Surface Recrystallization Model of Fully Amorphized C3H5-Molecular-Ion-Implanted Silicon Substrate
Abstract
1. Introduction
2. Experimental Methods
2.1. Sample Preparation and Evaluation Procedure
2.2. TCAD Process Simulation Procedure
3. Results and Discussion
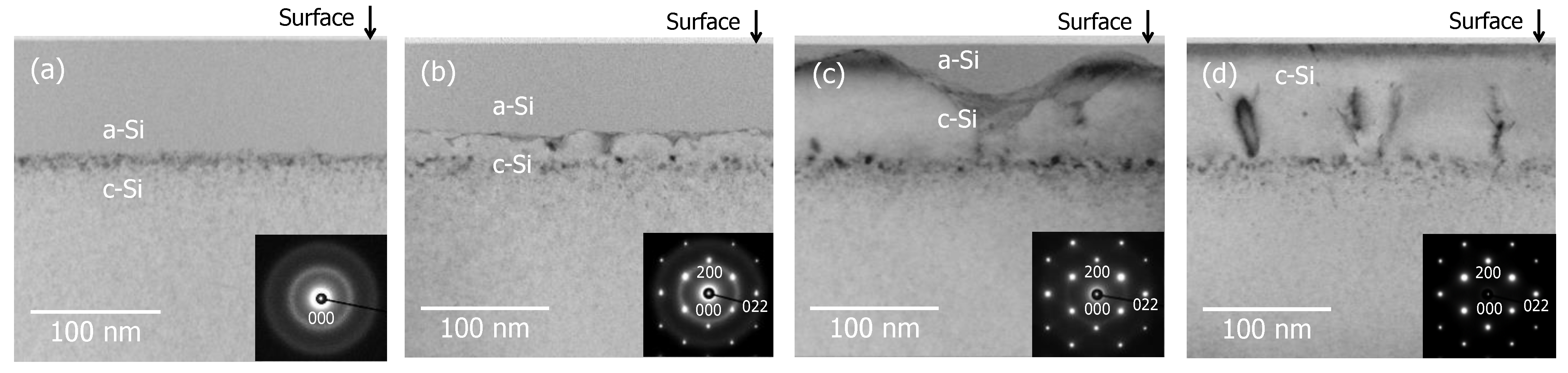
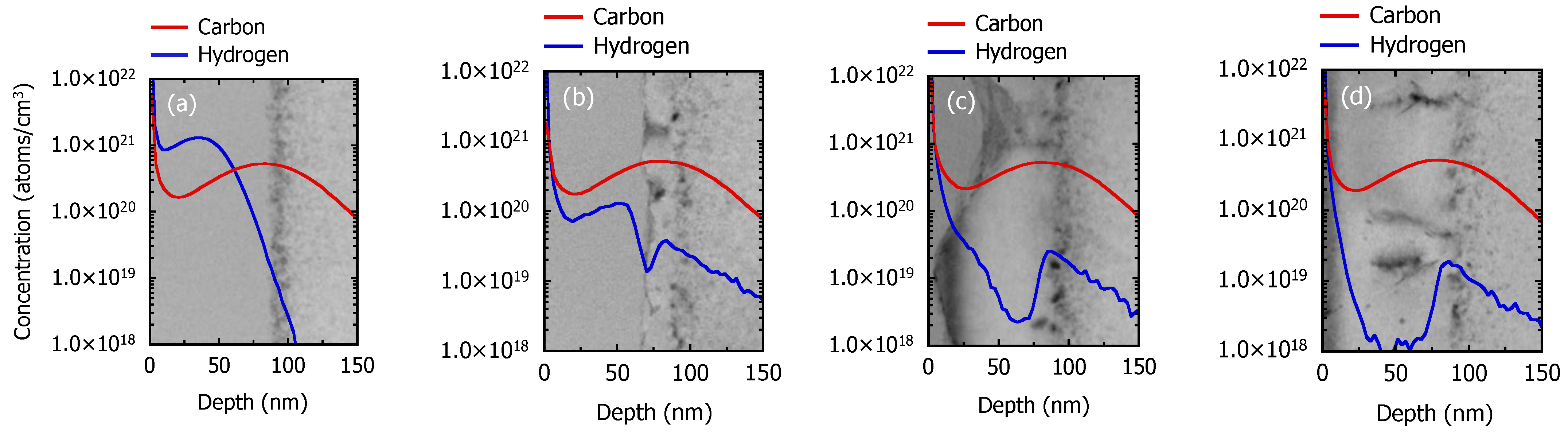
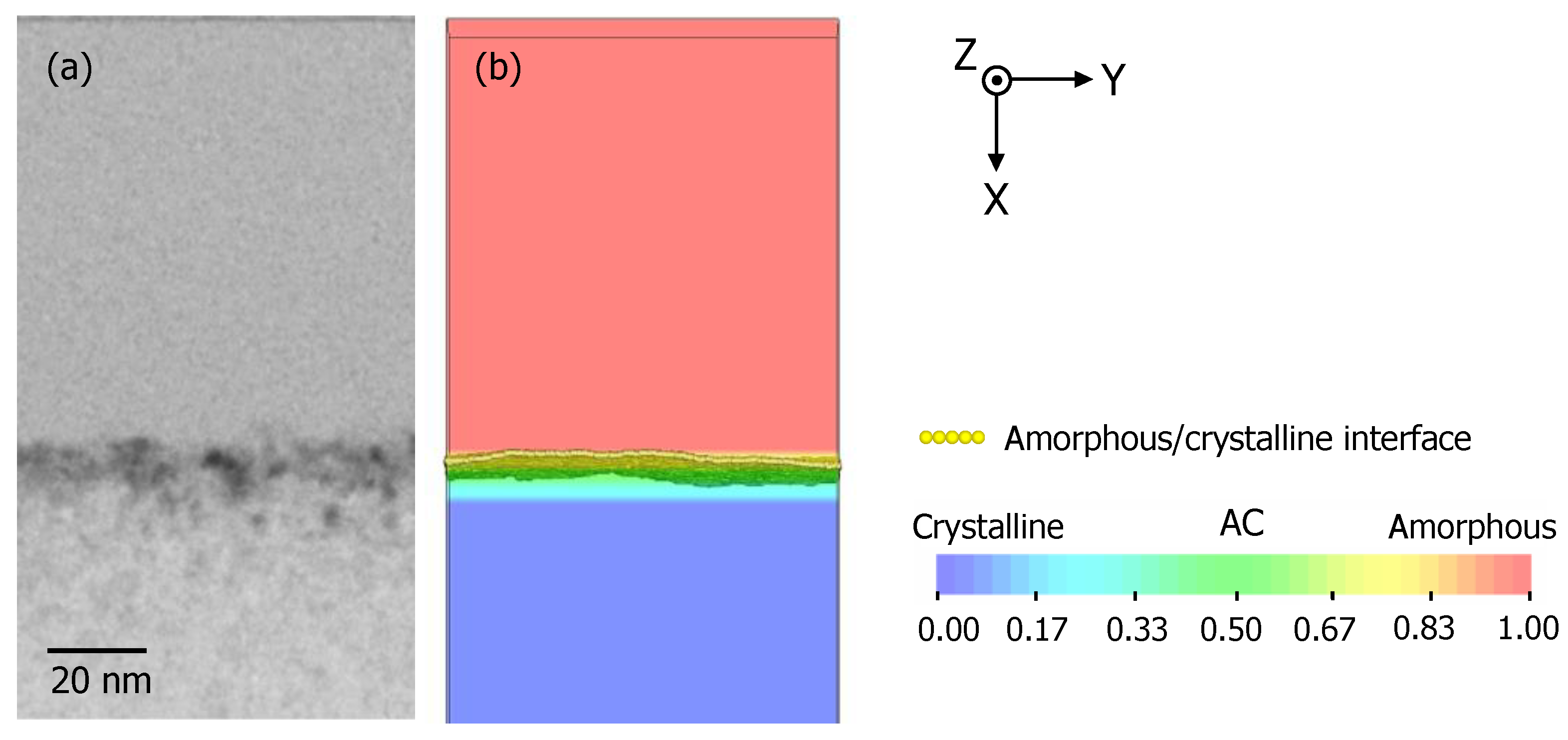
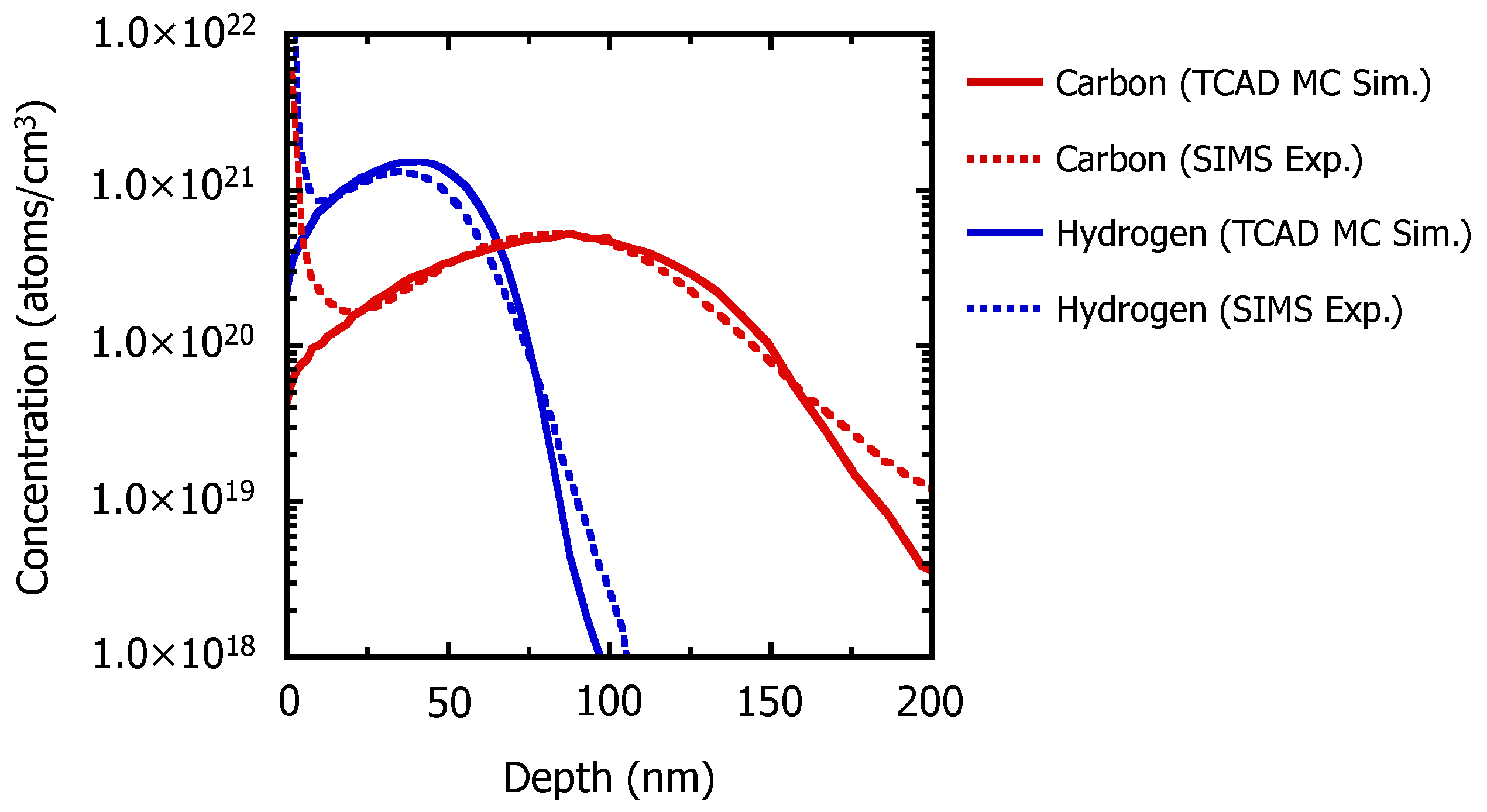
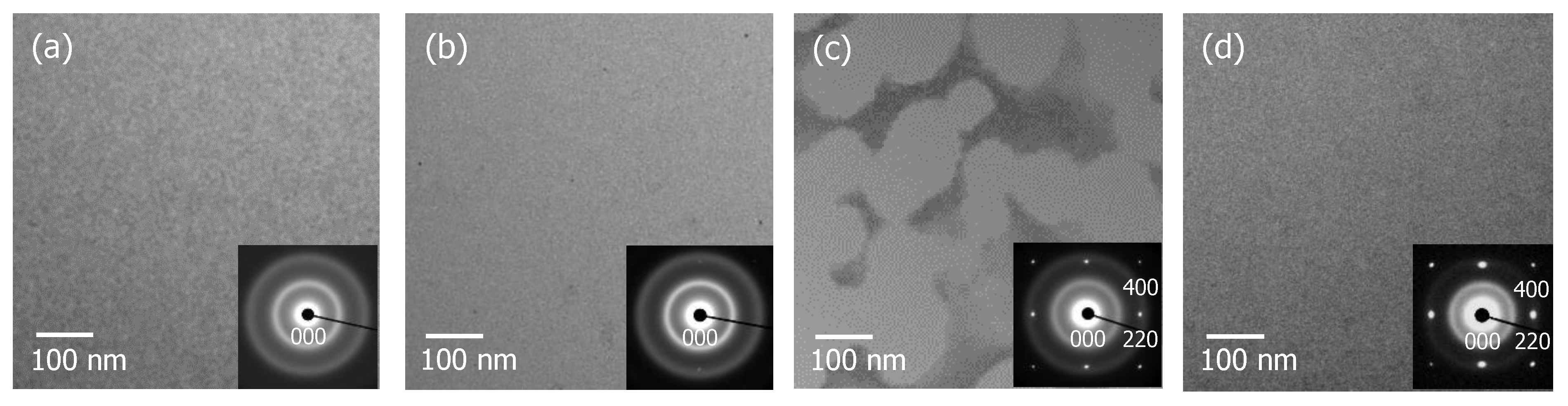
3.1. Recrystallization Behavior of Fully Amorphized C3H5-Molecular-Ion-Implanted Si Substrate under the Effect of Impurities after Thermal Annealing Treatment Analyzed by Cross-Sectional TEM and SIMS
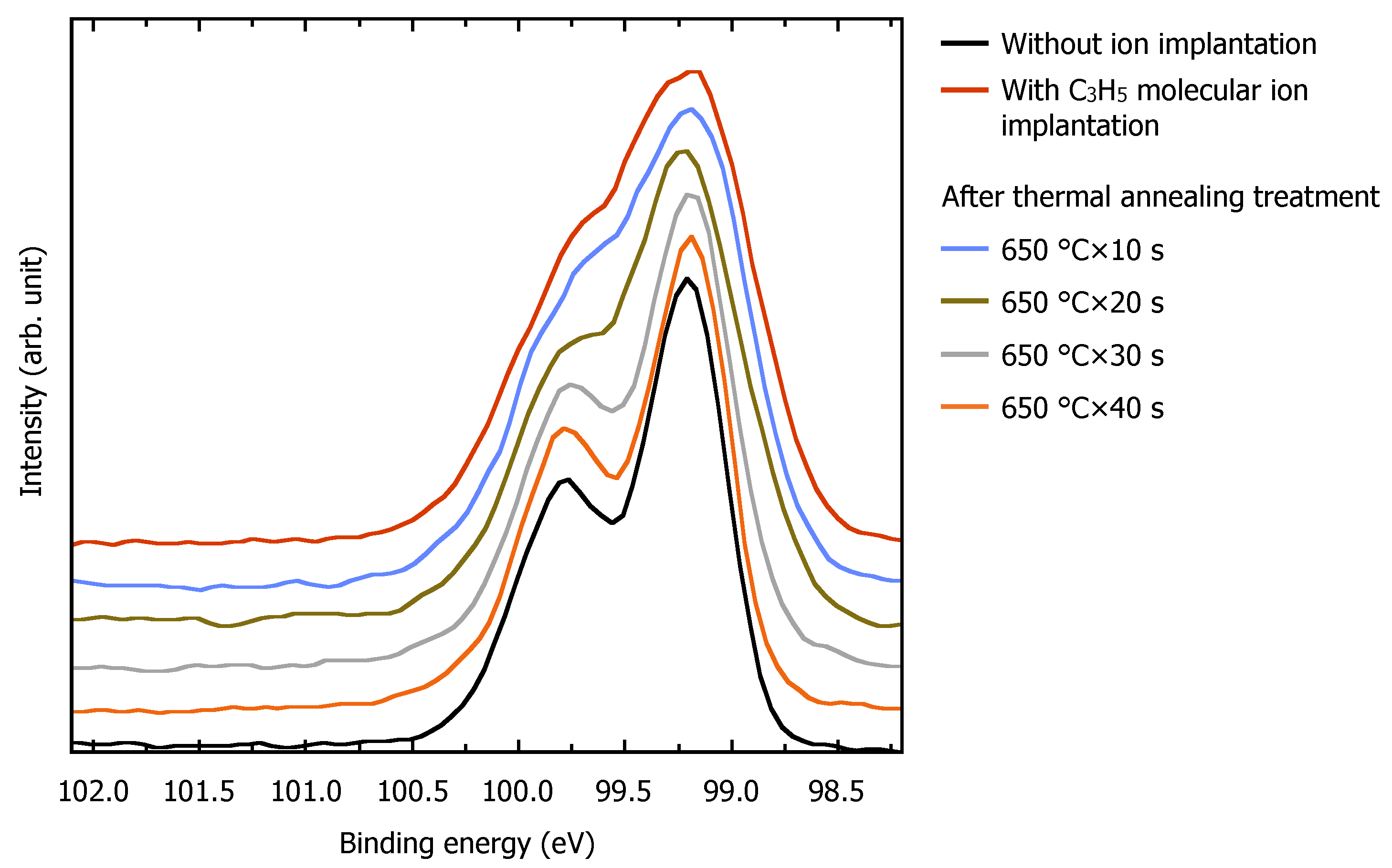
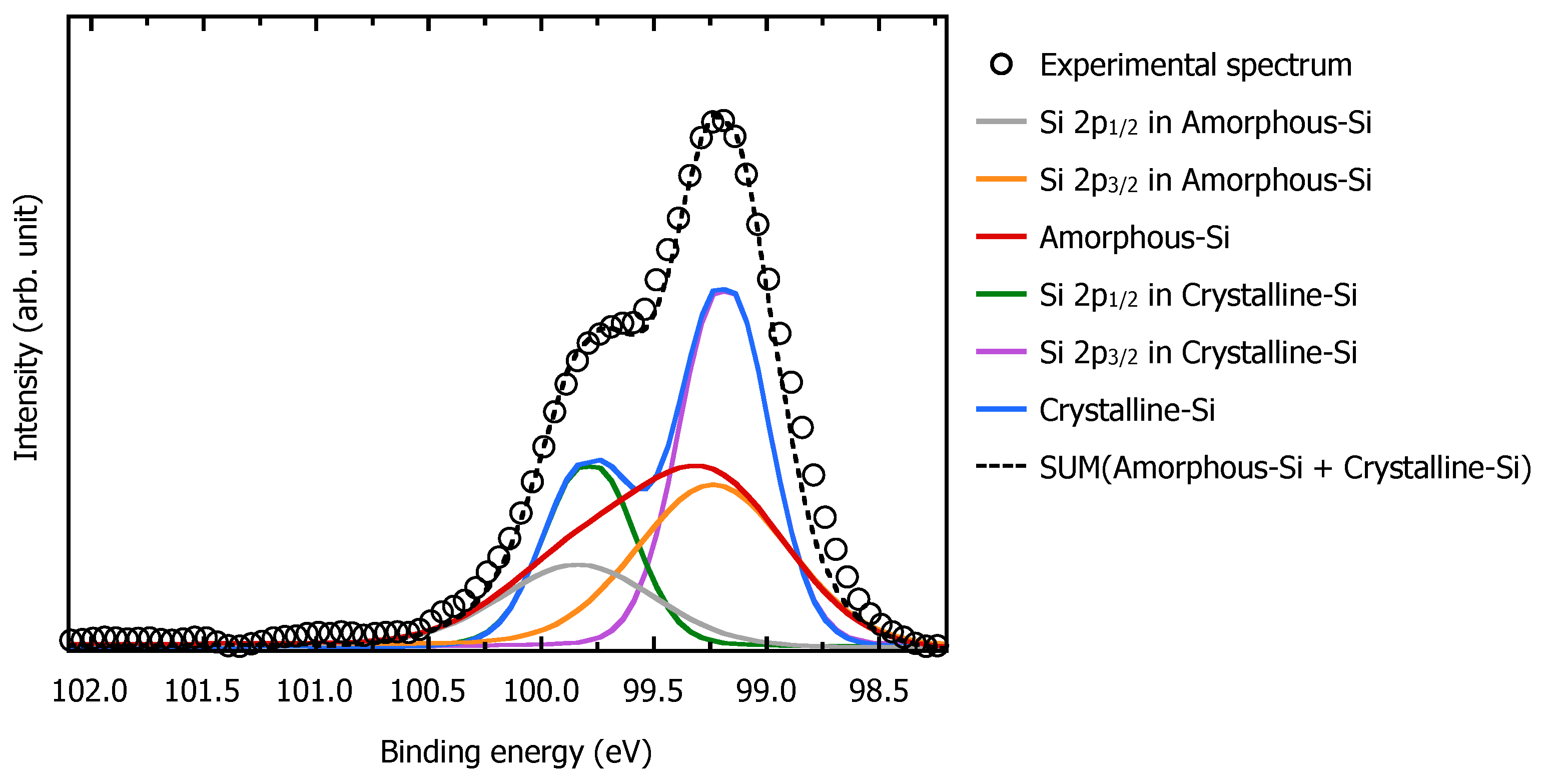
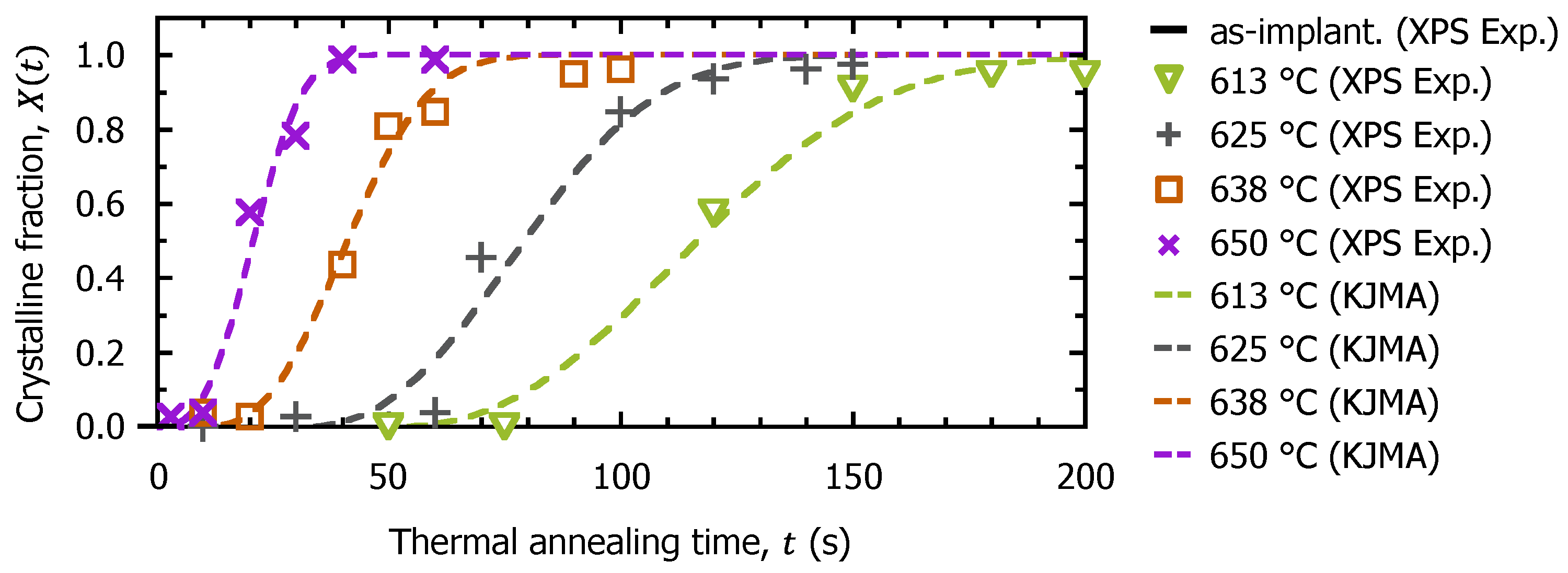
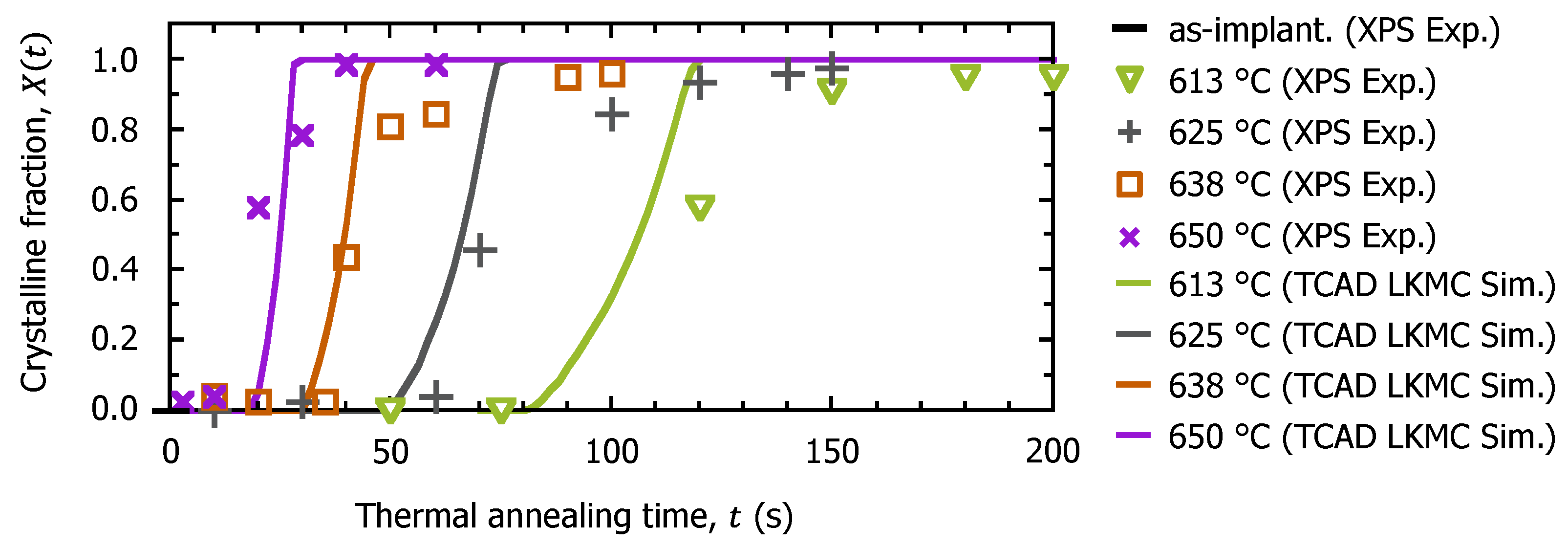
3.2. Surface Recrystallization Behavior of Fully Amorphized C3H5-Molecular-Ion-Implanted Si Substrate after Thermal Annealing Treatment by XPS Analysis
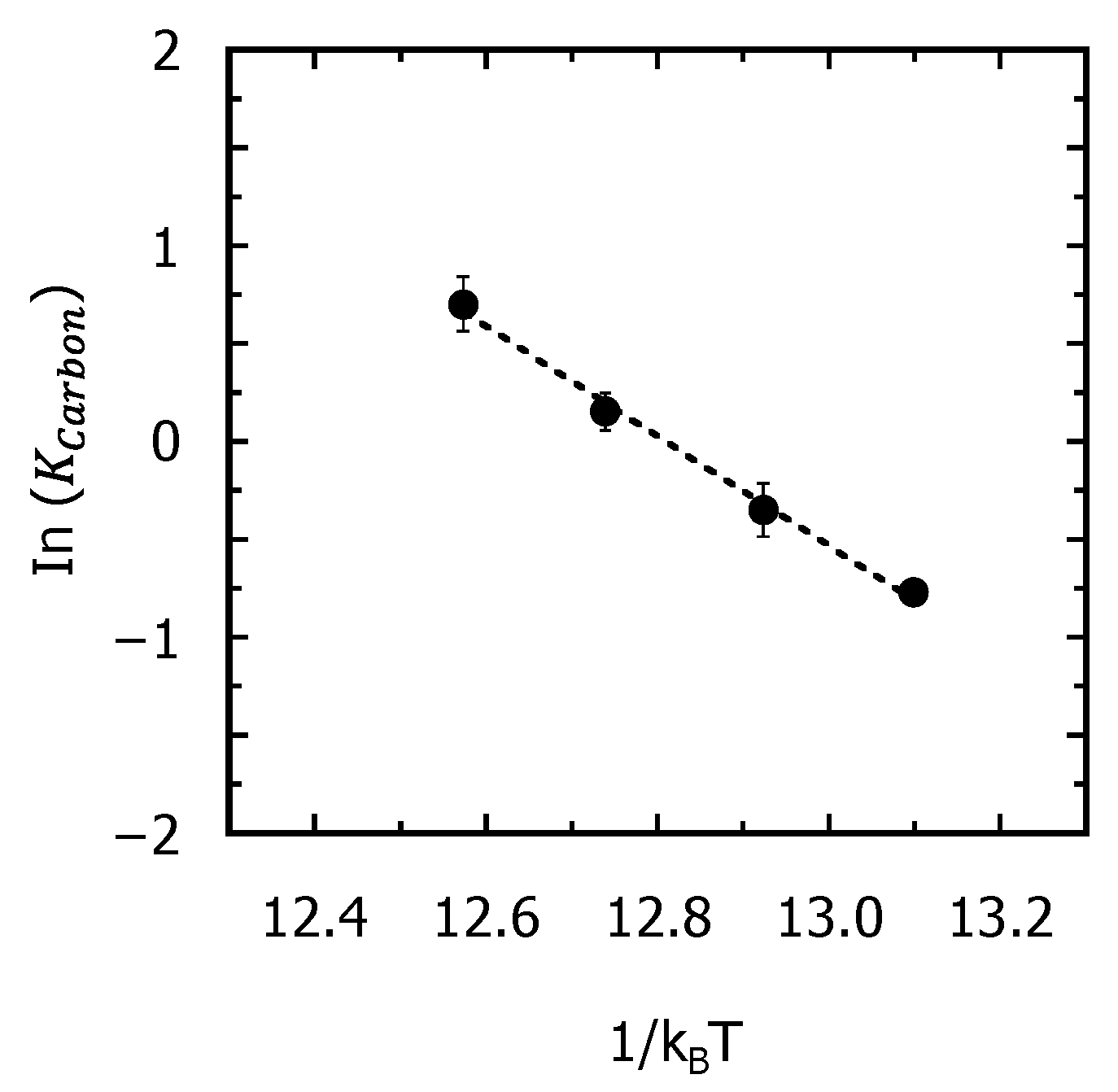
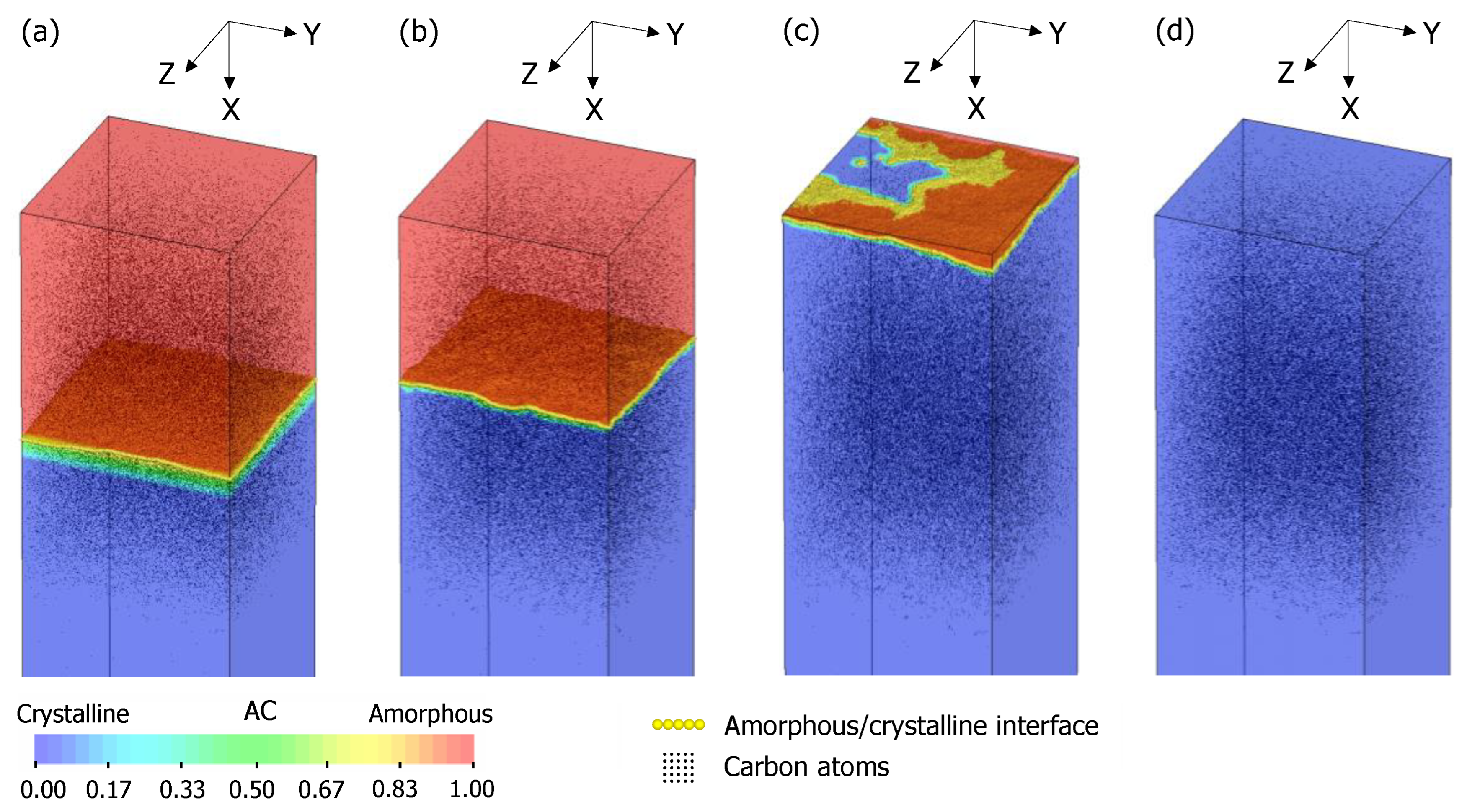
3.3. TCAD Process Simulation of Recrystallization of Fully Amorphized C3H5-Molecular-Ion-Implanted Si Substrate
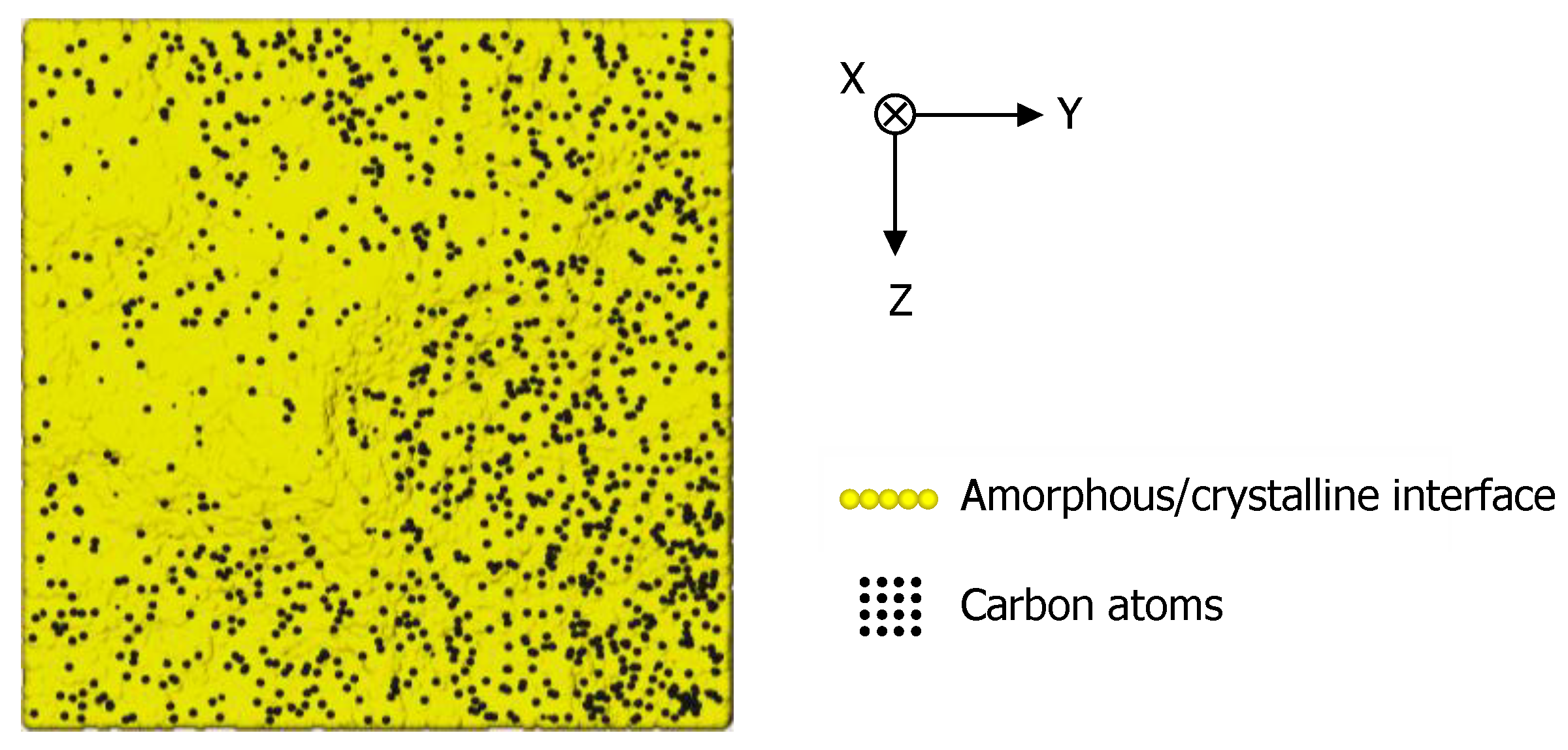
3.4. Surface Recrystallization Behavior of Fully Amorphized C3H5-Molecular-Ion-Implanted Si Substrate after Thermal Annealing Treatment Evaluated by Plan-View TEM Observation
3.5. Surface Recrystallization Model of Fully Amorphized C3H5-Molecular-Ion-Implanted Si Substrate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, F.; Nardone, G.; Polignano, M.L.; D’Ercole, A.; Pennella, F.; Felice, M.D.; Monte, A.D.; Matarazzo, A.; Moccia, G.; Polsinelli, G.; et al. Dark Current Spectroscopy of Transition Metals in CMOS Image Sensors. ECS J. Solid State Sci. Technol. 2017, 6, 217. [Google Scholar] [CrossRef]
- Domengie, F.; Regolini, J.L.; Bauza, D. Study of metal contamination in CMOS image sensors by dark-current and deep-level transient spectroscopies. J. Electron. Mater. 2010, 39, 625–629. [Google Scholar] [CrossRef]
- Kuroi, T.; Kawasaki, Y.; Komori, S.; Fukumoto, K.; Inuishi, M.; Tsukamoto, K.; Shinyashiki, H.; Shingyoji, T. Proximity Gettering of Heavy Metals by High-Energy Ion Implantation. Jpn. J. Appl. Phys. 1993, 32, 303. [Google Scholar] [CrossRef]
- Russo, F.; Moccia, G.; Nardone, G.; Alfonsetti, R.; Polsinelli, G.; D’Angelo, A.; Patacchiola, A.; Liverani, M.; Pianezza, P.; Lippa, T.; et al. Proximity Gettering of Slow Diffuser Contaminants in CMOS Image Sensors. Solid-State Electron. 2014, 91, 91–99. [Google Scholar] [CrossRef]
- Kurita, K.; Kadono, T.; Okuyama, R.; Hirose, R.; Onaka-Masada, A.; Koga, Y.; Okuda, H. Proximity gettering of C3H5 carbon cluster ion-implanted silicon wafers for CMOS image sensors: Gettering effects of transition metal, oxygen, and hydrogen impurities. Jpn. J. Appl. Phys. 2016, 55, 121301. [Google Scholar] [CrossRef]
- Kurita, K.; Kadono, T.; Okuyama, R.; Shigematsu, S.; Hirose, R.; Onaka-Masada, A.; Koga, Y.; Okuda, H. Proximity gettering technology for advanced CMOS image sensors using carbon cluster ion-implantation technique: A review. Phys. Status Solidi A 2017, 214, 1700216. [Google Scholar] [CrossRef]
- Kurita, K.; Kadono, T.; Shigematsu, S.; Hirose, R.; Okuyama, R.; Onaka-Masada, A.; Okuda, H.; Koga, Y. Proximity Gettering Design of Hydrocarbon-Molecular-Ion-Implanted Silicon Wafers Using Dark Current Spectroscopy for CMOS Image Sensors. Sensors 2019, 19, 2073. [Google Scholar] [CrossRef]
- Okuyama, R.; Masada, A.; Shigematsu, S.; Kadono, T.; Hirose, R.; Koga, Y.; Okuda, H.; Kurita, K. Effect of dose and size on defect engineering in carbon cluster implanted silicon wafers. Jpn. J. Appl. Phys. 2018, 57, 011301. [Google Scholar] [CrossRef]
- Shirasawa, S.; Sueoka, K.; Yamaguchi, T.; Maekawa, K. Density functional theory calculations for estimation of gettering sites of C, H, intrinsic point defects and related complexes in Si wafers. Mater. Sci. Semicond. Process. 2016, 44, 13–17. [Google Scholar] [CrossRef]
- Shigematsu, S.; Okuyama, R.; Hirose, R.; Kadono, T.; Onaka-Masada, A.; Suzuki, A.; Kobayashi, K.; Okuda, H.; Koga, Y.; Kurita, K. Influence of oxygen on copper gettering in hydrocarbon molecular ion implanted region using atom probe tomography. Nucl. Instrum. Methods Phys. Res. B 2020, 478, 99. [Google Scholar] [CrossRef]
- Kadono, T.; Okuyama, R.; Onaka-Masada, A.; Hirose, R.; Shigematsu, S.; Koga, Y.; Okuda, H.; Kurita, K. Effect of hydrocarbon molecular ion size for amorphous region formation analyzed by X-ray photoelectron spectroscopy. Jpn. J. Appl. Phys. 2020, 59, 025510. [Google Scholar] [CrossRef]
- Kobayashi, K.; Okuyama, R.; Kadono, T.; Onaka-Masada, A.; Hirose, R.; Suzuki, A.; Koga, Y.; Kurita, K. Recrystallization model of discrete amorphous regions in C3H5-molecular-ionimplanted silicon substrate surface analyzed by X-ray photoelectron spectroscopy. Jpn. J. Appl. Phys. 2022, 61, 115501. [Google Scholar] [CrossRef]
- Johnson, B.C.; McCallum, J.C.; Aziz, M.J. Solid-phase epitaxy. In Handbook of Crystal Growth; Elsevier: Boston, MA, USA, 2015; Chapter 7; pp. 31–363. [Google Scholar]
- Pelaz, L.; Marqués, L.A.; Aboy, M.; López, P.; Santos, I. Improved physical models for advanced silicon device processing. Mater. Sci. Semicond. Process. 2017, 62, 62. [Google Scholar] [CrossRef]
- Mok, K.R.C.; Benistant, F.; Jaraiz, M.; Rubio, J.E.; Castrillo, P.; Pinacho, R.; Srinivasan, M.P. Comprehensive model of damage accumulation in silicon. J. Appl. Phys. 2008, 103, 014911. [Google Scholar] [CrossRef]
- Liang, G.; Zhong, H.; Wang, Y.; Zhang, S.; Xu, M.; Kuang, S.; Ren, J.; Zhang, N.; Yan, S.; Yu, X.; et al. Molecular Dynamics Simulations of Vacancy Generation and Migration near a Monocrystalline Silicon Surface during Energetic Cluster Ion Implantation. Coatings 2020, 10, 146. [Google Scholar] [CrossRef]
- Pongrácz, A.; Szívós, J.; Ujhelyi, F.; Zolnai, Z.; Sepsi, Ö.; Nádudvari, G.; Byrnes, J.; Rubin, L.M.; Moore, E.D. Tilt angle and dose rate monitoring of low energy ion implantation processes with photomodulated reflectance measurement: AM: Advanced Metrology. In Proceedings of the 2020 31st Annual SEMI Advanced Semiconductor Manufacturing Conference (ASMC), Saratoga Springs, NY, USA, 24–26 August 2020; pp. 1–4. [Google Scholar]
- Julliard, P.L.; Dumas, P.; Monsieur, F.; Hilario, F.; Rideau, D.; Hémeryck, A.; Cristiano, F. Implant heating contribution to amorphous layer formation: A KMC approach. In Proceedings of the 2020 International Conference on Simulation of Semiconductor Processes and Devices (SISPAD), Kobe, Japan, 23 September–6 October 2020; pp. 43–46. [Google Scholar]
- Camara, O.; Tunes, M.A.; Greaves, G.; Mir, A.H.; Donnelly, S.; Hinks, J.A. Understanding amorphization mechanisms using ion irradiation in situ a TEM and 3D damage reconstruction. Ultramicroscopy 2019, 207, 112838. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, J.; Teuber, T.; Donner, A.; Radek, M.; Bougeard, D.; Böttger, R.; Hansen, J.L.; Larsen, A.H.; Posselt, M.; Bracht, H. Self-diffusion in amorphous silicon by local bond rearrangements. Phys. Rev. Lett. 2018, 120, 225902. [Google Scholar] [CrossRef]
- Posselt, M.; Bracht, H.; Radić, D. Atomistic simulations on the relationship between solid-phase epitaxial recrystallization and self-diffusion in amorphous silicon. J. Appl. Phys. 2022, 131, 035102. [Google Scholar] [CrossRef]
- Williams, J.S.; Elliman, R.G. Role of Electronic Processes in Epitaxial Recrystallization of Amorphous Semiconductors. Phys. Rev. Lett. 1983, 51, 1069. [Google Scholar] [CrossRef]
- Lampin, E.; Krzeminski, C. Molecular dynamics simulations of the solid phase epitaxy of Si: Growth mechanism and orientation effects. J. Appl. Phys. 2009, 106, 063519. [Google Scholar] [CrossRef]
- Johnson, B.C.; McCallum, J.C. Dopant-enhanced solid-phase epitaxy in buried amorphous silicon layers. Phys. Rev. B 2007, 76, 045216. [Google Scholar] [CrossRef]
- Strane, J.W.; Lee, S.R.; Stein, H.J.; Picraux, S.T.; Watanabe, J.K.; Mayer, J.W. Carbon incorporation into Si at high concentrations by ion implantation and solid phase epitaxy. J. Appl. Phys. 1996, 79, 637. [Google Scholar] [CrossRef]
- Werner, P.; Eichler, S.; Mariani, G.; Kögler, R.; Skorupa, W. Investigation of CxSi defects in C implanted silicon by transmission electron microscopy. Appl. Phys. Lett. 1997, 70, 252. [Google Scholar] [CrossRef]
- Rudawski, N.G.; Whidden, L.R.; Craciun, V.; Jones, K.S. Amorphization and Solid-Phase Epitaxial Growth of C-Cluster Ion-Implanted Si. J. Electron. Mater. 2009, 38, 1926. [Google Scholar] [CrossRef][Green Version]
- Roth, J.A.; Olson, G.L.; Jacobson, D.C.; Poate, J.M. Kinetics of solid phase epitaxy in thick amorphous Si layers formed by MeV ion implantation. Appl. Phys. Lett. 1990, 57, 1340. [Google Scholar] [CrossRef]
- Roth, J.A.; Olson, G.L.; Jacobson, D.C.; Poate, J.M.; Kirschbaum, C. The effect of hydrogen on the kinetics of solid phase epitaxy in amorphous silicon. Mater. Res. Soc. Symp. Proc. 1992, 205, 45. [Google Scholar] [CrossRef]
- Pyke, D.J.; McCallum, J.C.; Johnson, B.C. Hydrogen refinement during solid phase epitaxy of buried amorphous silicon layers. J. Appl. Phys. 2010, 108, 044901. [Google Scholar] [CrossRef]
- Lu, G.Q.; Nygren, E.; Aziz, M.J. Pressure-enhanced crystallization kinetics of amorphous Si and Ge: Implications for point-defect mechanisms. J. Appl. Phys. 1991, 70, 5323. [Google Scholar] [CrossRef]
- Sklenard, B.; Barbe, J.C.; Batude, P.; Rivallin, P.; Tavernier, C.; Cristoloveanu, S.; Martin-Bragado, I. An atomistic investigation of the impact of in-plane uniaxial stress during solid phase epitaxial regrowth. Appl. Phys. Lett. 2013, 102, 151907. [Google Scholar] [CrossRef]
- Yamamoto, M. Kinetics of Material; Uchida Rokakuho: Tokyo, Japan, 2015; Chapter 3; pp. 145–148. [Google Scholar]
- Shirzad, K.; Viney, C. A critical review on applications of the Avrami equation beyond materials science. J. R. Soc. Interface 2023, 20, 20230242. [Google Scholar] [CrossRef]
- Synopsys Sentaurus Process User’s Manual, Release p-2019.03; Synopsys Inc.: Zurich, Switzerland, 2019; pp. 104–129, 455–470.
- Tian, S. Predictive Monte Carlo ion implantation simulator from sub-keV to above 10 MeV. J. Appl. Phys. 2003, 93, 5893–5904. [Google Scholar] [CrossRef]
- Posselt, M.; Schmidt, B.; Murthy, C.S.; Feudel, T.; Suzuki, K. Modeling of Damage Accumulation during Ion Implantation into Single-Crystalline Silicon. J. Electrochem. Soc. 1997, 144, 1495–1504. [Google Scholar] [CrossRef]
- Caturla, M.-J.; Diaz de la Rubia, T.; Marques, L.A.; Gilmer, G.H. Ion-beam processing of silicon at keV energies: A molecular-dynamics study. Phys. Rev. B 1996, 54, 16683. [Google Scholar] [CrossRef]
- Martin-Bragado, I.; Moroz, V. Facet formation during solid phase epitaxy regrowth: A lattice kinetic Monte Carlo model. Appl. Phys. Lett. 2009, 95, 123123. [Google Scholar] [CrossRef]
- Martin-Bragado, I.; Sklenard, B. Understanding Si (111) solid phase epitaxial regrowth using Monte Carlo modeling: Bi-modal growth, defect formation, and interface topology. J. Appl. Phys. 2012, 112, 024327. [Google Scholar] [CrossRef]
- Schafer, R.W. What is a savitzky-golay filter? [lecture notes]. IEEE Signal Process. Mag. 2011, 28, 111. [Google Scholar] [CrossRef]
- Tougaard, S. Practical guide to the use of backgrounds in quantitative XPS. J. Vac. Sci. Technol. A 2021, 39, 011201. [Google Scholar] [CrossRef]
- Ogawa, S.; Tang, J.; Yoshigoe, A.; Ishidzuka, S.; Teraoka, Y.; Takakuwa, Y. Relation between oxidation rate and oxidation-induced strain at SiO2/Si (001) interfaces during thermal oxidation. Jpn. J. Appl. Phys. 2013, 52, 110128. [Google Scholar] [CrossRef]
- Kim, M.; Lippert, G.; Osten, H.J. X-ray photoelectron spectroscopic investigation of carbon incorporation and segregation during pseudomorphic growth of Si1−yCy on Si (001). J. Appl. Phys. 1996, 80, 5748. [Google Scholar] [CrossRef]
- Lu, Z.H.; Mitchell, D.F.; Graham, M.J. Quantitative analysis of low-energy Xe+ ion bombardment damage of Si (100) surfaces using x-ray photoelectron spectroscopy. Appl. Phys. Lett. 1994, 65, 552–554. [Google Scholar]
- Ishii, M.; Hirose, Y.; Sato, T.; Ohwaki, T.; Taga, Y. In situ analysis of Si (100) surface damage induced by low-energy rare-gas ion bombardment using x-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A 1997, 15, 820–824. [Google Scholar] [CrossRef]
- Masaki, Y.; LeComber, P.G.; Fitzgerald, A.G. Solid phase crystallization of thin films of Si prepared by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 1993, 74, 129. [Google Scholar] [CrossRef]
- Olson, G.L.; Roth, J.A. Kinetics of solid phase crystallization in amorphous silicon. Mater. Sci. Rep. 1988, 3, 1–77. [Google Scholar] [CrossRef]






| Process | Parameter | Input Value | Unit |
|---|---|---|---|
| MC ion implantation | |||
| LKMC recrystallization | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, K.; Okuyama, R.; Kadono, T.; Onaka-Masada, A.; Hirose, R.; Suzuki, A.; Nagatomo, S.; Koga, Y.; Sueoka, K.; Kurita, K. Surface Recrystallization Model of Fully Amorphized C3H5-Molecular-Ion-Implanted Silicon Substrate. Crystals 2024, 14, 748. https://doi.org/10.3390/cryst14090748
Kobayashi K, Okuyama R, Kadono T, Onaka-Masada A, Hirose R, Suzuki A, Nagatomo S, Koga Y, Sueoka K, Kurita K. Surface Recrystallization Model of Fully Amorphized C3H5-Molecular-Ion-Implanted Silicon Substrate. Crystals. 2024; 14(9):748. https://doi.org/10.3390/cryst14090748
Chicago/Turabian StyleKobayashi, Koji, Ryosuke Okuyama, Takeshi Kadono, Ayumi Onaka-Masada, Ryo Hirose, Akihiro Suzuki, Sho Nagatomo, Yoshihiro Koga, Koji Sueoka, and Kazunari Kurita. 2024. "Surface Recrystallization Model of Fully Amorphized C3H5-Molecular-Ion-Implanted Silicon Substrate" Crystals 14, no. 9: 748. https://doi.org/10.3390/cryst14090748
APA StyleKobayashi, K., Okuyama, R., Kadono, T., Onaka-Masada, A., Hirose, R., Suzuki, A., Nagatomo, S., Koga, Y., Sueoka, K., & Kurita, K. (2024). Surface Recrystallization Model of Fully Amorphized C3H5-Molecular-Ion-Implanted Silicon Substrate. Crystals, 14(9), 748. https://doi.org/10.3390/cryst14090748







