oxygen as Precursors. Chem. Vap. Depos. 2008, 14, 67–70. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, Y.; Mayer, M.T.; Simpson, Z.I.; McMahon, G.; Zhou, S.; Wang, D. Growth of p-Type Hematite by Atomic Layer Deposition and Its Utilization for Improved Solar Water Splitting. J. Am. Chem. Soc. 2012, 134, 5508–5511. [Google Scholar] [CrossRef] [PubMed]
- Riha, S.C.; Klahr, B.M.; Tyo, E.C.; Seifert, S.; Vajda, S.; Pellin, M.J.; Hamann, T.W.; Martinson, A.B.F. Atomic Layer Deposition of a Submonolayer Catalyst for the Enhanced Photoelectrochemical Performance of Water Oxidation with Hematite. ACS Nano 2013, 7, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.D.; Schlepütz, C.M.; Guo, P.; Riha, S.C.; Chang, R.P.H.; Martinson, A.B.F. Atomic Layer Deposition of Epitaxial Iron Oxides for Photoelectrochemical Water Oxidation. ECS Meet. Abstr. 2015, MA2015-02, 1719. [Google Scholar] [CrossRef]
- Li, X.; Fan, N.C.; Fan, H.J. A Micro-pulse Process of Atomic Layer Deposition of Iron Oxide Using Ferrocene and Ozone Precursors and Ti-Doping. Chem. Vap. Depos. 2013, 19, 104–110. [Google Scholar] [CrossRef]
- Bachmann, J.; Jing; Knez, M.; Barth, S.; Shen, H.; Mathur, S.; Gösele, U.; Nielsch, K. Ordered Iron Oxide Nanotube Arrays of Controlled Geometry and Tunable Magnetism by Atomic Layer Deposition. J. Am. Chem. Soc. 2007, 129, 9554–9555. [Google Scholar] [CrossRef]
- Riha, S.C.; Racowski, J.M.; Lanci, M.P.; Klug, J.A.; Hock, A.S.; Martinson, A.B.F. Phase Discrimination through Oxidant Selection in Low-Temperature Atomic Layer Deposition of Crystalline Iron Oxides. Langmuir 2013, 29, 3439–3445. [Google Scholar] [CrossRef] [PubMed]
- Klug, J.A.; Becker, N.G.; Riha, S.C.; Martinson, A.B.F.; Elam, J.W.; Pellin, M.J.; Proslier, T. Low temperature atomic layer deposition of highly photoactive hematite using iron(iii) chloride and water. J. Mater. Chem. A 2013, 1, 11607–11613. [Google Scholar] [CrossRef]
- de Ridder, M.; van de Ven, P.C.; van Welzenis, R.G.; Brongersma, H.H.; Helfensteyn, S.; Creemers, C.; Van Der Voort, P.; Baltes, M.; Mathieu, M.; Vansant, E.F. Growth of Iron Oxide on Yttria-Stabilized Zirconia by Atomic Layer Deposition. J. Phys. Chem. B 2002, 106, 13146–13153. [Google Scholar] [CrossRef]
- Selvaraj, S.; Moon, H.; Yun, J.-Y.; Kim, D.-H. Iron oxide grown by low-temperature atomic layer deposition. Korean J. Chem. Eng. 2016, 33, 3516–3522. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Hamann, T.; Bisquert, J. Water Oxidation at Hematite Photoelectrodes: The Role of Surface States. J. Am. Chem. Soc. 2012, 134, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Klahr, B.M.; Martinson, A.B.F.; Hamann, T.W. Photoelectrochemical Investigation of Ultrathin Film Iron Oxide Solar Cells Prepared by Atomic Layer Deposition. Langmuir 2011, 27, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Martinson, A.B.F.; DeVries, M.J.; Libera, J.A.; Christensen, S.T.; Hupp, J.T.; Pellin, M.J.; Elam, J.W. Atomic Layer Deposition of Fe2O3 Using Ferrocene and Ozone. J. Phys. Chem. C 2011, 115, 4333–4339. [Google Scholar] [CrossRef]
- Van Bui, H.; Grillo, F.; van Ommen, J.R. Atomic and molecular layer deposition: Off the beaten track. Chem. Commun. 2017, 53, 45–71. [Google Scholar] [CrossRef]
- Astruc, D. Why is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2016, 2017, 6–29. [Google Scholar] [CrossRef]
- Ramachandran, R.K.; Dendooven, J.; Detavernier, C. Plasma enhanced atomic layer deposition of Fe2O3 thin films. J. Mater. Chem. A 2014, 2, 10662–10667. [Google Scholar] [CrossRef]
- Choi, B.; Park, G.-W.; Jeong, J.-R.; Jeon, N. Comparative Study of Thermal and Plasma-Enhanced Atomic Layer Deposition of Iron Oxide Using Bis(N,N′-di-butylacetamidinato)iron(II). Nanomaterials 2023, 13, 1858. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fan, G.; Fu, H.; Li, Z.; Zou, Z. Tandem photoelectrochemical cells for solar water splitting. Adv. Phys. X 2018, 3, 1487267. [Google Scholar] [CrossRef]
- Wang, L.; Liang, K.; Wang, G.; Yang, Y. Interface-engineered hematite nanocones as binder-free electrodes for high-performance lithium-ion batteries. J. Mater. Chem. A 2018, 6, 13968–13974. [Google Scholar] [CrossRef]
- Yang, Y. A mini-review: Emerging all-solid-state energy storage electrode materials for flexible devices. Nanoscale 2020, 12, 3560–3573. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cao, J.; Gao, J.; Zhao, J.; Jiang, W.; Ahmad, W.; Jiang, J.; Ling, M.; Liang, C.; Chen, J. Unveiling the structure-activity relationship of hollow spindle-like α-Fe2O3 nanoparticles via phosphorus doping engineering for enhanced lithium storage. Sustain. Mater. Technol. 2023, 38, e00744. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, X.; Lu, L.; Wu, X.; Wang, F. Recent Developments of Nanomaterials and Nanostructures for High-Rate Lithium Ion Batteries. ChemSusChem 2020, 13, 5361–5407. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, Y.; Yu, M.; Zhai, T.; Liang, C.; Xie, S.; Balogun, M.S.; Tong, Y. Oxygen-Deficient Hematite Nanorods as High-Performance and Novel Negative Electrodes for Flexible Asymmetric Supercapacitors. Adv. Mater. 2020, 32, 3148–3155. [Google Scholar] [CrossRef]
- Lai, F.; Feng, J.; Heil, T.; Wang, G.-C.; Adler, P.; Antonietti, M.; Oschatz, M. Strong metal oxide-support interactions in carbon/hematite nanohybrids activate novel energy storage modes for ionic liquid-based supercapacitors. Energy Storage Mater. 2019, 20, 188–195. [Google Scholar] [CrossRef]
- Yadav, A.A.; Deshmukh, T.B.; Deshmukh, R.V.; Patil, D.D.; Chavan, U.J. Electrochemical supercapacitive performance of Hematite α-Fe2O3 thin films prepared by spray pyrolysis from non-aqueous medium. Thin Solid Film. 2016, 616, 351–358. [Google Scholar] [CrossRef]
- Hjiri, M.; Algessair, S.; Dhahri, R.; Mirzaei, A.; Neri, G. Gas sensing properties of hematite nanoparticles synthesized via different techniques. RSC Adv. 2024, 14, 17526–17534. [Google Scholar] [CrossRef]
- Garcia, D.; Picasso, G.; Hidalgo, P.; Peres, H.E.M.; Sun Kou, R.; Gonçalves, J.M. Sensors based on Ag-loaded hematite (α-Fe2O3) nanoparticles for methyl mercaptan detection at room temperature. Anal. Chem. Res. 2017, 12, 74–81. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, Y.; Guo, Q.; Das, A.; Sobrido, A.B.J.; Hing, K.A.; Zayats, A.V.; Krause, S. Photoelectrochemical Detection of Calcium Ions Based on Hematite Nanorod Sensors. ACS Appl. Nano Mater. 2022, 5, 17087–17094. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jang, H.W. α-Fe2O3 nanostructure-based gas sensors. J. Sens. Sci. Technol. 2021, 30, 210–217. [Google Scholar] [CrossRef]
- Tulliani, J.-M.; Baroni, C.; Zavattaro, L.; Grignani, C. Strontium-Doped Hematite as a Possible Humidity Sensing Material for Soil Water Content Determination. Sensors 2013, 13, 12070–12092. [Google Scholar] [CrossRef] [PubMed]
- Hjiri, M. Highly sensitive NO2 gas sensor based on hematite nanoparticles synthesized by sol–gel technique. J. Mater. Sci. Mater. Electron. 2020, 31, 5025–5031. [Google Scholar] [CrossRef]
- Shen, S.; Lindley, S.A.; Chen, X.; Zhang, J.Z. Hematite heterostructures for photoelectrochemical water splitting: Rational materials design and charge carrier dynamics. Energy Environ. Sci. 2016, 9, 2744–2775. [Google Scholar] [CrossRef]
- Park, J.; Kang, J.; Chaule, S.; Jang, J.-H. Recent progress and perspectives on heteroatom doping of hematite photoanodes for photoelectrochemical water splitting. J. Mater. Chem. A 2023, 11, 24551–24565. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Cheng, Y.; Zhang, X.; Du, H.; Zhu, C.; Jiang, D.; Yuan, Y. A highly efficient hematite photoelectrochemical fuel cell for solar-driven hydrogen production. Int. J. Hydrog. Energy 2023, 48, 32699–32707. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Bisquert, J.; Hamann, T.W. Electrochemical and photoelectrochemical investigation of water oxidation with hematite electrodes. Energy Environ. Sci. 2012, 5, 7626–7636. [Google Scholar] [CrossRef]
- Wang, D.; Chang, G.; Zhang, Y.; Chao, J.; Yang, J.; Su, S.; Wang, L.; Fan, C.; Wang, L. Hierarchical three-dimensional branched hematite nanorod arrays with enhanced mid-visible light absorption for high-efficiency photoelectrochemical water splitting. Nanoscale 2016, 8, 12697–12701. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Huang, H.; Wang, J.; Li, Q.; Chen, L.Q.; Wang, Q. Toward Wearable Cooling Devices: Highly Flexible Electrocaloric Ba0.67Sr0.33TiO3 Nanowire Arrays. Adv. Mater. 2016, 28, 4811–4816. [Google Scholar] [CrossRef] [PubMed]
- Steier, L.; Luo, J.; Schreier, M.; Mayer, M.T.; Sajavaara, T.; Grätzel, M. Low-Temperature Atomic Layer Deposition of Crystalline and Photoactive Ultrathin Hematite Films for Solar Water Splitting. ACS Nano 2015, 9, 11775–11783. [Google Scholar] [CrossRef]
- Dobbelaere, T.; Mattelaer, F.; Roy, A.K.; Vereecken, P.; Detavernier, C. Plasma-enhanced atomic layer deposition of titanium phosphate as an electrode for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 330–338. [Google Scholar] [CrossRef]
- Henderick, L.; Blomme, R.; Minjauw, M.; Keukelier, J.; Meersschaut, J.; Dendooven, J.; Vereecken, P.; Detavernier, C. Plasma-enhanced atomic layer deposition of nickel and cobalt phosphate for lithium ion batteries. Dalton Trans. 2022, 51, 2059–2067. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Lei, B.-X.; Rao, H.-S.; Xu, Y.-F.; Wang, Y.-F.; Su, C.-Y.; Kuang, D.-B. Hydrothermal Fabrication of Hierarchically Anatase TiO2 Nanowire arrays on FTO Glass for Dye-sensitized Solar Cells. Sci. Rep. 2013, 3, 1352. [Google Scholar] [CrossRef] [PubMed]
- Harris-Lee, T.R.; Zhang, Y.; Bowen, C.R.; Fletcher, P.J.; Zhao, Y.; Guo, Z.; Innocent, J.W.F.; Johnson, S.A.L.; Marken, F. Photo-Chlorine Production with Hydrothermally Grown and Vacuum-Annealed Nanocrystalline Rutile. Electrocatalysis 2021, 12, 65–77. [Google Scholar] [CrossRef]
- Harris-Lee, T.R.; Johnson, S.A.L.; Wang, L.; Fletcher, P.J.; Zhang, J.; Bentley, C.; Bowen, C.R.; Marken, F. TiO2 nanocrystal rods on titanium microwires: Growth, vacuum annealing, and photoelectrochemical oxygen evolution. New J. Chem. 2022, 46, 8385–8392. [Google Scholar] [CrossRef]
- Lianos, P. Review of recent trends in photoelectrocatalytic conversion of solar energy to electricity and hydrogen. Appl. Catal. B Environ. 2017, 210, 235–254. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Wu, W.; Tian, Q.; Cui, S.; Dai, Z.; Ren, F.; Xiao, X.; Jiang, C. 3D Flowerlike α-Fe2O3@TiO2 Core–Shell Nanostructures: General Synthesis and Enhanced Photocatalytic Performance. ACS Sustain. Chem. Eng. 2015, 3, 2975–2984. [Google Scholar] [CrossRef]
- Peng, L.; Xie, T.; Lu, Y.; Fan, H.; Wang, D. Synthesis, photoelectric properties and photocatalytic activity of the Fe2O3/TiO2 heterogeneous photocatalysts. Phys. Chem. Chem. Phys. 2010, 12, 8033–8041. [Google Scholar] [CrossRef] [PubMed]
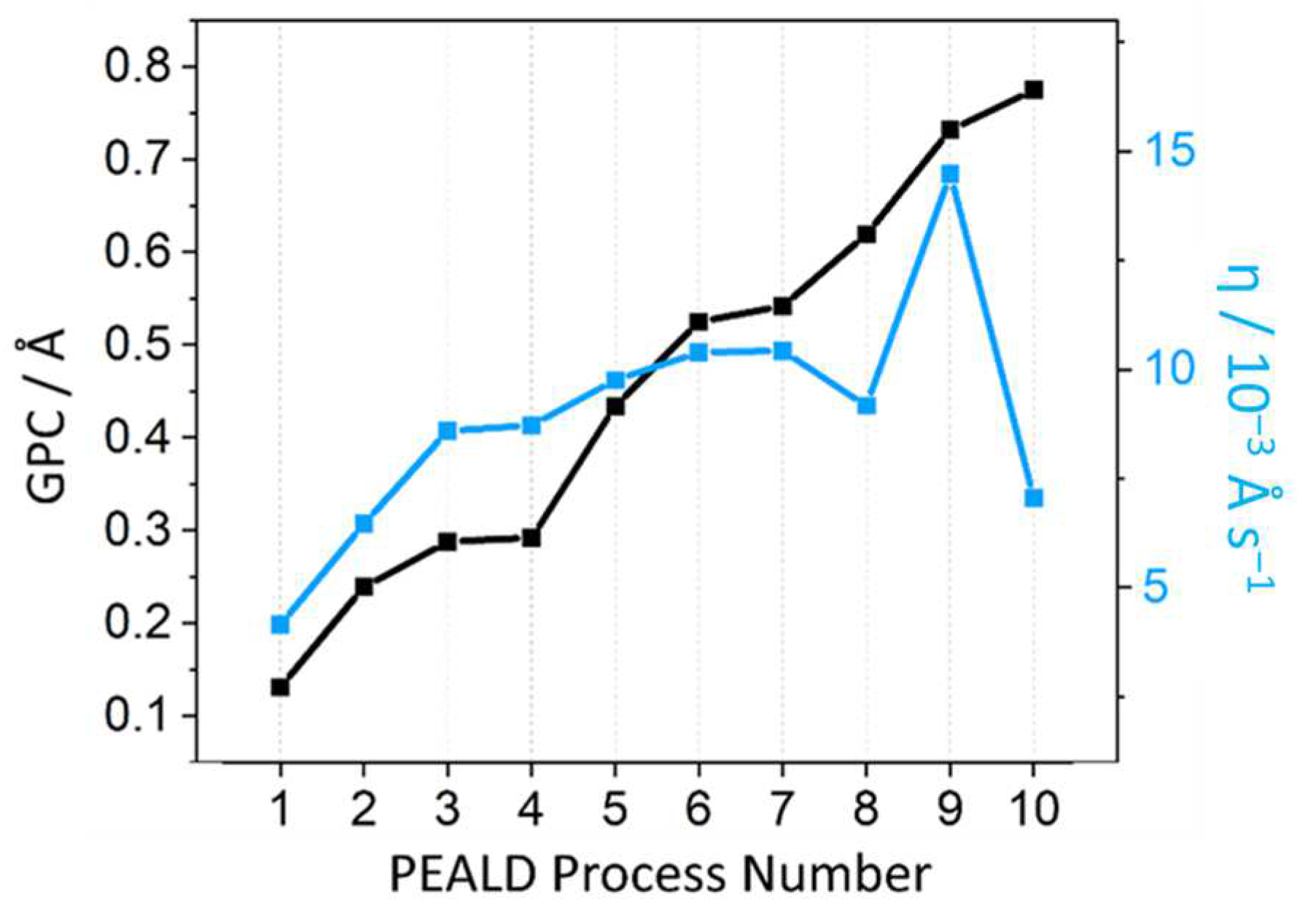



| Process Number | Sequence/s (Boost/Pulse) × n | Temp/°C (Pot/Chamber) | GPC/Å cycle−1 | η/10−3 Å s−1 |
|---|---|---|---|---|
| 1 | (0.5/1.0) × 1 | 60/240 | 0.13 | 4.16 |
| 2 | (2.0/5.0) × 1 | 60/240 | 0.24 | 6.46 |
| 3 | (0.5/3.0) × 1 | 90/240 | 0.29 | 8.60 |
| 4 | (0.5/3.0) × 1 | 90/300 | 0.29 | 8.72 |
| 5 | (0.5/1.0) × 3 | 90/240 | 0.43 | 9.75 |
| 6 | (0.5/3.0) × 3 | 90/240 | 0.53 | 10.40 |
| 7 | (1.0/3.0) × 3 | 90/240 | 0.54 | 10.42 |
| 8 | (0.5/3.0) × 5 | 90/240 | 0.62 | 9.17 |
| 9 | (0.5/3.0) × 3 | 120/240 | 0.73 | 14.49 |
| 10 | (0.5/3.0) × 10 | 120/240 | 0.78 | 7.05 |
| jphoto/µA cm−2 | ||||
|---|---|---|---|---|
| Illumination Side | TiO2 | 30 nm α-Fe2O3 | TiO2/Fe2O3-30 nm | TiO2/Fe2O3-60 nm |
| Front | 24 | 0.46 | 2.8 | 13 |
| Rear | 40 | 0.43 | 20 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris-Lee, T.R.; Brookes, A.; Zhang, J.; Bentley, C.L.; Marken, F.; Johnson, A.L. Plasma-Enhanced Atomic Layer Deposition of Hematite for Photoelectrochemical Water Splitting Applications. Crystals 2024, 14, 723. https://doi.org/10.3390/cryst14080723
Harris-Lee TR, Brookes A, Zhang J, Bentley CL, Marken F, Johnson AL. Plasma-Enhanced Atomic Layer Deposition of Hematite for Photoelectrochemical Water Splitting Applications. Crystals. 2024; 14(8):723. https://doi.org/10.3390/cryst14080723
Chicago/Turabian StyleHarris-Lee, Thom R., Andrew Brookes, Jie Zhang, Cameron L. Bentley, Frank Marken, and Andrew L. Johnson. 2024. "Plasma-Enhanced Atomic Layer Deposition of Hematite for Photoelectrochemical Water Splitting Applications" Crystals 14, no. 8: 723. https://doi.org/10.3390/cryst14080723
APA StyleHarris-Lee, T. R., Brookes, A., Zhang, J., Bentley, C. L., Marken, F., & Johnson, A. L. (2024). Plasma-Enhanced Atomic Layer Deposition of Hematite for Photoelectrochemical Water Splitting Applications. Crystals, 14(8), 723. https://doi.org/10.3390/cryst14080723








