Influence of the Acetylene Flow Rate and Process Pressure on the Carbon Deposition Behavior by Thermal Chemical Vapor Deposition Process
Abstract
:1. Introduction
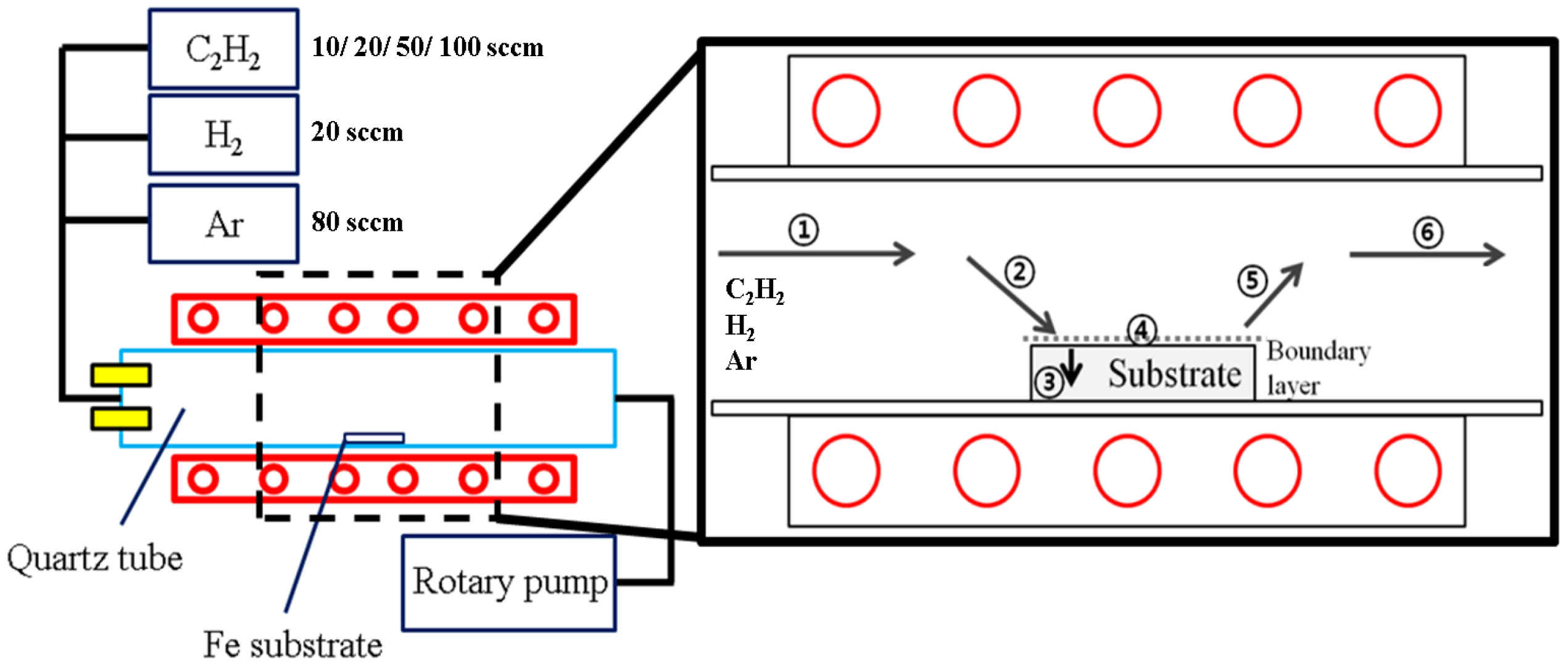
2. Experimental Method
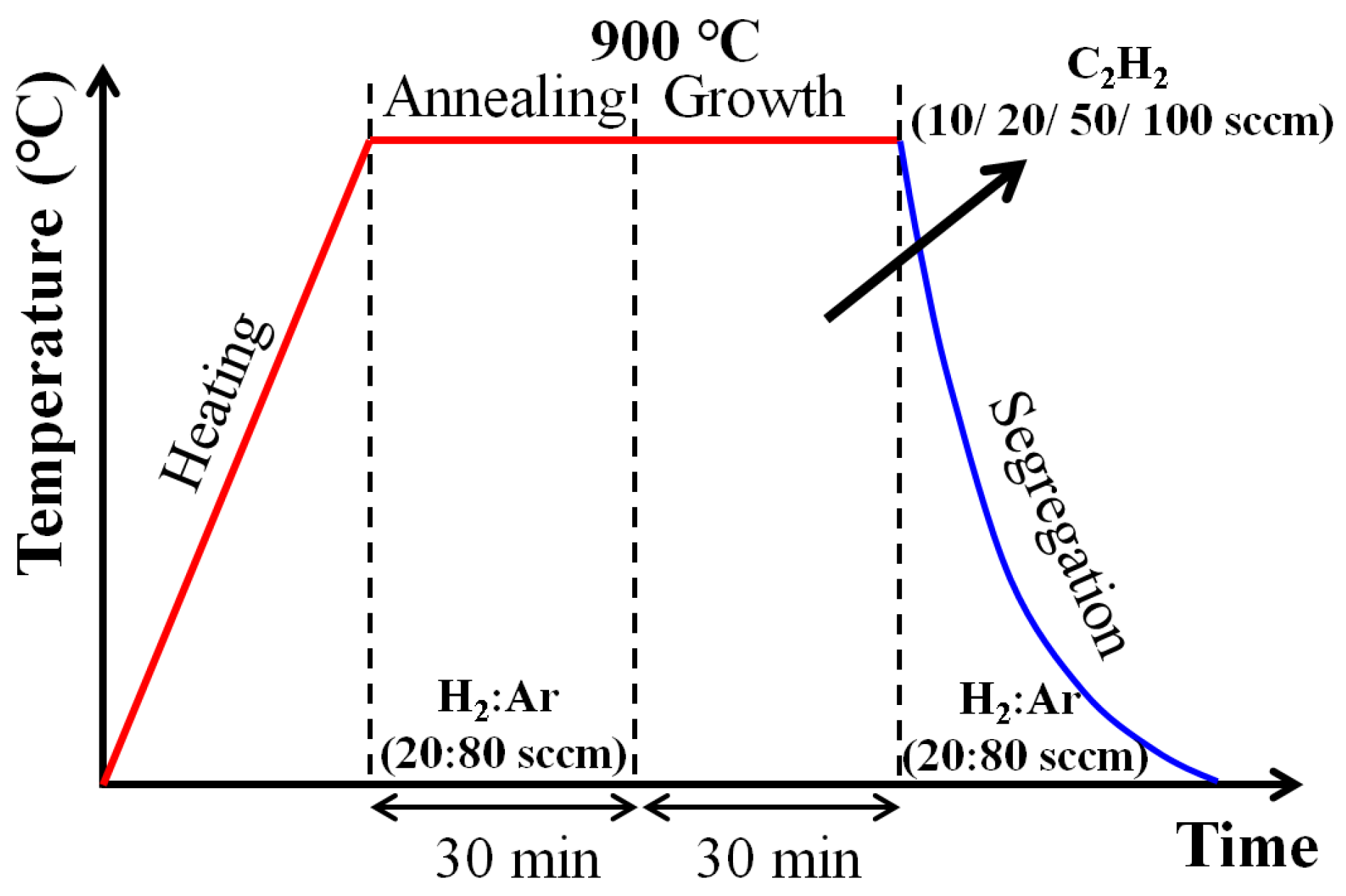
2.1. Graphene Synthesis
2.2. Graphene Characterization
3. Results and Discussion
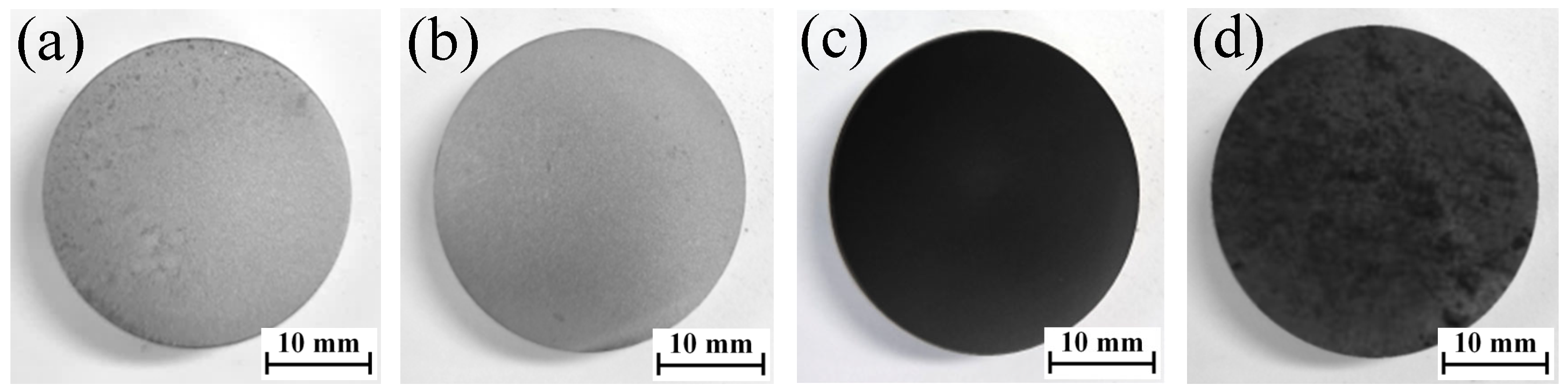
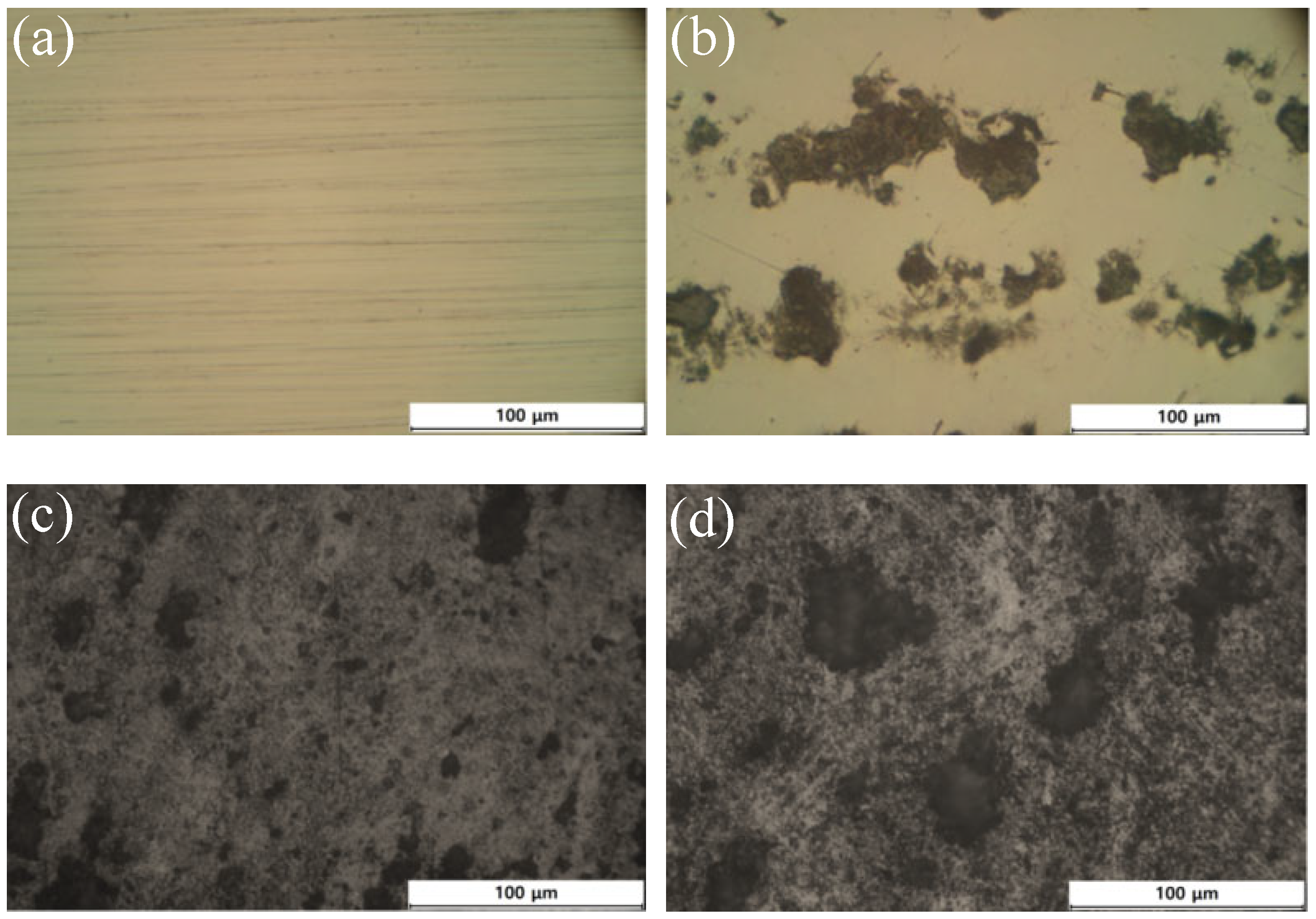
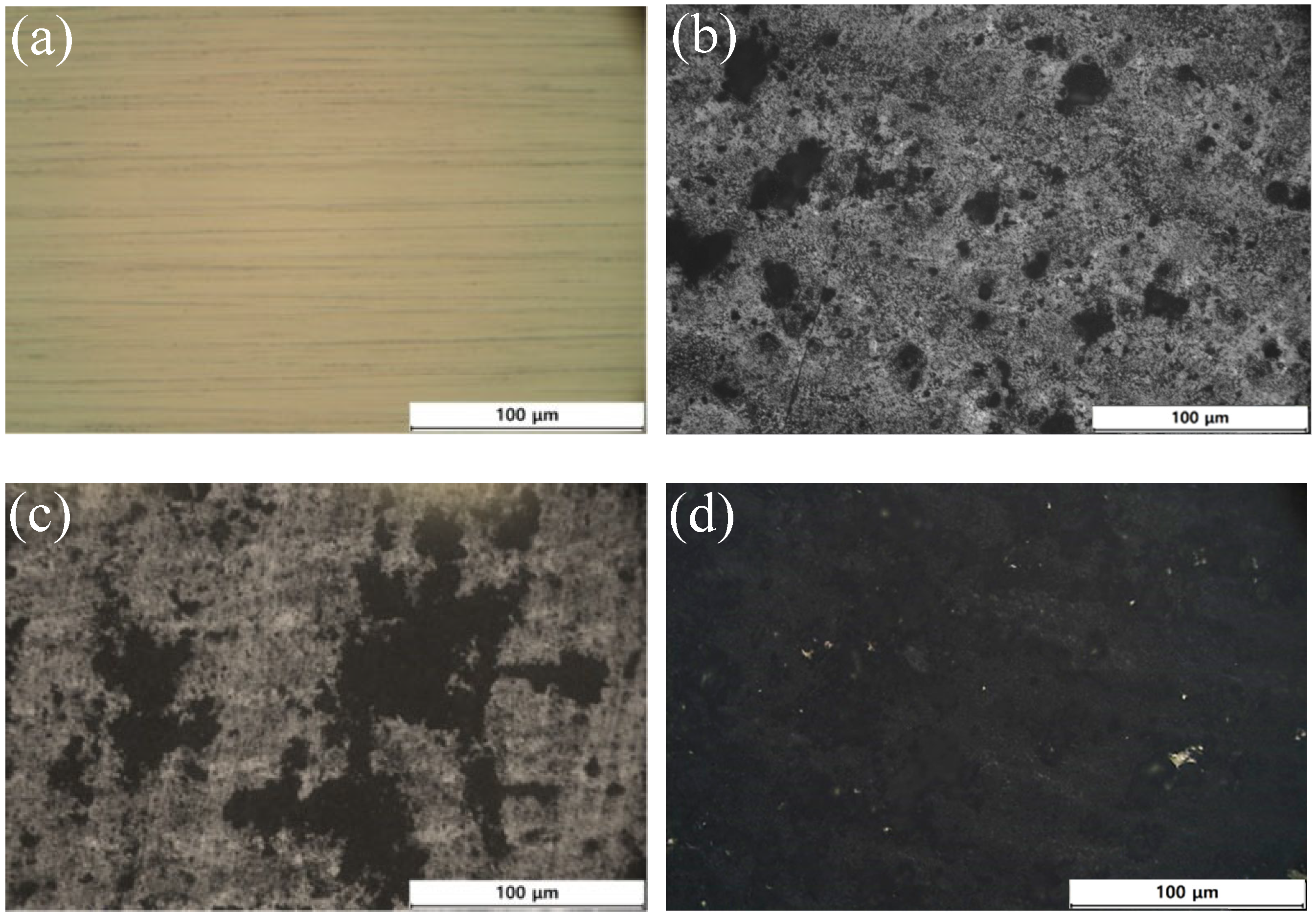
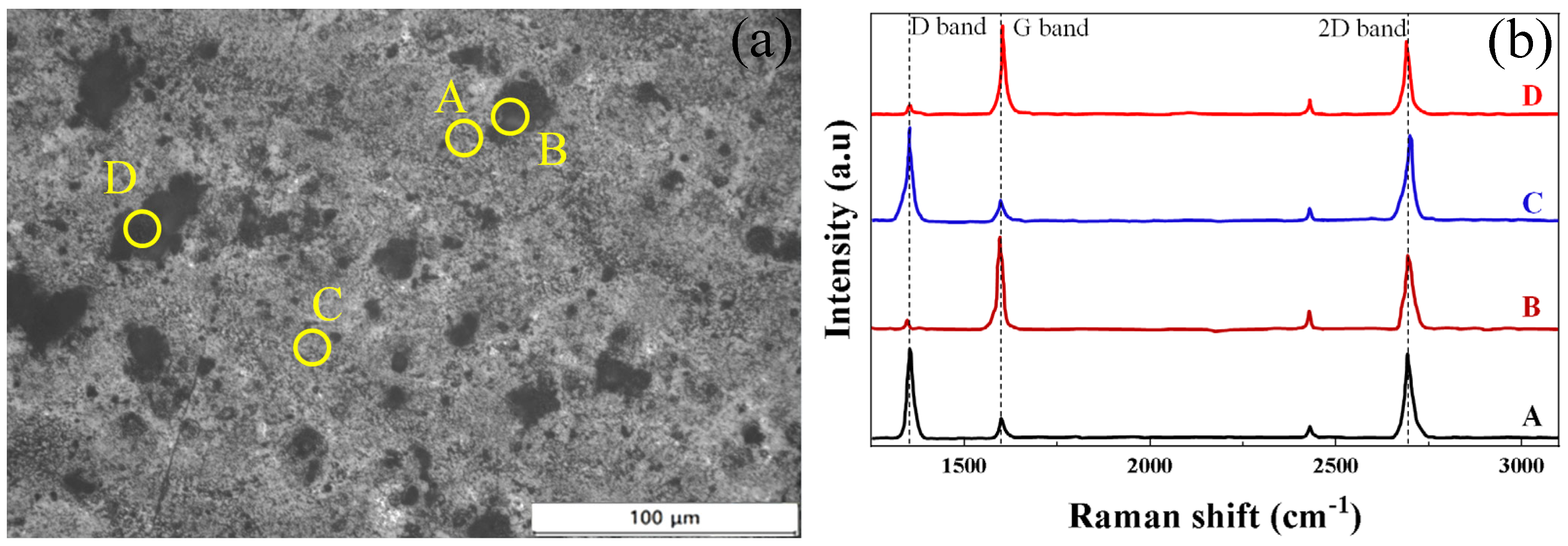
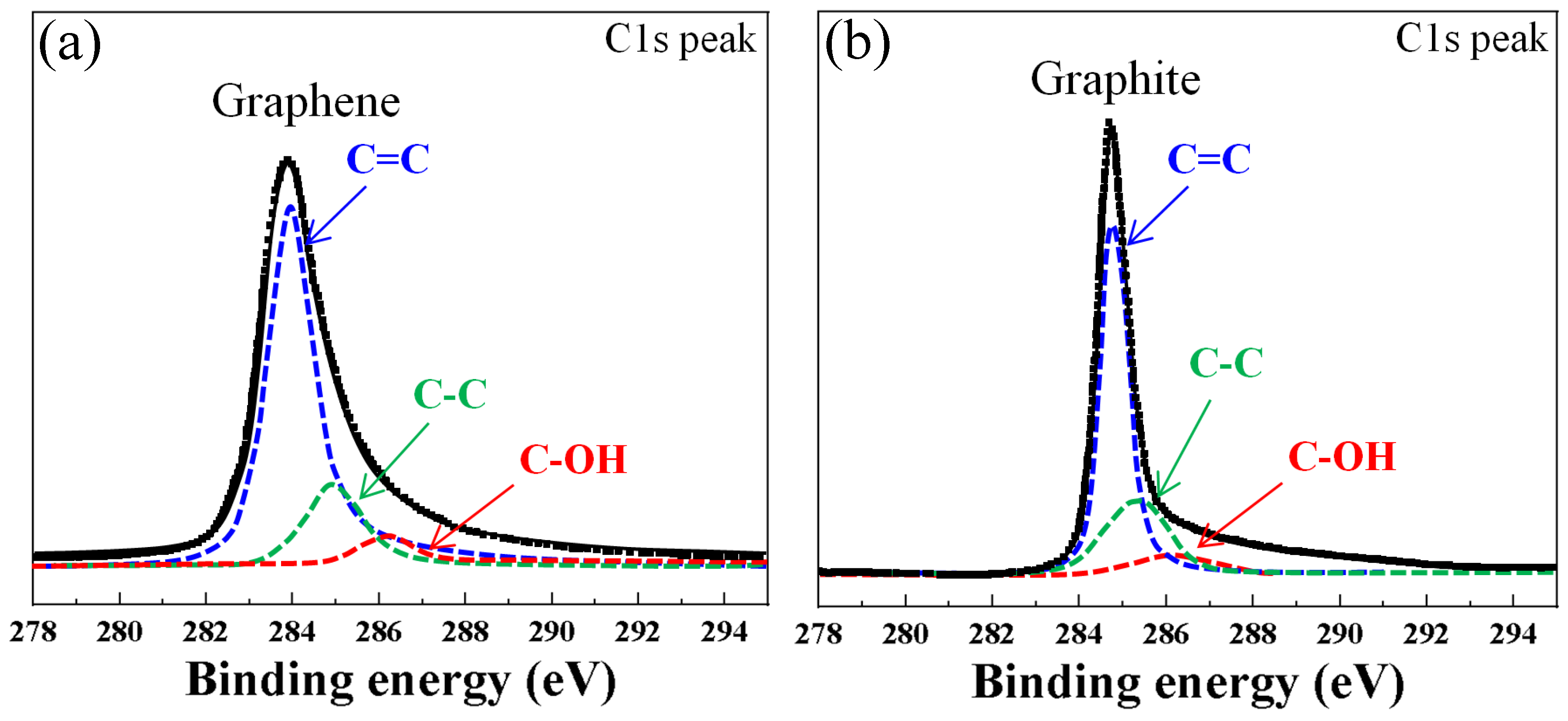
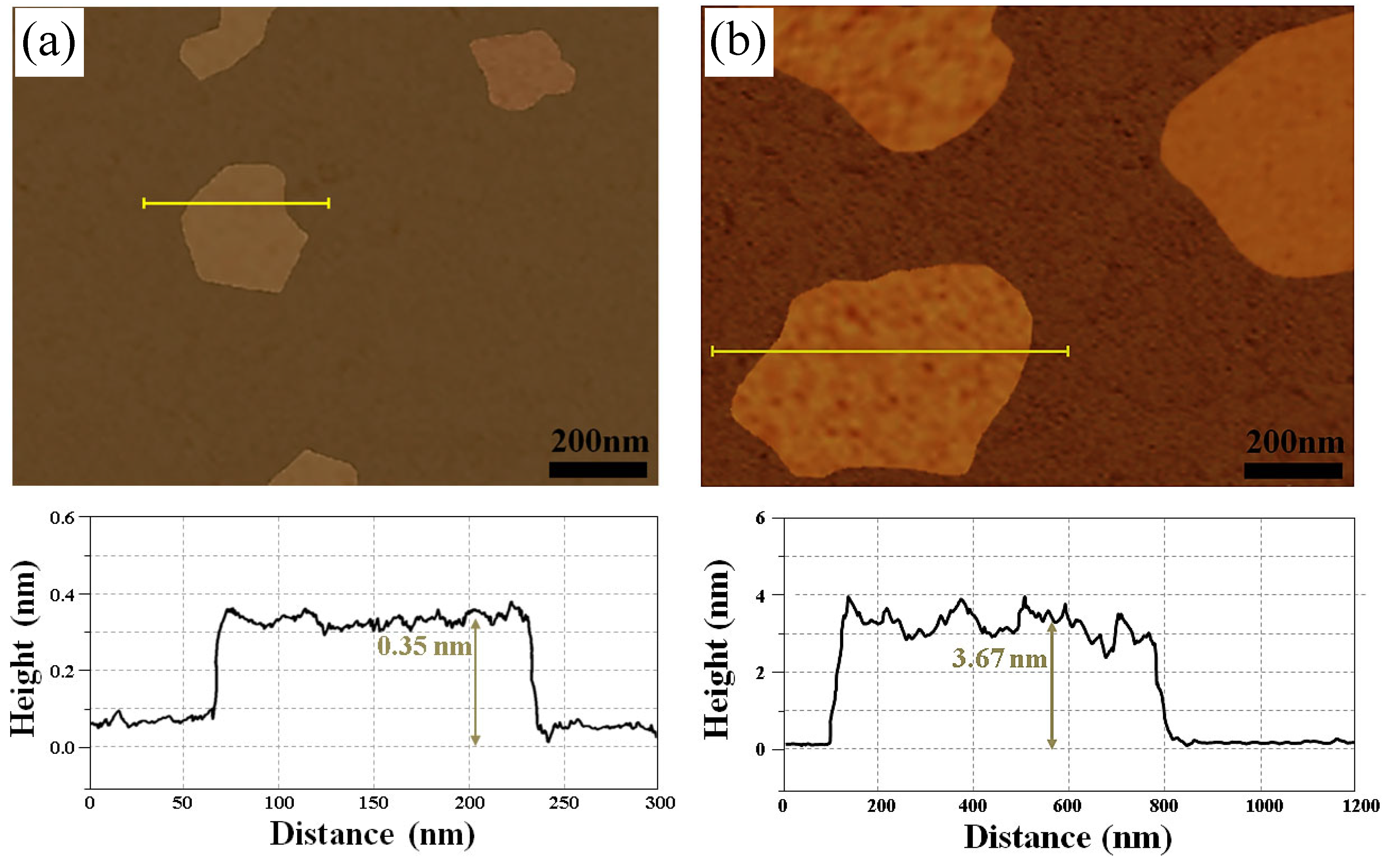
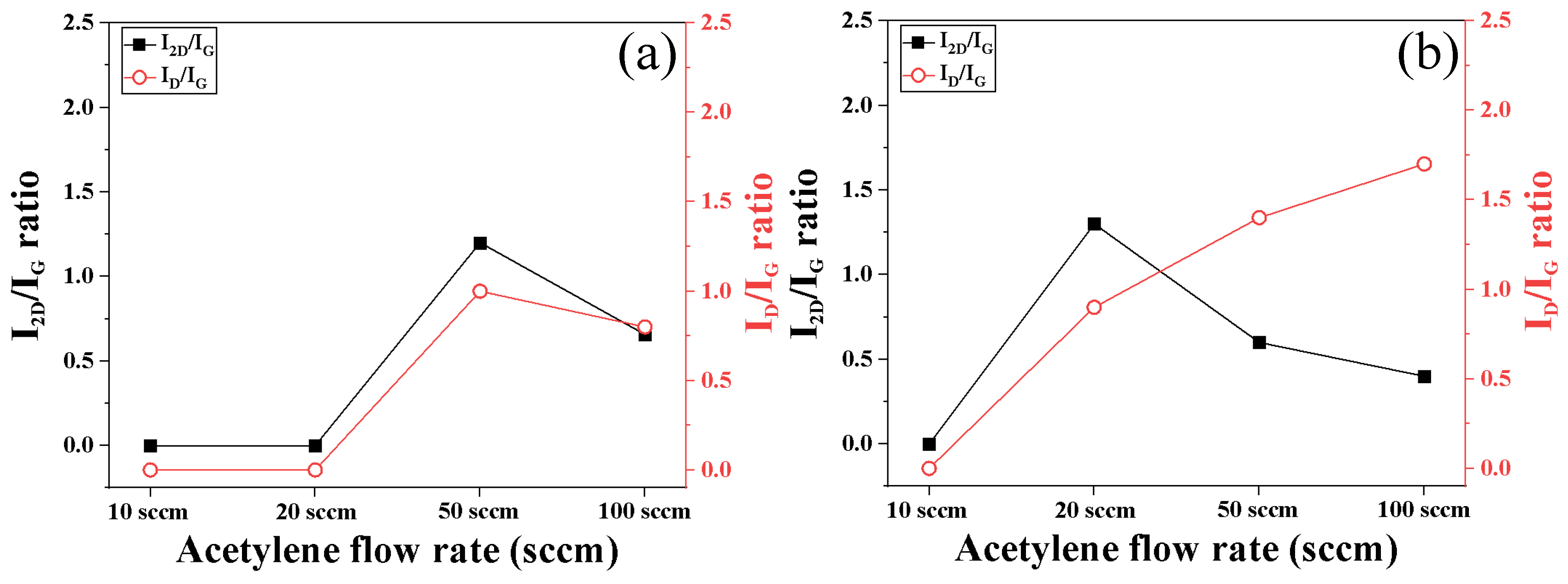
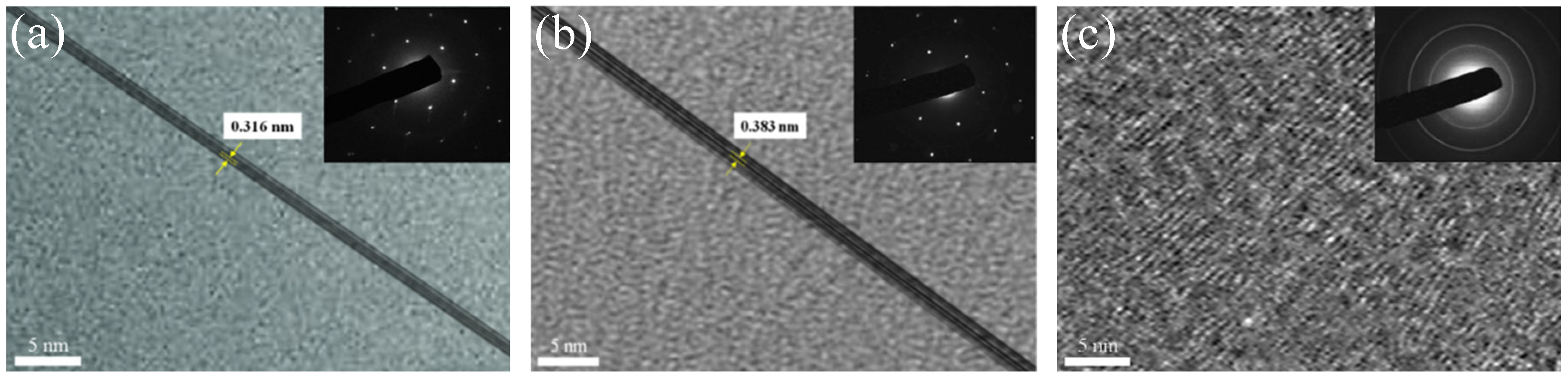
3.1. Surface Morphology
3.2. Mechanical Properties (Hardness)
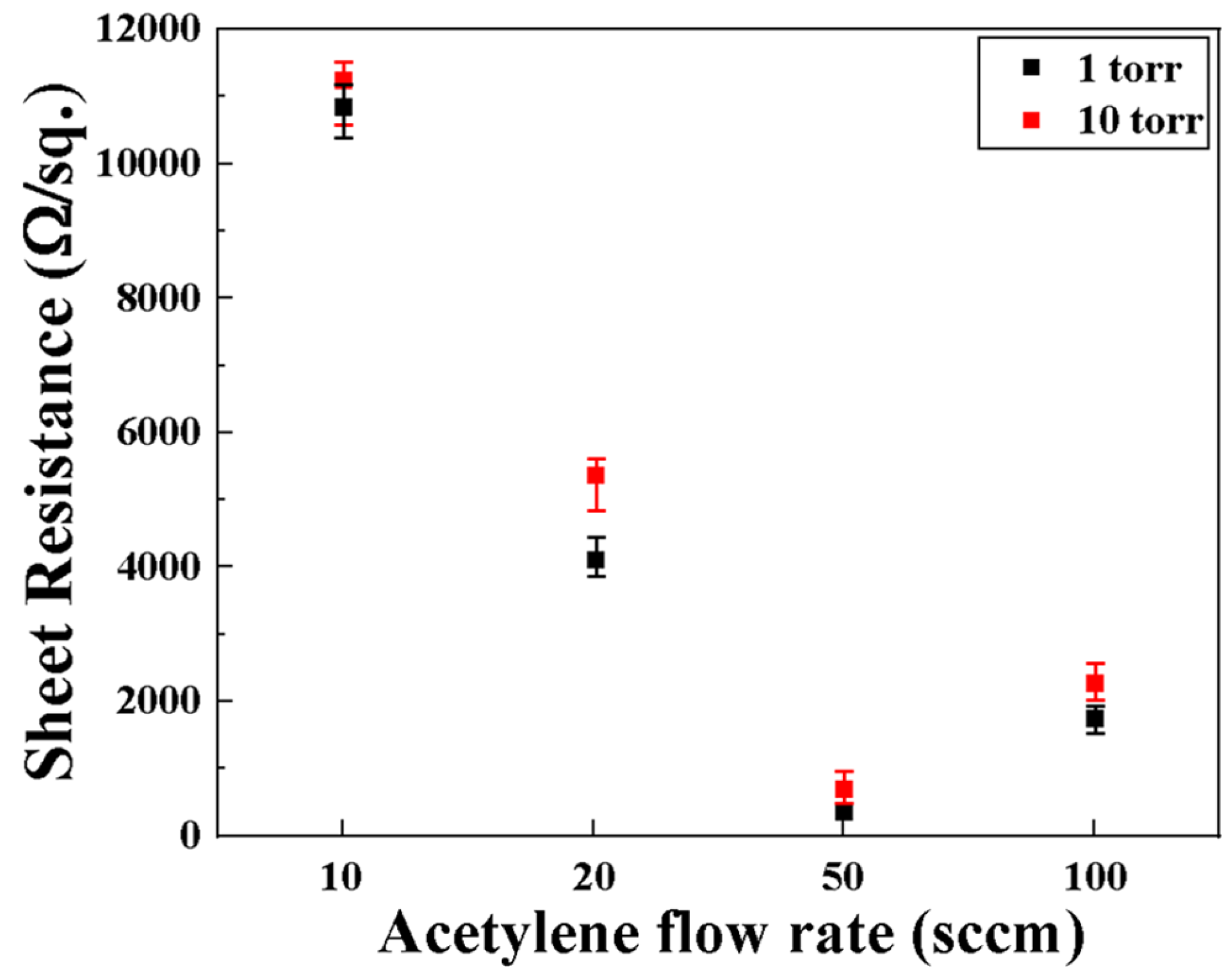
3.3. Electrical Properties (Sheet Resistance)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsovm, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Bhaviripudi, S.; Jia, X.T.; Dresselhaus, M.S.; Kong, J. Role of kinetic factors in chemical vapor deposition synthesis of uniform large area graphene using copper catalyst. Nano Lett. 2010, 10, 4128–4133. [Google Scholar] [CrossRef]
- Sun, B.; Pang, J.; Cheng, Q.; Zhang, S.; Li, Y.; Zhang, C. Synthesis of wafer-scale graphene with chemical vapor deposition for electronic device applications. Adv. Mater. Technol. 2021, 6, 2000744. [Google Scholar] [CrossRef]
- Wang, J.B.; Ren, Z.; Hou, Y.; Yan, X.L.; Liu, P.Z.; Zhang, H. A review of graphene synthesisatlow temperatures by CVD methods. New Carbon Mater. 2020, 35, 193–208. [Google Scholar] [CrossRef]
- Li, C.; Zheng, C.; Cao, F.; Zhang, Y.; Xia, X. The development trend of graphene derivatives. J. Electron. Mater. 2020, 51, 4107–4114. [Google Scholar] [CrossRef]
- Shi, Q.; Tokarska, K.; Ta, H.Q.; Yang, X.; Liu, Y. Substrate developments for the chemical vapor deposition synthesis of graphene. Adv. Mater. Interfaces 2020, 7, 1902024. [Google Scholar] [CrossRef]
- Ballestas, K.; Zapata, J.D.; Ramírez, D. Quantification of coverage, uniformity and residues for CVD monolayer graphene transfer process based on image analysis. Appl. Surf. Sci. 2023, 638, 158074. [Google Scholar] [CrossRef]
- Sutter, P.; Sutter, E. Microscopy of graphene growth, processing, and properties. Adv. Funct. Mater. 2013, 23, 2617–2634. [Google Scholar] [CrossRef]
- Yuan, Z.; He, G.; Faucher, S.; Kuehne, M.; Li, S.X. Direct chemical vapor deposition synthesis of porous single-layer graphene membranes with high gas permeances and selectivities. Adv. Mater. 2020, 33, 2104308. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.S.; Coleman, K.S. Graphene film growth on polycrystalline metals. Acc. Chem. Res. 2013, 46, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Lian, J.; Siriponglert, S.; Li, H.; Chen, Y.P.; Pei, S.S. Graphene segregated on Ni surfaces and transferred to insulators. Appl. Phys. Lett. 2008, 93, 113103–113106. [Google Scholar] [CrossRef]
- Li, N.; Li, D.; Zhen, Z.; Zhang, R.; Mu, R.; Xu, Z. Nucleation and growth of graphene at different temperatures by plasma enhanced chemical vapor deposition. Mater. Today Commun. 2023, 36, 106568. [Google Scholar] [CrossRef]
- Fang, W.; Hsu, A.L.; Song, Y.; Birdwell, A.G.; Amani, M.; Dubey, M.; Dresselhaus, M.S.; Palacios, T.; Kong, J. Asymmetric growth of bilayer graphene on copper enclosures using low-pressure chemical vapor deposition. ACS Nano 2014, 8, 6491–6499. [Google Scholar] [CrossRef]
- Zhao, Z.; Shan, Z.; Zhang, C.; Li, Q.; Tian, B.; Huang, Z.; Lin, W.; Chen, X.; Ji, H.; Zhang, W.; et al. Study on the diffusion mechanism of graphene grown on copper pockets. Small 2015, 11, 1418–1422. [Google Scholar] [CrossRef]
- Addou, R.; Dahal, A.; Sutter, P.; Batzill, M. Monolayer graphene growth on Ni(111) by low temperature chemical vapor deposition. Appl. Phys. Lett. 2012, 100, 21601. [Google Scholar] [CrossRef]
- Ramón, M.E.; Gupta, A.; Corbet, C.; Ferrer, D.A.; Movva, H.C.P.; Carpenter, G.; Colombo, L.; Bourianoff, G.; Doczy, M.; Akinwande, D.; et al. CMOS-compatible synthesis of large-area, high-mobility graphene by chemical vapor deposition of acetylene on cobalt thin films. ACS Nano 2011, 5, 7198–7204. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Ciobanu, C.V.; Petrova, V.; Shenoy, V.B.; Bareño, J.; Gambin, V.; Petrov, I.; Kodambaka, S. Growth of semiconducting graphene on palladium. Nano Lett. 2009, 9, 3985–3990. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Starodub, E.; Kappes, B.B.; Ciobanu, C.V.; Bartelt, N.C.; McCarty, K.F.; Kodambaka, S. Orientation-dependent work function of graphene on Pd (111). Appl. Phys. Lett. 2010, 97, 143114. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, B.; Guo, Y.; Huang, L.; Jiang, L.; Chen, J.; Geng, D.; Liu, Y.; Hu, W.; Yu, G. Synthesis of large-area, few-layer graphene on iron foil by chemical vapor deposition. Nano Res. 2011, 4, 1208–1214. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, E.K.; Joo, W.-J.; Jang, Y.; Kim, B.-S.; Lim, J.Y.; Choi, S.-H.; Ahn, S.J.; Ahn, J.R.; Park, M.-H.; et al. Wafer-scale growth of single-crystal monolayer graphene on reusable hydrogen-terminated germanium. Science 2014, 344, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, M.; Zhu, Y.; Ding, G.; Jiang, D.; Guo, Q.; Liu, S.; Xie, X.; Chu, P.K.; Di, Z.; et al. Direct growth of graphene film on germanium substrate. Sci. Rep. 2013, 3, 2465. [Google Scholar] [CrossRef]
- An, H.; Lee, W.J.; Jung, J. Graphene synthesis on Fe foil using thermal CVD. Curr. Appl. Phys. 2011, 11, S81–S85. [Google Scholar] [CrossRef]
- Chen, W.Q.; Cheong, Y.H.; Fu, X.; Ge, L.Y.; Veksha, A.; Lisak, G. Fe-assisted catalytic chemical vapor deposition of graphene-like carbon nanosheets over SrO. Carbon 2021, 171, 444–454. [Google Scholar] [CrossRef]
- Medina-Llamas, V.L.; Fajardo-Díaz, J.L.; Morelos-Gomez, A.; Endo, M.; López-Urías, F.; Muñoz-Sandoval, E. Crystalline multilayer graphene nanoflakes synthesized by catalytic chemical vapor deposition using reduced nanostructured hematite as catalyst precursor and 1,2-dichlorobenzene and benzylamine mixture as carbon source. Carbon 2023, 203, 813–826. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Bruno, G. Graphene CVD growth on copper and nickel: Role of hydrogen in kinetics and structure. Phys. Chem. Chem. Phys. 2011, 13, 20836–20843. [Google Scholar] [CrossRef]
- Sun, X.; Lin, L.; Sun, L.; Zhang, J.; Rui, D.; Li, J.; Wang, M.; Tan, C.; Kang, N.; Wei, D.; et al. Low-temperature and rapid growth of large single-crystalline graphene with ethane. Small 2018, 14, 1702916. [Google Scholar] [CrossRef]
- Chen, C.S.; Hsieh, C.K. Effects of acetylene flow rate and processing temperature on graphene films grown by thermal chemical vapor deposition. Thin Solid Films 2015, 584, 265–269. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Hbiriq, Y.; Ammar, M.R.; Fantini, C.; Hennet, L.; Zaghrioui, M. Probing symmetry-breaking defects in polished graphitizable s p 2 carbons using angle-resolved polarized Raman scattering. Phys. Rev. B 2023, 107, 134305. [Google Scholar] [CrossRef]
- Haridas, H.; Kader, A.K.A.; Sellathurai, A.; Barz, D.P.; Kontopoulou, M. Noncovalent Functionalization of Graphene Nanoplatelets and Their Applications in Supercapacitors. ACS Appl. Mater. Interfaces 2024, 16, 16630–16640. [Google Scholar] [CrossRef] [PubMed]
- Castriota, M.; Cazzanelli, E.; Pacilè, D.; Papagno, L.; Girit, O.; Meyer, J.C.; Zettl, A.; Giarola, M.; Mariotto, G. Spatial dependence of Raman frequencies in ordered and disordered monolayer graphene. Diamond Relat. Mater. 2010, 19, 608. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Lu, F.; Li, K.; Xu, Y.; Ma, C. Synthesis of turbostratic graphene by direct carbon ions implantation on LiNbO3. Appl. Surf. Sci. 2019, 493, 1255–1259. [Google Scholar] [CrossRef]
- Garlow, J.A.; Barrett, L.K.; Wu, L.; Kisslinger, K.; Zhu, Y.; Pulecio, J.F. Large-Area Growth of Turbostratic Graphene on Ni (111) via Physical Vapor Deposition. Sci. Rep. 2016, 6, 19804. [Google Scholar] [CrossRef]
- Yu, Q.; Jauregui, L.A.; Wu, W.; Colby, R.; Tian, J.; Su, Z.; Cao, H.; Liu, Z.; Pandey, D.; Wei, D.; et al. Control and characterization of individual grains and grain boundaries in graphene grown by chemical vapour deposition. Nat. Mater. 2011, 10, 443–449. [Google Scholar] [CrossRef]



| Transition Metal | Carbon Solubility at 1000 °C | Growth Mechanism |
|---|---|---|
| Copper (Cu) | 0.03 wt.% | Penetration [15,16] |
| Nickel (Ni) | 2.02 wt.% | Surface deposition [17] |
| Cobalt (Co) | 3.41 wt.% | Segregation [18] |
| Platinum (Pd) | 5.98 wt.% | Segregation [19,20] |
| Iron (Fe) | 7.91 wt.% | Segregation [21] |
| Germanium (Ge) | 0.00 wt.% | Surface deposition [22,23] |
| wt.% | C | Si | Mn | P | S | Ni | Cr | Mo |
|---|---|---|---|---|---|---|---|---|
| AISI 1006 | 0.049 | 0.003 | 0.232 | 0.013 | 0.008 | 0.085 | 0.056 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, G.-H.; Choi, B.; Lee, Y.-K.; Moon, K. Influence of the Acetylene Flow Rate and Process Pressure on the Carbon Deposition Behavior by Thermal Chemical Vapor Deposition Process. Crystals 2024, 14, 782. https://doi.org/10.3390/cryst14090782
Kwon G-H, Choi B, Lee Y-K, Moon K. Influence of the Acetylene Flow Rate and Process Pressure on the Carbon Deposition Behavior by Thermal Chemical Vapor Deposition Process. Crystals. 2024; 14(9):782. https://doi.org/10.3390/cryst14090782
Chicago/Turabian StyleKwon, Gi-Hoon, Byoungho Choi, Young-Kook Lee, and Kyoungil Moon. 2024. "Influence of the Acetylene Flow Rate and Process Pressure on the Carbon Deposition Behavior by Thermal Chemical Vapor Deposition Process" Crystals 14, no. 9: 782. https://doi.org/10.3390/cryst14090782






