Effect of Ceria Doping on the Mechanical Properties and Phase Stability of Partially Samaria-Stabilized Zirconia Crystals
Abstract
:1. Introduction
2. Materials and Methods
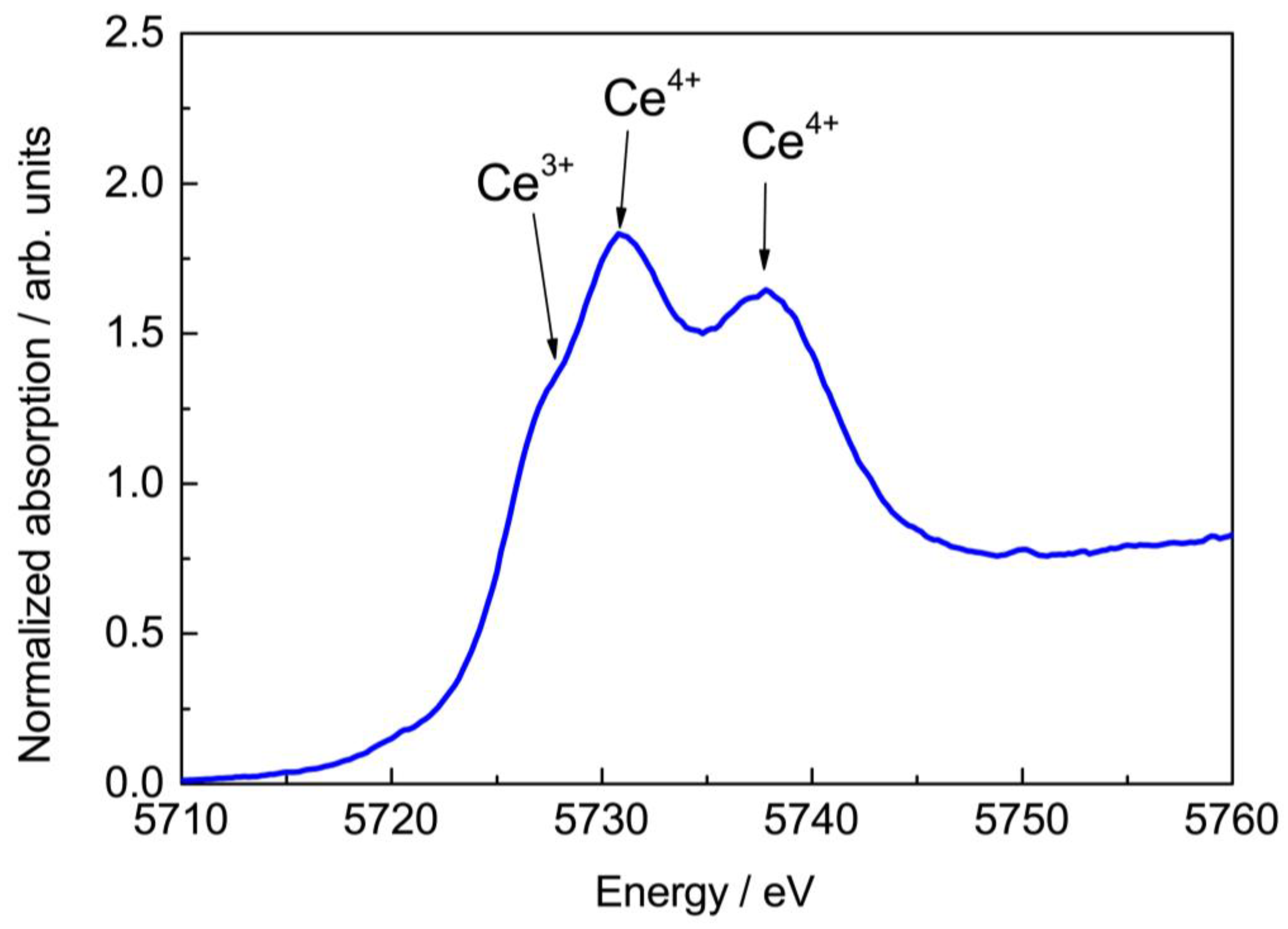
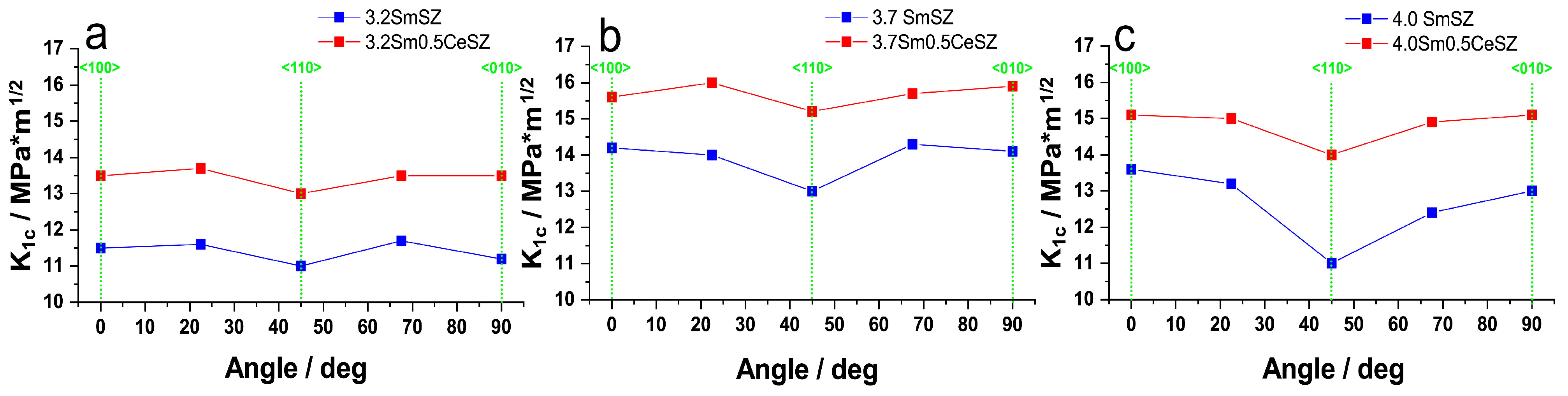
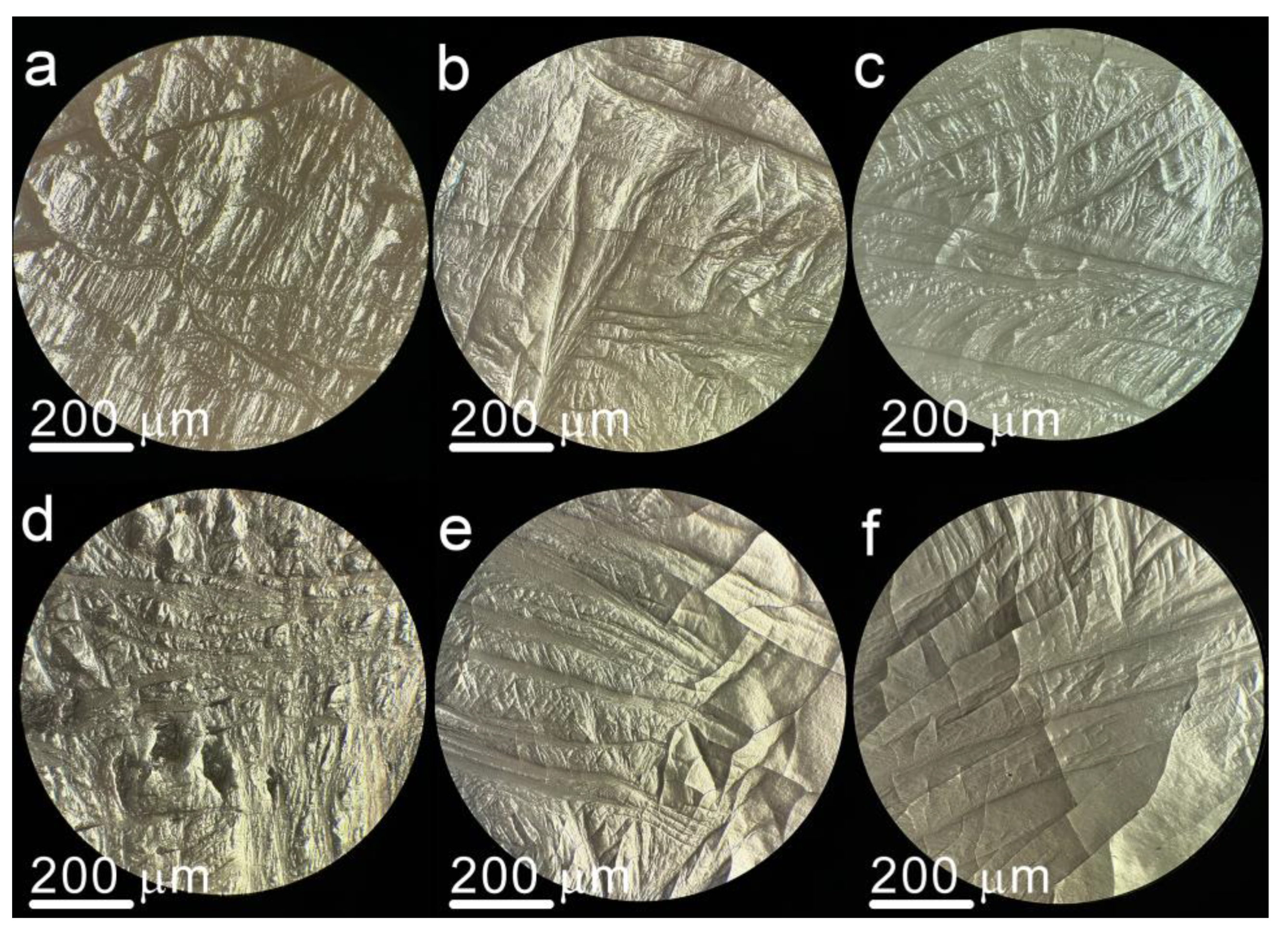
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Hannink, R.H.J.; Kelly, P.M.; Muddle, B.C. Transformation toughening in zirconia-containing ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Qi, B.; Liang, S.; Li, Y.; Zhou, C.; Yu, H.; Li, J. ZrO2 Matrix Toughened Ceramic Material-Strength and Toughness. Adv. Eng. Mater. 2022, 24, 2101278. [Google Scholar] [CrossRef]
- Hisbergues, M.; Vendeville, S.; Vendeville, P. Review zirconia. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88B, 519–529. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, C.; Xu, X.; Li, H. Machine learning potential for Ab Initio phase transitions of zirconia. Theor. Appl. Mech. Lett. 2023, 13, 100481. [Google Scholar] [CrossRef]
- Heuer, A.H. Transformation Toughening in ZrO2-Containing Ceramics. J. Am. Ceram. Soc. 1987, 70, 689–698. [Google Scholar] [CrossRef]
- Song, X.; Ding, Y.; Zhang, J.; Jiang, C.; Liu, Z.; Lin, C.; Zheng, W.; Zeng, Y. Thermophysical and mechanical properties of cubic, tetragonal and monoclinic ZrO2. J. Mater. Res. Technol. 2023, 23, 648–655. [Google Scholar] [CrossRef]
- Cheng, J.; Tian, C.; Yang, J.; He, J. Electrical and mechanical properties of Sm2O3 doped Y-TZP electrolyte ceramics. Ceram. Int. 2018, 44, 17033–17037. [Google Scholar] [CrossRef]
- Bejugama, S.; Chameettachal, S.; Pati, F.; Pandey, A.K. Tribology and in-vitro biological characterization of samaria doped ceria stabilized zirconia ceramics. Ceram. Int. 2021, 47, 17580–17588. [Google Scholar] [CrossRef]
- Rauchs, G.; Fett, T.; Munz, D.; Oberacker, R. Tetragonal-to-monoclinic phase transformation in CeO2-stabilised zirconia under uniaxial loading. J. Eur. Ceram. Soc. 2001, 21, 2229–2241. [Google Scholar] [CrossRef]
- Palmero, P.; Fornabaio, M.; Montanaro, L.; Reveron, H.; Esnouf, C.; Chevalier, J. Towards long lasting zirconia-based composites for dental implants: Part I: Innovative synthesis, microstructural characterization and invitro stability. Biomaterials 2015, 50, 38–46. [Google Scholar] [CrossRef]
- Savin, A.; Craus, M.L.; Turchenko, V.; Bruma, A.; Dubos, P.A.; Malo, S.; Konstantinova, T.E.; Burkhovetsky, V.V. Monitoring techniques of cerium stabilized zirconia for medical prosthesis. Appl. Sci. 2015, 5, 1665. [Google Scholar] [CrossRef]
- Reveron, H.; Fornabaio, M.; Palmero, P.; Fürderer, T.; Adolfsson, E.; Lughi, V.; Bonifacio, A.; Sergo, V.; Montanaro, L.; Chevalier, J. Towards long lasting zirconia-based composites for dental implants: Transformation induced plasticity and its consequence on ceramic reliability. Acta Biomater. 2017, 48, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zinkevich, M.; Aldinger, F. Phase diagrams and thermodynamics of rare-earth-doped zirconia ceramics. Pure Appl. Chem. 2007, 79, 1731–1753. [Google Scholar] [CrossRef]
- Yoshimura, M.; Yashima, M.; Noma, T.; Sōmiya, S. Formation of diffusionlessly transformed tetragonal phases by rapid quenching of melts in ZrO2-RO1.5 systems (R = rare earths). J. Mater. Sci. 1990, 25, 2011–2016. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.; Zhang, C.; Huang, X.; Ye, F. Dy2O3 stabilized ZrO2 as a toughening agent for Gd2Zr2O7 ceramic. Mater. Lett. 2017, 188, 142–144. [Google Scholar] [CrossRef]
- Chislov, A.S.; Borik, M.A.; Kulebyakin, A.V.; Lomonova, E.E.; Milovich, F.O.; Myzina, V.A.; Ryabochkina, P.A.; Sidorova, N.V.; Tabachkova, N.Y. Comparison of the structure and physicochemical properties of ZrO2 based crystals partially stabilized with. Y2O3, Gd2O3 and Sm2O3. Mod. Electron. Mater. 2024, 10, 3–10. [Google Scholar] [CrossRef]
- Zhu, W.; Nakashima, S.; Marin, E.; Gu, H.; Pezzotti, G. Annealing-induced off-stoichiometric and structural alterations in Ca2+-and Y3+-stabilized zirconia ceramics. Materials 2021, 14, 5555. [Google Scholar] [CrossRef]
- Schulz, U. Phase transformation in EB-PVD yttria partially stabilized zirconia thermal barrier coatings during annealing. J. Am. Ceram. Soc. 2000, 83, 904–910. [Google Scholar] [CrossRef]
- Cao, X.; Vassen, R.; Wang, J.; Zou, B.; Li, S.; Hui, Y.; Yuan, J.; Song, W.; Cao, Q.; Jiang, J.; et al. Degradation of zirconia in moisture. Corros. Sci. 2020, 176, 109038. [Google Scholar] [CrossRef]
- Zhu, W.; Nakashima, S.; Marin, E.; Gu, H.; Pezzotti, G. Microscopic mapping of dopant content and its link to the structural and thermal stability of yttria-stabilized zirconia polycrystals. J. Mater. Sci. 2020, 55, 524–534. [Google Scholar] [CrossRef]
- Turon-Vinas, M.; Roa, J.J.; Marro, F.G.; Anglada, M. Mechanical properties of 12Ce-ZrO2/3Y-ZrO2 composites. Ceram. Int. 2015, 41, 14988–14997. [Google Scholar] [CrossRef]
- Borik, M.; Kulebyakin, V.; Myzina, V.; Lomonova, E.E.; Milovich, F.O.; Ryabochkina, P.A.; Sidorova, N.V.; Shulga, N.Y.; Tabachkova, N.Y. Mechanical characteristics, structure, and phase stability of tetragonal crystals of ZrO2–Y2O3 solid solutions doped with cerium and neodymium oxides. J. Phys. Chem. Solids 2021, 150, 109808. [Google Scholar] [CrossRef]
- Borik, M.; Chislov, A.; Kulebyakin, A.; Lomonova, E.E.; Milovich, F.O.; Myzina, V.; Ryabochkina, P.A.; Sidorova, N.V.; Tabachkova, N.Y. Phase Composition and Mechanical Properties of Sm2O3 Partially Stabilized Zirconia Crystals. Crystals 2022, 12, 1630. [Google Scholar] [CrossRef]
- Niihara, K. A fracture mechanics analysis of indentation-induced Palmqvist crack in ceramics. J. Mater. Sci. Lett. 1983, 2, 221–223. [Google Scholar] [CrossRef]
- Niihara, K.; Morena, R.; Hasselman, D.P.H. Evaluation of KIc of brittle solids by the indentation method with low crack-to-indent ratios. J. Mater. Sci. Lett. 1982, 1, 13–16. [Google Scholar] [CrossRef]
- Moradkhani, A.; Baharvandi, H. Effects of additive amount, testing method, fabrication process and sintering temperature on the mechanical properties of Al2O3/3Y-TZP composites. Eng. Fract. Mech. 2018, 191, 446–460. [Google Scholar] [CrossRef]
- Orera, V.M.; Merino, R.I.; Peña, F. Ce3+↔Ce4+ conversion in ceria-doped zirconia single crystals induced by oxido-reduction treatments. Solid State Ion. 1994, 72 Pt 2, 103002. [Google Scholar] [CrossRef]
- Kozlova, A.P.; Kasimova, V.M.; Buzanov, O.A.; Chernenko, K.; Klementiev, K.; Pankratov, V. Luminescence and vacuum ultraviolet excitation spectroscopy of cerium doped Gd3Ga3Al2O12 single crystalline scintillators under synchrotron radiation excitations. Results Phys. 2020, 16, 103002. [Google Scholar] [CrossRef]
- Kern, F. Ytterbia-neodymia-costabilized TZP-Breaking the limits of strength-toughness correlations for zirconia? J. Eur. Ceram. Soc. 2013, 33, 965–973. [Google Scholar] [CrossRef]
- Borik, M.A.; Chislov, A.S.; Kulebyakin, A.V.; Lomonova, E.E.; Milovich, F.O.; Myzina, V.A.; Ryabochkina, P.A.; Sidorova, N.V.; Tabachkova, N.Y. Effect of heat treatment on the structure and mechanical properties of partially gadolinia-stabilized zirconia crystals. J. Asian Ceram. Soc. 2021, 9, 559–569. [Google Scholar] [CrossRef]
- Evans, A.G.; Cannon, R.M. Overview no. 48. Toughening of brittle solids by martensitic transformations. Acta Metall. 1986, 34, 761–800. [Google Scholar] [CrossRef]
- Chien, F.R.; Ubic, F.J.; Prakash, V.; Heuer, A.H. Stress-induced martensitic transformation and ferroelastic deformation adjacent microhardness indents in tetragonal zirconia single crystals. Acta Mater. 1998, 46, 2151–2171. [Google Scholar] [CrossRef]
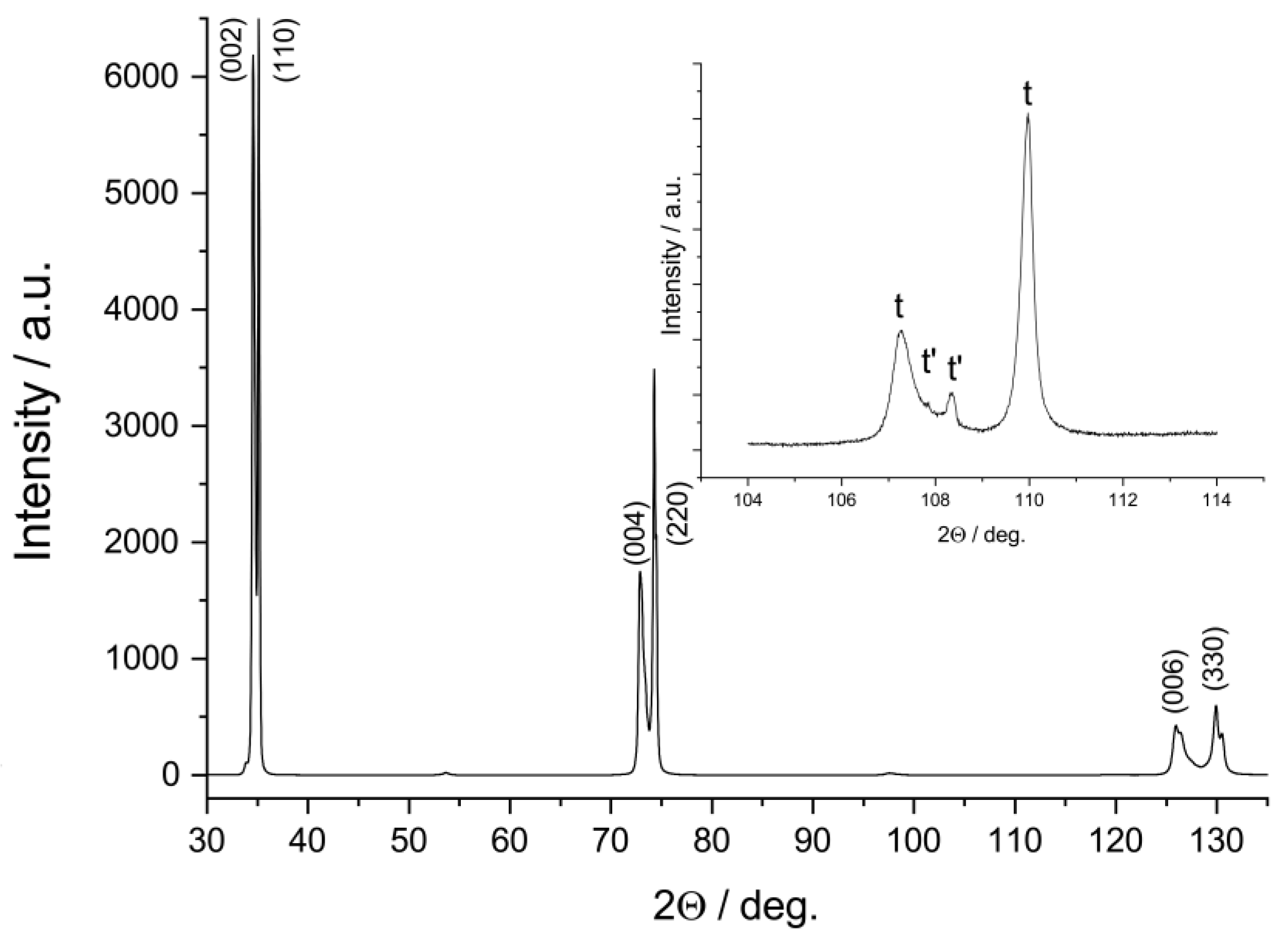



| Specimen | Phase | Content, wt.% | Lattice Parameters, Å | c/√2a |
|---|---|---|---|---|
| 3.7SmSZ [24] | t t’ | 85 ± 5 15 ± 5 | a = 3.6062(2) c = 5.1866(2) a = 3.6426(5) c = 5.1695(2) | 1.0170 1.0035 |
| 3.7Sm0.5CeSZ | t t’ | 90 ± 5 10 ± 5 | a = 3.6062(2) c = 5.1886(2) a = 3.6426(5) c = 5.1682(2) | 1.0174 1.0032 |
| 4.0SmSZ [24] | t t’ | 75 ± 5 25 ± 5 | a = 3.6063(2); c = 5.1854(2) a = 3.6429(5); c = 5.1692(2) | 1.0167 1.0034 |
| 4.0Sm0.5CeSZ | t t’ | 85 ± 5 15 ± 5 | a = 3.6063(1) c = 5.1877(2) a = 3.6427(5) c = 5.1685(2) | 1.0172 1.0033 |
| Composition | Microhardness, GPa | Composition | Microhardness, GPa |
|---|---|---|---|
| 3.2SmSZ | 10.75 ± 0.30 | 3.2Sm0.5CeSZ | 10.54 ± 0.25 |
| 3.7SmSZ | 11.30 ± 0.30 | 3.7Sm0.5CeSZ | 11.15 ± 0.25 |
| 4.0SmSZ | 12.15 ± 0.30 | 4.0Sm0.5CeSZ | 11.70 ± 0.25 |
| Specimen | HV, GPa | ||
|---|---|---|---|
| As-Grown | Vacuum-Annealed | Air-Annealed | |
| 3.2Sm0.5CeSZ | 10.54 ± 0.25 | 8.55 ± 0.30 | 8.50 ± 0.30 |
| 3.7Sm0.5CeSZ | 11.15 ± 0.25 | 8.65 ± 0.30 | 8.60 ± 0.30 |
| 4.0Sm0.5CeSZ | 11.70 ± 0.25 | 9.25 ± 0.30 | 8.70 ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borik, M.; Chislov, A.; Kulebyakin, A.; Lomonova, E.; Milovich, F.; Myzina, V.; Pankratov, V.; Poselennov, A.; Ryabochkina, P.; Sidorova, N.; et al. Effect of Ceria Doping on the Mechanical Properties and Phase Stability of Partially Samaria-Stabilized Zirconia Crystals. Crystals 2024, 14, 736. https://doi.org/10.3390/cryst14080736
Borik M, Chislov A, Kulebyakin A, Lomonova E, Milovich F, Myzina V, Pankratov V, Poselennov A, Ryabochkina P, Sidorova N, et al. Effect of Ceria Doping on the Mechanical Properties and Phase Stability of Partially Samaria-Stabilized Zirconia Crystals. Crystals. 2024; 14(8):736. https://doi.org/10.3390/cryst14080736
Chicago/Turabian StyleBorik, Mikhail, Artem Chislov, Alexej Kulebyakin, Elena Lomonova, Filipp Milovich, Valentina Myzina, Vladimir Pankratov, Alexandr Poselennov, Polina Ryabochkina, Natalia Sidorova, and et al. 2024. "Effect of Ceria Doping on the Mechanical Properties and Phase Stability of Partially Samaria-Stabilized Zirconia Crystals" Crystals 14, no. 8: 736. https://doi.org/10.3390/cryst14080736
APA StyleBorik, M., Chislov, A., Kulebyakin, A., Lomonova, E., Milovich, F., Myzina, V., Pankratov, V., Poselennov, A., Ryabochkina, P., Sidorova, N., Tabachkova, N., Zakharov, D., & Kiselev, D. (2024). Effect of Ceria Doping on the Mechanical Properties and Phase Stability of Partially Samaria-Stabilized Zirconia Crystals. Crystals, 14(8), 736. https://doi.org/10.3390/cryst14080736











