Properties and Applications of Supersaturated Metastable Alloys Obtained via Electrodeposition
Abstract
:1. Introduction
1.1. Supersaturation and Metastability
1.2. Supersaturated Alloys
1.3. Electrodeposition and Electroless Deposition
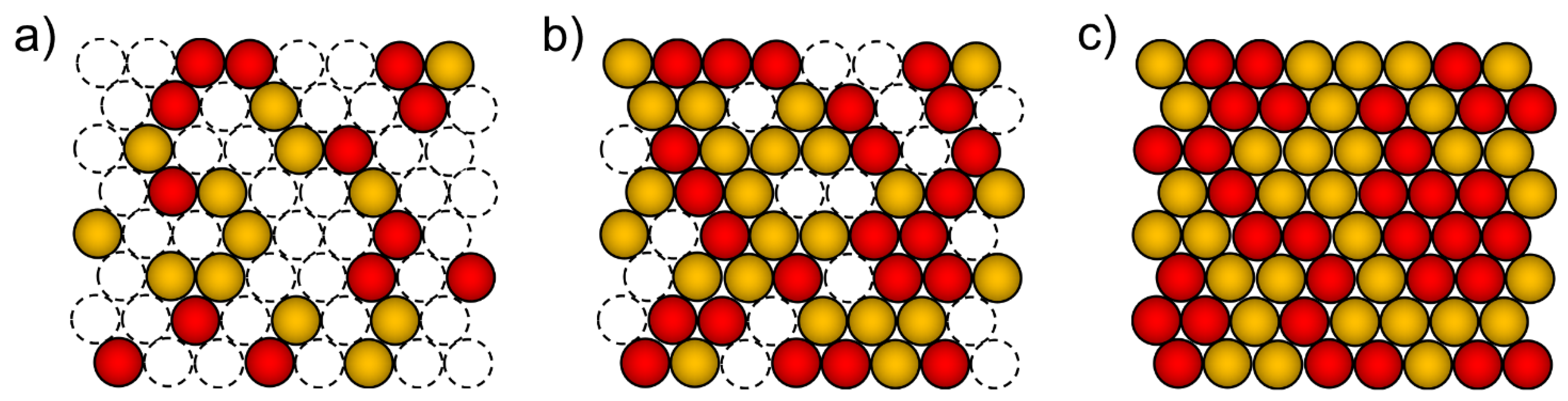
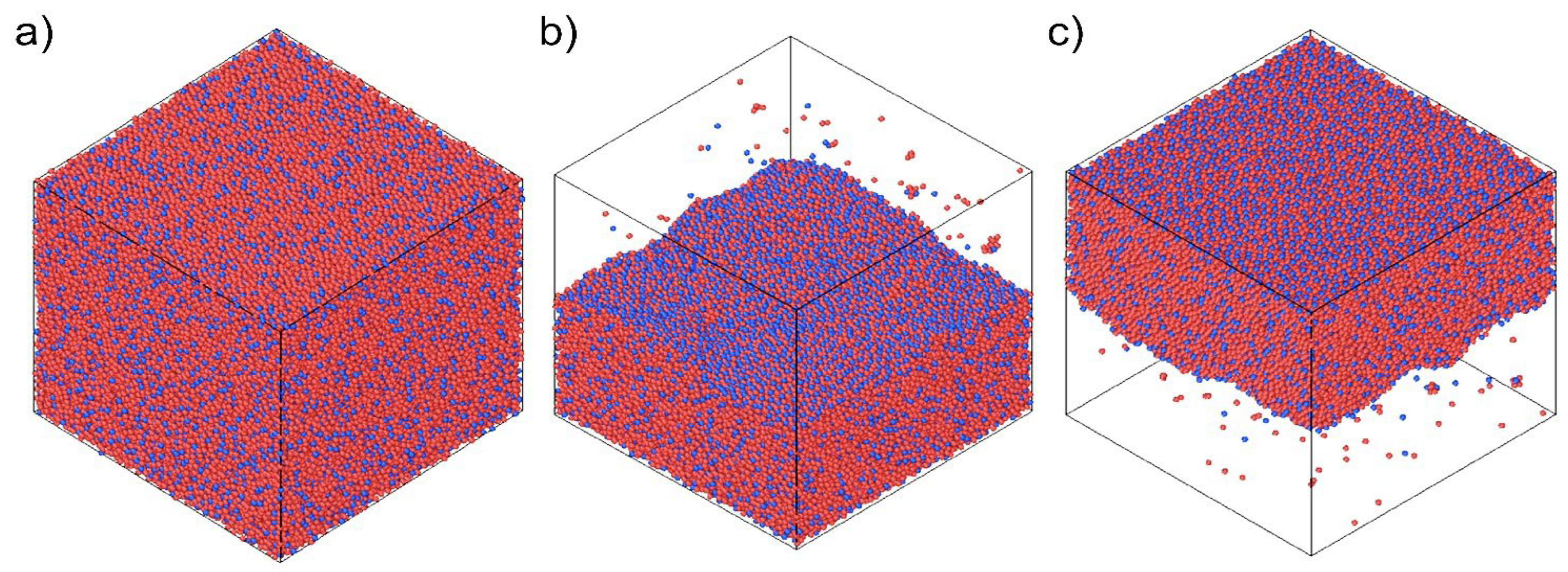
2. Electrodeposition of Metastable Alloys
3. Properties and Applications of Some Electrodeposited Supersaturated Alloys
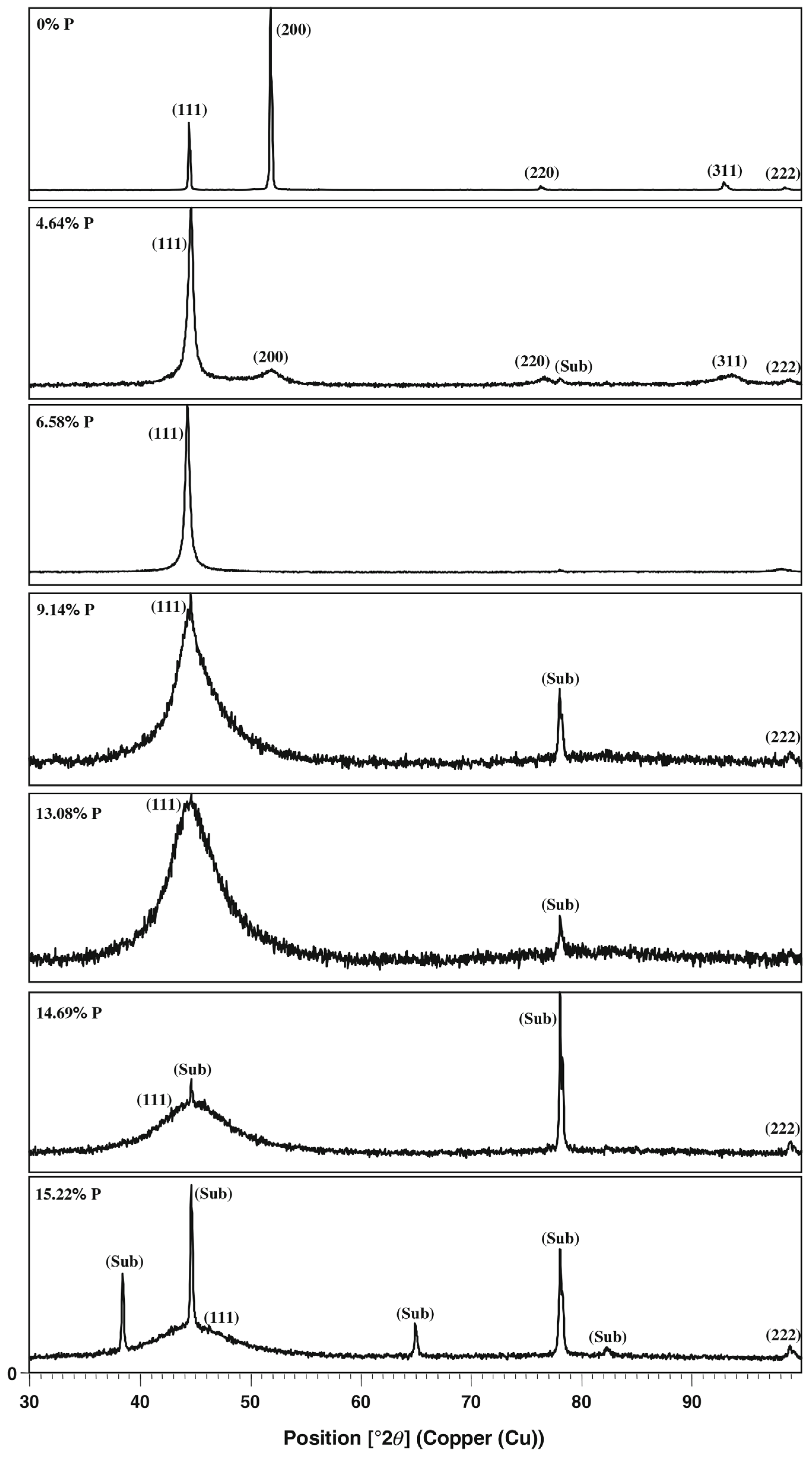
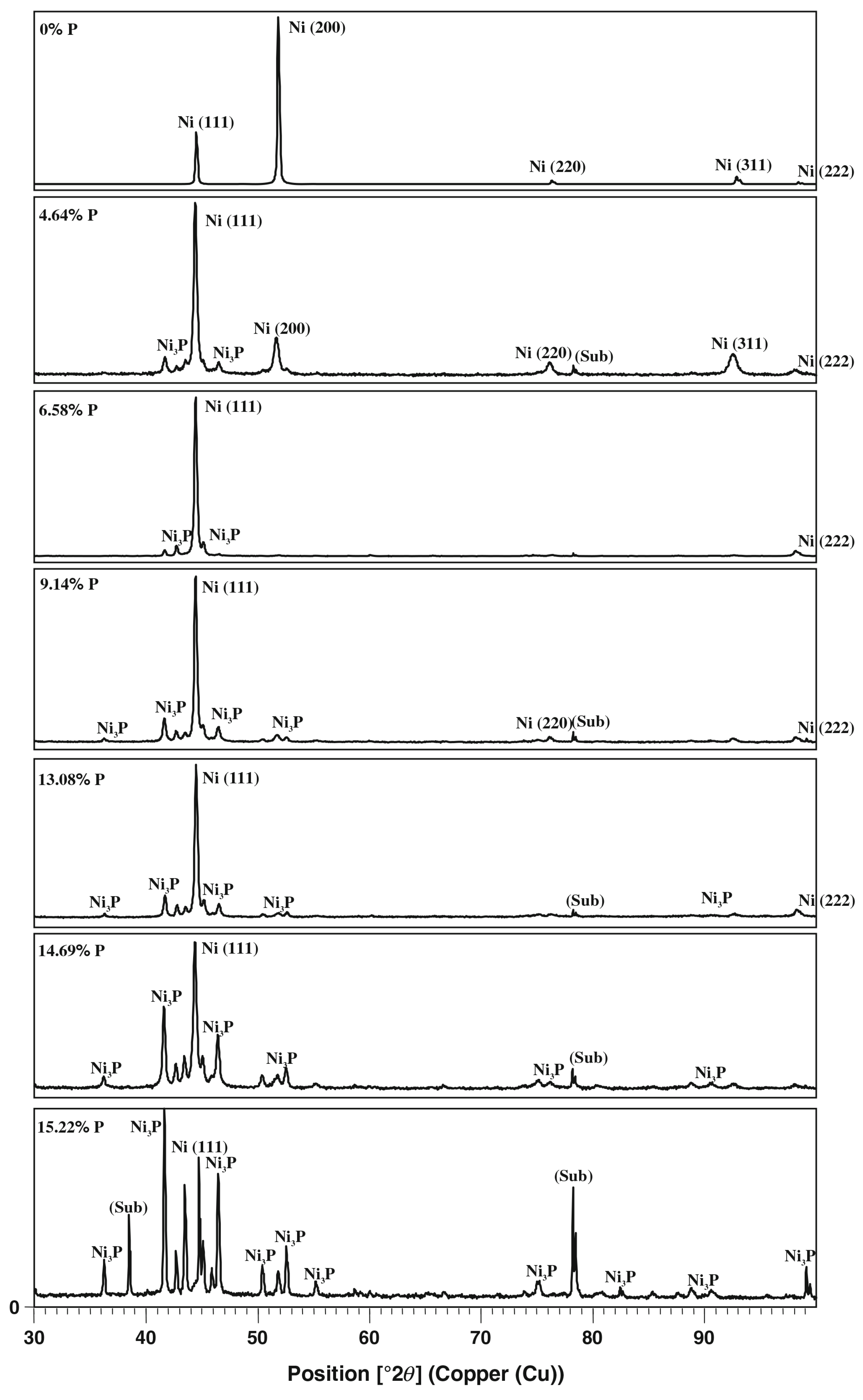
3.1. Ni-P and Co-P
3.2. Fe-P
3.3. Fe-Zn
3.4. Co-Cu
3.5. Cu-Ag
3.6. Ag-Ni
3.7. Other Alloys
4. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balakrishna Bhat, T.; Arunachalam, V.S. Strengthening Mechanisms in Alloys. Proc. Indian Acad. Sci. Sect. C Eng. Sci. 1980, 3, 275–296. [Google Scholar] [CrossRef]
- Dieter, G.E.; Bacon, D. Mechanical Metallurgy; McGraw-Hill: New York, NY, USA, 1976; Volume 3. [Google Scholar]
- Haasen, P. Mechanical Properties of Solid Solutions. In Fundamental Aspects of Structural Alloy Design; Springer: Berlin/Heidelberg, Germany, 1977; pp. 3–25. [Google Scholar]
- Borgioli, F. From Austenitic Stainless Steel to Expanded Austenite-S Phase: Formation, Characteristics and Properties of an Elusive Metastable Phase. Metals 2020, 10, 187. [Google Scholar] [CrossRef]
- Borgioli, F. The “Expanded” Phases in the Low-Temperature Treated Stainless Steels: A Review. Metals 2022, 12, 331. [Google Scholar] [CrossRef]
- Wang, W.-H.; Dong, C.; Shek, C.H. Bulk Metallic Glasses. Mater. Sci. Eng. R Rep. 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of Metallic Supercooled Liquid and Bulk Amorphous Alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Shojaei, Z.; Khayati, G.R.; Darezereshki, E. Review of electrodeposition methods for the preparation of high-entropy alloys. Int. J. Miner. Metall. Mater. 2022, 29, 1683–1696. [Google Scholar] [CrossRef]
- Xu, Z.F.; Wang, Y.; Gao, X.M.; Peng, L.Y.; Qiao, Q.; Xiao, J.J.; Guo, F.Y.; Wang, R.G.; Yu, J.K. Electrochemical Deposition and Corrosion Resistance Characterization of FeCoNiCr High-Entropy Alloy Coatings. Coatings 2023, 13, 1167. [Google Scholar] [CrossRef]
- Serban, B.A.; Olaru, M.T.; Badea, I.C.; Mitrica, D.; Burada, M.; Anasiei, I.; Ghita, M.; Tudor, A.I.; Matei, C.A.; Popescu, A.M.J.; et al. Non-Aqueous Electrodeposition and Characterization of AlCrCuFeNi High Entropy Alloy Thin Films. Materials 2022, 15, 6007. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, C.L.P.; Janardhana, R.K.S.K.; Reddy, K.M.; Murapaka, C.; Joardar, J.; Sarada, B.; Tamboli, R.R.; Hu, Y.X.; Zhang, Y.M.; Wang, X.D.; et al. An advancement in the synthesis of unique soft magnetic CoCuFeNiZn high entropy alloy thin films. Sci. Rep. 2021, 11, 8836. [Google Scholar] [CrossRef]
- Dehestani, M.; Sharafi, S.; Khayati, G.R. The effect of pulse current density on the microstructure, magnetic, mechanical, and corrosion properties of high-entropy alloy coating Fe-Co-Ni-Mo-W, achieved through electro co-deposition. Intermetallics 2022, 147, 107610. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, S.F.; Zuo, L.H.; Li, J.K.; Guo, J.X.; Wang, P.; Sun, J.F.; Dai, L. Recent advances of high-entropy electrocatalysts for water electrolysis by electrodeposition technology: A short review. Rare Met. 2024, 43, 2371–2390. [Google Scholar] [CrossRef]
- Bian, H.W.; Wang, R.; Zhang, K.Z.; Zheng, H.L.; Wen, M.J.; Li, Z.M.; Li, Z.H.; Wang, G.X.; Xie, G.W.; Liu, X.; et al. Facile electrodeposition synthesis and super performance of nano-porous Ni-Fe-Cu-Co-W high entropy alloy electrocatalyst. Surf. Coat. Technol. 2023, 459, 129407. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 1483223116. [Google Scholar]
- Zargarnezhad, H.; Dolati, A. A 3D Continuum-Kinetic Monte Carlo Simulation Study of Early Stages of Nucleation and Growth in Ni Electrodeposition. Electrochim. Acta 2017, 236, 1–9. [Google Scholar] [CrossRef]
- Treeratanaphitak, T.; Pritzker, M.D.; Abukhdeir, N.M. Kinetic Monte Carlo Simulation of Electrodeposition Using the Embedded-Atom Method. Electrochim. Acta 2014, 121, 407–414. [Google Scholar] [CrossRef]
- Ataalite, H.; Dardouri, M.; Arbaoui, A.; Fathi, A.; Hasnaoui, A.; Sbiaai, K. Kinetic Monte Carlo Simulation of Polycrystalline Silver Metal Electrodeposition: Scaling of Roughness and Effects of Deposition Parameters. Phys. Chem. Chem. Phys. 2023, 25, 4216–4229. [Google Scholar] [CrossRef] [PubMed]
- Ataalite, H.; Dardouri, M.; Hasnaoui, A.; Sbiaai, K. Morphological Surface Study of Silver Electrodeposition by Kinetic Monte Carlo-Embedded Atom Method. Phys. Status Solidi B 2022, 259, 2100559. [Google Scholar] [CrossRef]
- Onabuta, Y.; Kunimoto, M.; Wang, S.; Fukunaka, Y.; Nakai, H.; Homma, T. Multiscale Simulation of Irregular Shape Evolution during the Initial Stage of Zn Electrodeposition on a Negative Electrode Surface. J. Phys. Chem. C 2022, 126, 5224–5232. [Google Scholar] [CrossRef]
- Cheimarios, N.; To, D.; Kokkoris, G.; Memos, G.; Boudouvis, A.G. Monte Carlo and Kinetic Monte Carlo Models for Deposition Processes: A Review of Recent Works. Front. Phys. 2021, 9, 631918. [Google Scholar] [CrossRef]
- Mascaró, F.V.; McCrum, I.T.; Koper, M.T.M.; Rost, M.J. Nucleation and Growth of Dendritic Islands during Platinum Oxidation-Reduction Cycling. J. Electrochem. Soc. 2022, 169, 112506. [Google Scholar] [CrossRef]
- Zhu, J.Z.; Liu, Z.K.; Vaithyanathan, V.; Chen, L.Q. Linking phase-field model to CALPHAD: Application to precipitate shape evolution in Ni-base alloys. Scr. Mater. 2002, 46, 401–406. [Google Scholar] [CrossRef]
- Schwen, D.; Jiang, C.; Aagesen, L.K. A sublattice phase-field model for direct CALPHAD database coupling. Comput. Mater. Sci. 2021, 195, 110466. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Zhang, X.; Lu, X.-G.; Wang, C.; Zhang, Y. Microscopic Phase-Field Simulation of γ’ Precipitation in Ni-Based Binary Alloys Coupled with CALPHAD Method. Crystals 2022, 12, 971. [Google Scholar] [CrossRef]
- Okamoto, H.; Okamoto, H. Phase Diagrams for Binary Alloys; ASM international: Materials Park, OH, USA, 2000; Volume 44. [Google Scholar]
- Mallory, G.O.; Hajdu, J.B. Electroless Plating: Fundamentals and Applications; William Andrew: Norwich, NY, USA, 1990; ISBN 0936569077. [Google Scholar]
- Lewis, D.B.; Marshall, G.W. Investigation into the Structure of Electrodeposited Nickel-Phosphorus Alloy Deposits. Surf. Coat. Technol. 1996, 78, 150–156. [Google Scholar] [CrossRef]
- Ishida, K.; Nishizawa, T. The Co-P (Cobalt-Phosphorus) System. Bull. Alloy Phase Diagr. 1990, 11, 555–560. [Google Scholar] [CrossRef]
- Brenner, A.; Couch, D.E.; Williams, E.K. Electrodeposition of Alloys of Phosphorus with Nickel or Cobalt. J. Res. Nat. Bur. Stand 1950, 44, 109. [Google Scholar] [CrossRef]
- Brüning, R.; Brown, D.A.; Bera, H.; Jakse, N. Molecular Dynamics Simulations of Amorphous Ni–P Alloy Formation by Rapid Quenching and Atomic Deposition. J. Phys. Condens. Matter 2020, 32, 154001. [Google Scholar] [CrossRef]
- Sha, W.; Wu, X.; Keong, K.G. Electroless Copper and Nickel-Phosphorus Plating: Processing, Characterisation and Modelling; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 0857090968. [Google Scholar]
- Pillai, A.M.; Rajendra, A.; Sharma, A.K. Electrodeposited Nickel–Phosphorous (Ni–P) Alloy Coating: An in-Depth Study of Its Preparation, Properties, and Structural Transitions. J. Coat. Technol. Res. 2012, 9, 785–797. [Google Scholar] [CrossRef]
- Li, G.; Gao, Y.P.; Liu, R.P. Binary Ni–P Bulk Amorphous Glass Prepared by Electrodeposition Method. J. Non. Cryst. Solids 2007, 353, 4199–4202. [Google Scholar] [CrossRef]
- Hu, C.-C.; Bai, A. Influences of the Phosphorus Content on Physicochemical Properties of Nickel–Phosphorus Deposits. Mater. Chem. Phys. 2003, 77, 215–225. [Google Scholar] [CrossRef]
- Hu, C.-C.; Bai, A. Composition Control of Electroplated Nickel–Phosphorus Deposits. Surf. Coat. Technol. 2001, 137, 181–187. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chung, S.-T.; Pan, S.-J.; Tsai, W.-T.; Lin, C.-S. Microstructure Evolution and Hardening Mechanisms of Ni–P Electrodeposits. Surf. Coat. Technol. 2010, 205, 2097–2103. [Google Scholar] [CrossRef]
- Mahalingam, T.; Raja, M.; Thanikaikarasan, S.; Sanjeeviraja, C.; Velumani, S.; Moon, H.; Kim, Y.D. Electrochemical Deposition and Characterization of Ni–P Alloy Thin Films. Mater. Charact. 2007, 58, 800–804. [Google Scholar] [CrossRef]
- Hou, K.-H.; Jeng, M.-C.; Ger, M.-D. A Study on the Wear Resistance Characteristics of Pulse Electroforming Ni–P Alloy Coatings as Plated. Wear 2007, 262, 833–844. [Google Scholar] [CrossRef]
- Seo, M.H.; Kim, J.S.; Hwang, W.S.; Kim, D.J.; Hwang, S.S.; Chun, B.S. Characteristics of Ni–P Alloy Electrodeposited from a Sulfamate Bath. Surf. Coat. Technol. 2004, 176, 135–140. [Google Scholar] [CrossRef]
- Lin, C.S.; Lee, C.Y.; Chen, F.J.; Chien, C.T.; Lin, P.L.; Chung, W.C. Electrodeposition of Nickel-Phosphorus Alloy from Sulfamate Baths with Improved Current Efficiency. J. Electrochem. Soc. 2006, 153, C387. [Google Scholar] [CrossRef]
- Chang, L.; Chen, C.-H.; Fang, H. Electrodeposition of Ni–P Alloys From a Sulfamate Electrolyte: Relationship Between Bath PH and Structural Characteristics. J. Electrochem. Soc. 2007, 155, D57. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, F.; Chang, L.; Zheng, J.; Zhang, Z.; Zhang, J.; Cao, C. Electrodeposition of High Corrosion Resistance Cu/Ni–P Coating on AZ91D Magnesium Alloy. Appl. Surf. Sci. 2011, 257, 9213–9220. [Google Scholar] [CrossRef]
- Vijayan, S.; Luo, N.; Aindow, M. Microstructural Stability and Phase Transformations in Electrodeposited Cobalt-Phosphorus Coatings. J. Alloys Compd. 2017, 719, 142–150. [Google Scholar] [CrossRef]
- Bernasconi, R.; Allievi, F.; Sadeghi, H.; Magagnin, L. Codeposition of Nickel–Phosphorus Alloys Reinforced with Boron Carbide Microparticles: Direct and Pulse Plating. Trans. IMF 2017, 95, 52–59. [Google Scholar] [CrossRef]
- Wang, F.; Itoh, K.; Watanabe, T. Relationship between the Crystallographic Structure of Electrodeposited Fe-P Alloy Film and Its Thermal Equilibrium Phase Diagram. Mater. Trans. 2003, 44, 127–132. [Google Scholar] [CrossRef]
- Sridharan, K.; Sheppard, K. Crystallization of Amorphous Iron-Nickel-Phosphorus Alloys Prepared by Electrodeposition. J. Mater. Process. Technol. 1997, 68, 109–116. [Google Scholar] [CrossRef]
- Schlesinger, M.; Paunovic, M. Modern Electroplating, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 1118063147. [Google Scholar]
- Kuznetsov, A.N.; Cherstiouk, O.V.; Zaikovskii, V.I.; Rudina, N.A.; Kvon, R.I.; Oshchepkov, A.G. Electrodeposited Ni-P Electrodes: An Effect of Amorphous Structure on the Electrochemical Behavior and Electrocatalytic Activity in the Hydrogen Oxidation Reaction in Alkaline Media. J. Electroanal. Chem. 2023, 944, 117676. [Google Scholar] [CrossRef]
- Armyanov, S.; Vitkova, S.; Blajiev, O. Internal Stress and Magnetic Properties of Electrodeposited Amorphous Fe–P Alloys. J. Appl. Electrochem. 1997, 27, 185–191. [Google Scholar] [CrossRef]
- Mita, K.; Ikeda, T.; Maeda, M. Phase Diagram Study of Fe-Zn Intermetallics. J. Phase Equilibria 2001, 22, 122–125. [Google Scholar] [CrossRef]
- Hara, T.; Adaniya, T.; Sagiyama, M.; Honma, T.; Tonouchi, A.; Watanabe, T.; Ohmura, M. The Phase Composition and Wor Kability of Electrodeposited Fe-Zn Alloy. Trans. Iron Steel Inst. Jpn. 1983, 23, 954–958. [Google Scholar] [CrossRef]
- Kondo, K.; Hinotani, S.; Ohmori, Y. Crystal Structure and Morphology of Electrodeposited Zinc-Iron Binary Alloys. J. Appl. Electrochem. 1988, 18, 154–161. [Google Scholar] [CrossRef]
- Gu, M.; Marder, A.R. Morphological Changes of Electrodeposited Zn and Zn-Fe Coatings during Heating. J. Mater. Sci. 1991, 26, 4588–4598. [Google Scholar] [CrossRef]
- Ieffa, S.; Bernasconi, R.; Nobili, L.; Cavallotti, P.L.; Magagnin, L. Direct and Pulse Plating of Metastable Zn–Ni Alloys. Trans. IMF 2014, 92, 321–324. [Google Scholar] [CrossRef]
- Drewien, C.A.; Marder, A.R. Effect of Annealing upon Decomposition of Electrodeposited Iron-Zinc Alloy Coatings. J. Mater. Sci. 1994, 29, 965–972. [Google Scholar] [CrossRef]
- Drewien, C.A.; Goldstein, J.I.; Marder, A.R. η to G Phase Transformation in Electrodeposited Iron-Zinc Alloy Coatings. Metall. Mater. Trans. A 1994, 25, 1119–1125. [Google Scholar] [CrossRef]
- Kanamura, T.; Morita, J.; Nakayama, M.; Arai, K.; Ogawa, Y. Properties of Iron-Zinc Alloy-Electroplated Galvannealed Steel Sheet. Nippon. Steel Technol. Rep. 1994, 63, 23–26. [Google Scholar]
- Xu, Y.; Wang, W.; Yu, F.; Wang, Y.; Qi, M.; Zhao, Y.; Wang, Y. Effects of Pulse Frequency and Current Density on Microstructure and Properties of Biodegradable Fe-Zn Alloy. J. Mater. Res. Technol. 2022, 18, 44–58. [Google Scholar] [CrossRef]
- Yuasa, M.; Kajikawa, K.; Hakamada, M.; Mabuchi, M. Saturation Magnetization in Supersaturated Solid Solution of Co–Cu Alloy. Appl. Phys. Lett. 2009, 95, 162502. [Google Scholar] [CrossRef]
- Pratama, K.; Barrirero, J.; Mücklich, F.; Motz, C. Microstructure Evolution and Mechanical Stability of Supersaturated Solid Solution Co-Rich Nanocrystalline Co-Cu Produced by Pulsed Electrodeposition. Materials 2020, 13, 2616. [Google Scholar] [CrossRef] [PubMed]
- Kreye, H.; Hornbogen, E. Recrystallisation of Supersaturated Copper-Cobalt Solid Solutions. J. Mater. Sci. 1970, 5, 89–95. [Google Scholar] [CrossRef]
- Berkowitz, A.E.; Mitchell, J.R.; Carey, M.J.; Young, A.P.; Rao, D.; Starr, A.; Zhang, S.; Spada, F.E.; Parker, F.T.; Hutten, A. Giant Magnetoresistance in Heterogeneous Cu–Co and Ag–Co Alloy Films. J. Appl. Phys. 1993, 73, 5320–5325. [Google Scholar] [CrossRef]
- Liu, K.; Nagodawithana, K.; Searson, P.C.; Chien, C.L. Perpendicular Giant Magnetoresistance of Multilayered Co/Cu Nanowires. Phys. Rev. B 1995, 51, 7381. [Google Scholar] [CrossRef]
- Ram, S.; Frankwicz, P.S. Granular GMR Sensors of Co–Cu and Co–Ag Nanoparticles Synthesized through a Chemical Route Using NaBH4. Phys. Status Solidi 2001, 188, 1129–1140. [Google Scholar] [CrossRef]
- Prieto, A.G.; Fdez-Gubieda, M.L.; Meneghini, C.; Garcia-Arribas, A.; Mobilio, S. Microstructural and Magnetic Evolution upon Annealing of Giant Magnetoresistance Melt-Spun Co-Cu Granular Alloys. Phys. Rev. B 2003, 67, 224415. [Google Scholar] [CrossRef]
- Pratama, K.; Tian, C.; Sharma, A.; Watroba, M.; Gubicza, J.; Dilasari, B.; Schwiedrzik, J.; Michler, J. Pulsed Electrodeposition of Homogenous and Heterogeneous Solid Solution Layered Structure in High Strength Nanocrystalline CoCu Alloys. Surf. Coat. Technol. 2024, 480, 130613. [Google Scholar] [CrossRef]
- Giancoli, D.C. Physics for Scientists and Engineers with Modern Physics; Pearson Education: London, UK, 2008; Volume 2, ISBN 0131495089. [Google Scholar]
- Kim, M.J.; Lee, H.J.; Yong, S.H.; Kwon, O.J.; Kim, S.-K.; Kim, J.J. Facile Formation of Cu-Ag Film by Electrodeposition for the Oxidation-Resistive Metal Interconnect. J. Electrochem. Soc. 2012, 159, D253. [Google Scholar] [CrossRef]
- Kim, M.J.; Yong, S.H.; Ko, H.S.; Lim, T.; Park, K.J.; Kwon, O.J.; Kim, J.J. Superfilling of Cu-Ag Using Electrodeposition in Cyanide-Based Electrolyte. J. Electrochem. Soc. 2012, 159, D656–D658. [Google Scholar] [CrossRef]
- Volov, I.; Swanson, E.; O’Brien, B.; Novak, S.W.; Boom, R.V.D.; Dunn, K.; West, A.C. Pulse-Plating of Copper-Silver Alloys for Interconnect Applications. J. Electrochem. Soc. 2012, 159, D677–D683. [Google Scholar] [CrossRef]
- Strehle, S.; Menzel, S.; Wendrock, H.; Acker, J.; Gemming, T.; Wetzig, K. Thermo-Mechanical Behavior and Microstructural Evolution of Electrochemically Deposited Low-Alloyed Cu(Ag) Thin Films. Microelectron. Eng. 2004, 76, 205–211. [Google Scholar] [CrossRef]
- Strehle, S.; Menzel, S.; Bartha, J.W.; Wetzig, K. Electroplating of Cu(Ag) Thin Films for Interconnect Applications. Microelectron. Eng. 2010, 87, 180–186. [Google Scholar] [CrossRef]
- Bernasconi, R.; Hart, J.L.; Lang, A.C.; Magagnin, L.; Nobili, L.; Taheri, M.L. Structural Properties of Electrodeposited Cu-Ag Alloys. Electrochim. Acta 2017, 251, 475–481. [Google Scholar] [CrossRef]
- Lee, K.H.; Kong, W.; Han, M.; Park, D.J.; Ahn, J.H.; Han, S.Z.; Park, Y.-B.; Lee, K.H.; Choe, S. Properties of Nanocrystalline CuAg Foil Prepared via Electrodeposition. J. Alloys Compd. 2021, 881, 160522. [Google Scholar] [CrossRef]
- Gambino, J.P. Improved Reliability of Copper Interconnects Using Alloying. In Proceedings of the 2010 17th IEEE International Symposium on the Physical and Failure Analysis of Integrated Circuits, Singapore, 5–9 July 2010; IEEE: New York, NY, USA, 2010; pp. 1–7. [Google Scholar]
- Swingler, J. Performance and Arcing Characteristics of Ag/Ni Contact Materials under DC Resistive Load Conditions. IET Sci. Meas. Technol. 2011, 5, 37–45. [Google Scholar] [CrossRef]
- Proux, O.; Mimault, J.; Revenant-Brizard, C.; Regnard, J.R.; Mevel, B. Structural Evolution of NiAg Heterogeneous Alloys upon Annealing. J. Phys. Condens. Matter 1999, 11, 147. [Google Scholar] [CrossRef]
- Barthelemy, A.; Cros, V.; Duvail, J.L.; Fert, A.; Morel, R.; Parent, F.; Petroff, F.; Steren, L.B. Giant Magnetoresistance in Magnetic Nanostructures. Nanostructured Mater. 1995, 6, 217–226. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Chen, X.; Wen, R.; Yue, G.-H.; Peng, D.-L. Facile Synthesis of Near-Monodisperse Ag@Ni Core–Shell Nanoparticles and Their Application for Catalytic Generation of Hydrogen. Nanotechnology 2011, 22, 195604. [Google Scholar] [CrossRef]
- Greeley, J.; Nørskov, J.K. Large-Scale, Density Functional Theory-Based Screening of Alloys for Hydrogen Evolution. Surf. Sci. 2007, 601, 1590–1598. [Google Scholar] [CrossRef]
- Tyler, E.H.; Clinton, J.R.; Luo, H.L. Electrical Resistivity of Metastable Ag-Ni Alloys. Solid State Commun. 1973, 13, 1409–1411. [Google Scholar] [CrossRef]
- Cavallotti, P.L.; Nobili, L.; Vicenzo, A. Phase Structure of Electrodeposited Alloys. Electrochim. Acta 2005, 50, 4557–4565. [Google Scholar] [CrossRef]
- Foster, D.G.; Shapir, Y.; Jorné, J. Scaling of Roughness in Silver Electrodeposition. J. Electrochem. Soc. 2003, 150, C375. [Google Scholar] [CrossRef]
- Gómez, E.; García-Torres, J.; Vallés, E. Study and Preparation of Silver Electrodeposits at Negative Potentials. J. Electroanal. Chem. 2006, 594, 89–95. [Google Scholar] [CrossRef]
- Liang, D.; Liu, Z.; Hilty, R.D.; Zangari, G. Electrodeposition of Ag–Ni Films from Thiourea Complexing Solutions. Electrochim. Acta 2012, 82, 82–89. [Google Scholar] [CrossRef]
- Xie, B.-G.; Sun, J.-J.; Lin, Z.-B.; Chen, G.-N. Electrodeposition of Mirror-Bright Silver in Cyanide-Free Bath Containing Uracil as Complexing Agent without a Separate Strike Plating Process. J. Electrochem. Soc. 2008, 156, D79. [Google Scholar] [CrossRef]
- Dhanapal, K.; Vasumathi, M.; Santhi, K.; Narayanan, V.; Stephen, A. Double Dumbbell Shaped AgNi Alloy by Pulsed Electrodeposition. In Proceedings of the AIP Conference Proceedings, Shymkent, Kazakhstan, 11–13 September 2014; American Institute of Physics: College Park, MD, USA, 2014; Volume 1576, pp. 95–97. [Google Scholar]
- Santhi, K.; Karthick, S.N.; Kim, H.-J.; Nidhin, M.; Narayanan, V.; Stephen, A. Microstructure Analysis of the Ferromagnetic Ag–Ni System Synthesized by Pulsed Electrodeposition. Appl. Surf. Sci. 2012, 258, 3126–3132. [Google Scholar] [CrossRef]
- Eom, H.; Jeon, B.; Kim, D.; Yoo, B. Electrodeposition of Silver-Nickel Thin Films with a Galvanostatic Method. Mater. Trans. 2010, 51, 1842–1846. [Google Scholar] [CrossRef]
- Tang, M.H.; Hahn, C.; Klobuchar, A.J.; Ng, J.W.D.; Wellendorff, J.; Bligaard, T.; Jaramillo, T.F. Nickel–Silver Alloy Electrocatalysts for Hydrogen Evolution and Oxidation in an Alkaline Electrolyte. Phys. Chem. Chem. Phys. 2014, 16, 19250–19257. [Google Scholar] [CrossRef] [PubMed]
- Yamachika, N.; Musha, Y.; Sasano, J.; Senda, K.; Kato, M.; Okinaka, Y.; Osaka, T. Electrodeposition of Amorphous Au–Ni Alloy Film. Electrochim. Acta 2008, 53, 4520–4527. [Google Scholar] [CrossRef]
- Rouya, E.; Stafford, G.R.; Bertocci, U.; Mallett, J.J.; Schad, R.; Begley, M.R.; Kelly, R.G.; Reed, M.L.; Zangari, G. Electrodeposition of Metastable Au–Ni Alloys. J. Electrochem. Soc. 2010, 157, D396. [Google Scholar] [CrossRef]
- Dolati, A.; Ghorbani, M.; Ahmadi, M.R. An Electrochemical Study of Au–Ni Alloy Electrodeposition from Cyanide–Citrate Electrolytes. J. Electroanal. Chem. 2005, 577, 1–8. [Google Scholar] [CrossRef]
- Li, J.C.; Nan, S.H.; Jiang, Q. Study of the Electrodeposition of Al–Mn Amorphous Alloys from Molten Salts. Surf. Coat. Technol. 1998, 106, 135–139. [Google Scholar] [CrossRef]
- Tsuda, T.; Hussey, C.L.; Stafford, G.R. Electrodeposition of Al–Mo alloys from the Lewis acidic aluminum chloride-1-ethyl-3-methylimidazolium chloride molten salt. J. Electrochem. Soc. 2004, 151, C379–C384. [Google Scholar] [CrossRef]
- Tsuda, T.; Hussey, C.L. Electrochemistry of Vanadium (II) and the Electrodeposition of Aluminum-Vanadium Alloys in the Aluminum Chloride-1-Ethyl-3-Methylimidazolium Chloride Molten Salt. J. Min. Metall. Sect. B Metall. 2003, 39, 3–22. [Google Scholar] [CrossRef]
- Barthel, T.; Klimanek, P.; Raub, C.J.; Zielonka, A. Microstructure Formation in Electrodeposition of Highly Supersaturated Cu-Pb and Ag-Pb Layers. In Materials Science Forum; Trans Tech Publications: Zurich, Switzerland, 1996; Volume 228, pp. 475–480. [Google Scholar]
- Revathy, T.A.; Santhi, K.; Narayanan, V.; Stephen, A. Synthesis and Characterization of Fe-Ag Alloy by Pulsed Electrodeposition. Chem. Sci. Trans. 2013, 2, S153. [Google Scholar]
- Noce, R.D.; Gomes, O.D.M.; De Magalhaes, S.D.; Wolf, W.; Guimarães, R.B.; De Castro, A.C.; Pires, M.J.M.; Macedo, W.A.A.; Givord, D.; Barthem, V.M.T.S. Magnetic Properties of Fe–Cu Alloys Prepared by Pulsed Electrodeposition. J. Appl. Phys. 2009, 106, 093907. [Google Scholar] [CrossRef]
- Mallett, J.J.; Svedberg, E.B.; Sayan, S.; Shapiro, A.J.; Wielunski, L.; Madey, T.E.; Egelhoff, W.F.; Moffat, T.P. Compositional Control in Electrodeposition of FePt Films. Electrochem. Solid-State Lett. 2004, 7, C121. [Google Scholar] [CrossRef]
- Bernasconi, R.; Nova, A.; Pané, S.; Magagnin, L. Electrodeposition of Equiatomic FePt Permanent Magnets from Non-Aqueous Electrolytes Based on Ethylene Glycol. J. Electrochem. Soc. 2022, 169, 072506. [Google Scholar] [CrossRef]
- Ying, Y.; Wang, H.; Zheng, J.; Yu, J.; Li, W.; Qiao, L.; Cai, W.; Che, S. Preparation, Microstructure, and Magnetic Properties of Electrodeposited Nanocrystalline L10 FePt Films. J. Supercond. Nov. Magn. 2020, 33, 3563–3570. [Google Scholar] [CrossRef]
- Krasikov, A.V.; Kastsova, A.G.; Markov, M.A.; Bykova, A.D.; Kravchenko, I.N.; Galinovskii, A.L. Electrochemical Synthesis of Amorphous Layers from a Nonequilibrium Co–W Alloy as a Precursor for Nanocomposite Coating Formation. Russ. Metall. 2022, 2022, 666–673. [Google Scholar] [CrossRef]
- Krasikov, A.V.; Merkulova, M.V.; Mikhailov, M.S.; Vasilyeva, E.A.; Petrov, S.N.; Drozdova, N.F.; Fedoseev, M.L. Formation of a Composite Coating by Crystallization of a Supersaturated Solid Solution in the Ni–W System. Trans. Indian Inst. Met. 2024, 1–8. [Google Scholar] [CrossRef]
- Esquivel, J.; Gupta, R.K. Review—Corrosion Resistant Metastable Al Alloys: An Overview of Corrosion Mechanisms. J. Electrochem. Soc. 2020, 167, 081504. [Google Scholar] [CrossRef]
- Sziráki, L.; Kuzmann, E.; El-Sharif, M.; Chisholm, C.U.; Stichleutner, S.; Lak, G.B.; Süvegh, K.; Tatár, E.; Homonnay, Z.; Vértes, A. Electrodeposition of Novel Sn–Ni–Fe Ternary Alloys with Amorphous Structure. Appl. Surf. Sci. 2010, 256, 7713–7716. [Google Scholar] [CrossRef]
| Alloy | Type of Electrolyte | Application | References |
|---|---|---|---|
| Au-Ni | Acidic cyanide-citrate | Contacts and switches, substrate protection | [93,94,95] |
| Al-Mn | Molten salts | Corrosion protection, protective coatings | [96] |
| Al-Mo | Ionic liquid | Corrosion protection | [97] |
| Al-V | Ionic liquid | Corrosion protection | [98] |
| Cu-Pb | Alkaline citrate | Metal bearing coating | [99] |
| Ag-Pb | Alkaline cyanide | Metal bearing coating | [99] |
| Fe-Ag | Acidic perchlorate | Magnetic applications | [100] |
| Fe-Cu | Sulfate | Magnetic applications | [101] |
| Fe-Pt | Acidic chloride; acidic sulfate; non-aqueous | Magnetic properties | [102,103,104] |
| Co-W | Citrate | Wear protection | [105] |
| Ni-W | Citrate | Wear protection | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernasconi, R.; Nobili, L.; Magagnin, L. Properties and Applications of Supersaturated Metastable Alloys Obtained via Electrodeposition. Crystals 2024, 14, 761. https://doi.org/10.3390/cryst14090761
Bernasconi R, Nobili L, Magagnin L. Properties and Applications of Supersaturated Metastable Alloys Obtained via Electrodeposition. Crystals. 2024; 14(9):761. https://doi.org/10.3390/cryst14090761
Chicago/Turabian StyleBernasconi, Roberto, Luca Nobili, and Luca Magagnin. 2024. "Properties and Applications of Supersaturated Metastable Alloys Obtained via Electrodeposition" Crystals 14, no. 9: 761. https://doi.org/10.3390/cryst14090761






