A Review of Deep-Red (650–700 nm)-Emitting Semiconductor Nanocrystals
Abstract
:1. Introduction
2. Different Types of Deep-Red Nanocrystals
2.1. II-VI Nanocrystals
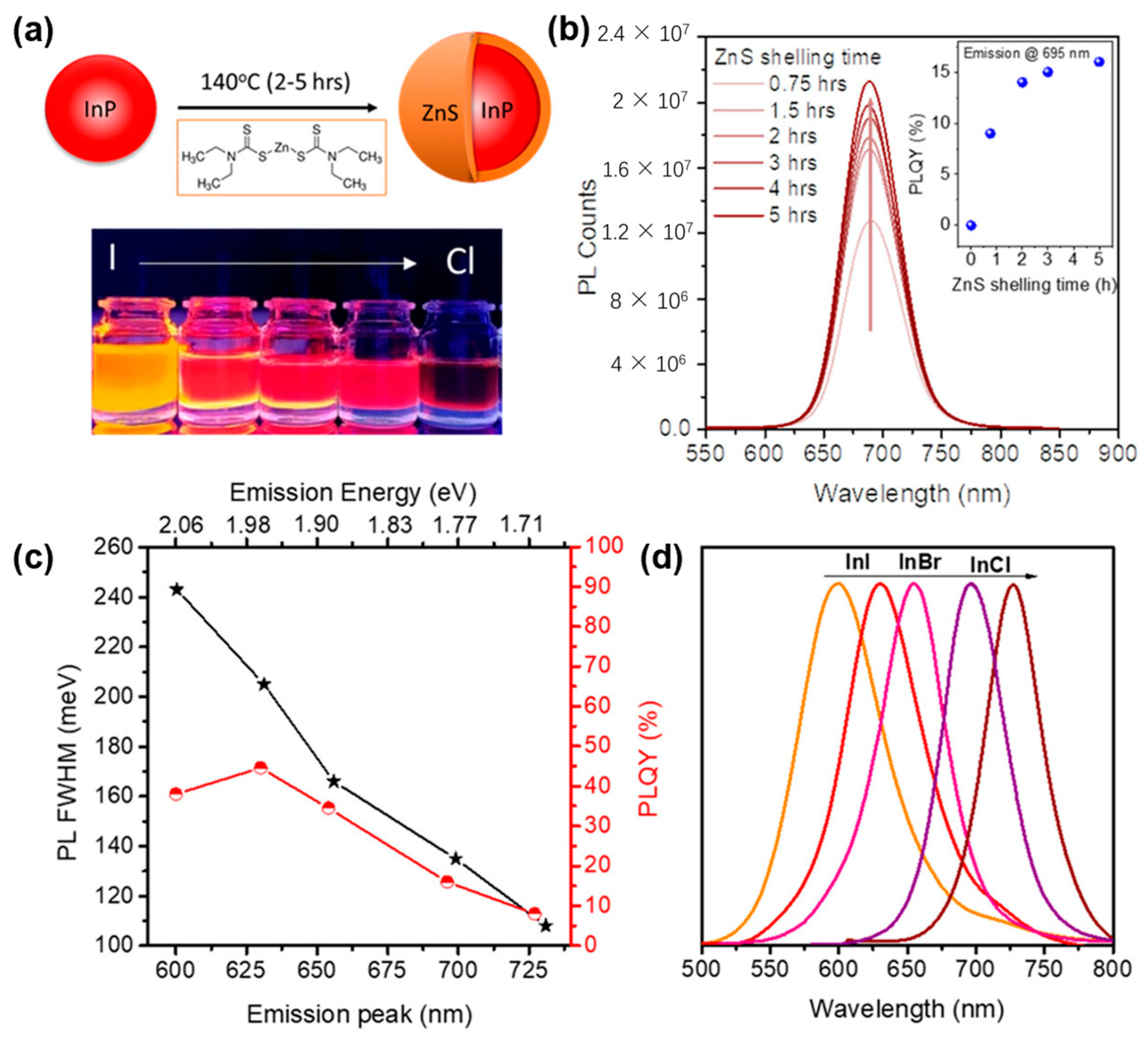
2.2. III-V Nanocrystals
2.3. I-III-VI Nanocrystals
2.4. Perovskite Nanocrystals
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shinhmar, H.; Hogg, C.; Neveu, M.; Jeffery, G. Weeklong Improved Colour Contrasts Sensitivity after Single 670 Nm Exposures Associated with Enhanced Mitochondrial Function. Sci. Rep. 2021, 11, 22872. [Google Scholar] [CrossRef]
- Silverå Ejneby, M.; Jakešová, M.; Ferrero, J.J.; Migliaccio, L.; Sahalianov, I.; Zhao, Z.; Berggren, M.; Khodagholy, D.; Đerek, V.; Gelinas, J.N.; et al. Chronic Electrical Stimulation of Peripheral Nerves via Deep-Red Light Transduced by an Implanted Organic Photocapacitor. Nat. Biomed. Eng. 2022, 6, 741–753. [Google Scholar] [CrossRef]
- Quang, N.V.; Huyen, N.T.; Tu, N.; Trung, D.Q.; Anh, D.D.; Tran, M.T.; Hung, N.D.; Viet, D.X.; Huy, P.T. A High Quantum Efficiency Plant Growth LED by Using a Deep-Red-Emitting α-Al2O3:Cr3+ Phosphor. Dalton Trans. 2021, 50, 12570–12582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, L.; Han, Y.; Li, H.; Ji, Z.; Zhang, Z. Luminescent Properties of Zn2+-Doped CaAl12O19:Mn4+ Deep-Red Phosphor for Indoor Plant Cultivation. Ceram. Int. 2019, 45, 8265–8270. [Google Scholar] [CrossRef]
- Xuan, T.-T.; Liu, J.-Q.; Xie, R.-J.; Li, H.-L.; Sun, Z. Microwave-Assisted Synthesis of CdS/ZnS:Cu Quantum Dots for White Light-Emitting Diodes with High Color Rendition. Chem. Mater. 2015, 27, 1187–1193. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Li, W.; Sun, K. Doped Quantum Dots for White-Light-Emitting Diodes Without Reabsorption of Multiphase Phosphors. Adv. Mater. 2012, 24, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, X.; Molokeev, M.S.; Zheng, H.; Wang, Q.; Xu, C.; Li, J.-G. Modulation of Bi3+ Luminescence from Broadband Green to Broadband Deep Red in Lu2WO6 by Gd3+ Doping and Its Applications in High Color Rendering Index White LED and Near-Infrared LED. Dalton Trans. 2023, 52, 2619–2630. [Google Scholar] [CrossRef]
- Zhu, R.; Luo, Z.; Chen, H.; Dong, Y.; Wu, S.-T. Realizing Rec. 2020 Color Gamut with Quantum Dot Displays. Opt. Express OE 2015, 23, 23680–23693. [Google Scholar] [CrossRef]
- Efros, A.L.; Brus, L.E. Nanocrystal Quantum Dots: From Discovery to Modern Development. ACS Nano 2021, 15, 6192–6210. [Google Scholar] [CrossRef]
- Sowers, K.L.; Swartz, B.; Krauss, T.D. Chemical Mechanisms of Semiconductor Nanocrystal Synthesis. Chem. Mater. 2013, 25, 1351–1362. [Google Scholar] [CrossRef]
- García De Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor Quantum Dots: Technological Progress and Future Challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Zhang, Z.; Ge, J.; Deng, Y.; Chen, X.; Zhang, J.-R.; Deng, Z.; Kambe, Y.; Talapin, D.V.; Wang, Y. Surface Passivation of Intensely Luminescent All-Inorganic Nanocrystals and Their Direct Optical Patterning. Nat. Commun. 2023, 14, 49. [Google Scholar] [CrossRef]
- Zhang, W.; Li, B.; Chang, C.; Chen, F.; Zhang, Q.; Lin, Q.; Wang, L.; Yan, J.; Wang, F.; Chong, Y.; et al. Stable and Efficient Pure Blue Quantum-Dot LEDs Enabled by Inserting an Anti-Oxidation Layer. Nat. Commun. 2024, 15, 783. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Peng, F.; Lu, Y.; Zhu, X.; Jin, W.; Qiu, J.; Dong, J.; Hao, Y.; Di, D.; Gao, Y.; et al. Solution-Processed Green and Blue Quantum-Dot Light-Emitting Diodes with Eliminated Charge Leakage. Nat. Photon. 2022, 16, 505–511. [Google Scholar] [CrossRef]
- Song, J.; Wang, O.; Shen, H.; Lin, Q.; Li, Z.; Wang, L.; Zhang, X.; Li, L.S. Over 30% External Quantum Efficiency Light-Emitting Diodes by Engineering Quantum Dot-Assisted Energy Level Match for Hole Transport Layer. Adv. Funct. Mater. 2019, 29, 1808377. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Gao, Y.; Zeng, Y.; Liu, F.; Shen, H.; Manna, L.; Li, H. Ultrastable and High-Efficiency Deep Red QLEDs through Giant Continuously Graded Colloidal Quantum Dots with Shell Engineering. Nano Lett. 2023, 23, 6689–6697. [Google Scholar] [CrossRef]
- Almeida, G.; Ubbink, R.F.; Stam, M.; Du Fossé, I.; Houtepen, A.J. InP Colloidal Quantum Dots for Visible and Near-Infrared Photonics. Nat. Rev. Mater. 2023, 8, 742–758. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; Zeng, H. Surface Chemistry of All Inorganic Halide Perovskite Nanocrystals: Passivation Mechanism and Stability. Adv. Mater. Interfaces 2018, 5, 1701662. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef]
- Diroll, B.T.; Guzelturk, B.; Po, H.; Dabard, C.; Fu, N.; Makke, L.; Lhuillier, E.; Ithurria, S. 2D II–VI Semiconductor Nanoplatelets: From Material Synthesis to Optoelectronic Integration. Chem. Rev. 2023, 123, 3543–3624. [Google Scholar] [CrossRef]
- Jiang, Y.; Cho, S.-Y.; Shim, M. Light-Emitting Diodes of Colloidal Quantum Dots and Nanorod Heterostructures for Future Emissive Displays. J. Mater. Chem. C 2018, 6, 2618–2634. [Google Scholar] [CrossRef]
- Jia, G.; Pang, Y.; Ning, J.; Banin, U.; Ji, B. Heavy-Metal-Free Colloidal Semiconductor Nanorods: Recent Advances and Future Perspectives. Adv. Mater. 2019, 31, 1900781. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse CdE (E = Sulfur, Selenium, Tellurium) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental Determination of the Extinction Coefficient of CdTe, CdSe, and CdS Nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Bailey, R.E.; Nie, S. Alloyed Semiconductor Quantum Dots: Tuning the Optical Properties without Changing the Particle Size. J. Am. Chem. Soc. 2003, 125, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Singhal, A.; Zheng, J.; Wang, C.; Chan, W.C.W. Optimizing the Synthesis of Red- to Near-IR-Emitting CdS-Capped CdTexSe1-x Alloyed Quantum Dots for Biomedical Imaging. Chem. Mater. 2006, 18, 4845–4854. [Google Scholar] [CrossRef]
- Mao, W.; Guo, J.; Yang, W.; Wang, C.; He, J.; Chen, J. Synthesis of High-Quality near-Infrared-Emitting CdTeS Alloyed Quantum Dots via the Hydrothermal Method. Nanotechnology 2007, 18, 485611. [Google Scholar] [CrossRef]
- Bang, J.H.; Suh, W.H.; Suslick, K.S. Quantum Dots from Chemical Aerosol Flow Synthesis: Preparation, Characterization, and Cellular Imaging. Chem. Mater. 2008, 20, 4033–4038. [Google Scholar] [CrossRef]
- Gurusinghe, N.P.; Hewa-Kasakarage, N.N.; Zamkov, M. Composition-Tunable Properties of CdSxTe1−x Alloy Nanocrystals. J. Phys. Chem. C 2008, 112, 12795–12800. [Google Scholar] [CrossRef]
- Pons, T.; Lequeux, N.; Mahler, B.; Sasnouski, S.; Fragola, A.; Dubertret, B. Synthesis of Near-Infrared-Emitting, Water-Soluble CdTeSe/CdZnS Core/Shell Quantum Dots. Chem. Mater. 2009, 21, 1418–1424. [Google Scholar] [CrossRef]
- Liang, G.-X.; Li, L.-L.; Liu, H.-Y.; Zhang, J.-R.; Burda, C.; Zhu, J.-J. Fabrication of Near-Infrared-Emitting CdSeTe/ZnS Core/Shell Quantum Dots and Their Electrogenerated Chemiluminescence. Chem. Commun. 2010, 46, 2974–2976. [Google Scholar] [CrossRef] [PubMed]
- Kunstman, P.; Coulon, J.; Kolmykov, O.; Moussa, H.; Balan, L.; Medjahdi, G.; Lulek, J.; Schneider, R. One Step Synthesis of Bright Luminescent Core/Shell CdTexS1−x/ZnS Quantum Dots Emitting from the Visible to the near Infrared. J. Lumin. 2018, 194, 760–767. [Google Scholar] [CrossRef]
- Loghina, L.; Houdek, J.; Slang, S.; Frumarova, B.; Cieslar, M.; Vlcek, M. Disubstituted Thiourea as a Suitable Sulfur Source in the Gram-Scale Synthesis of Yellow- and Red-Emitting CdTeS/CdxZn1−x S Core/Shell Quantum Dots. Nanoscale Adv. 2024, 6, 3377–3390. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Mohs, A.M.; Nie, S. Tuning the Optical and Electronic Properties of Colloidal Nanocrystals by Lattice Strain. Nat. Nanotech 2009, 4, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; He, Z.; Chan, W.H.; Choi, M.M.F. Synthesis and Characterization of High-Quality Water-Soluble Near-Infrared-Emitting CdTe/CdS Quantum Dots Capped by N-Acetyl-l-Cysteine Via Hydrothermal Method. J. Phys. Chem. C 2009, 113, 1293–1300. [Google Scholar] [CrossRef]
- Dai, M.-Q.; Zheng, W.; Huang, Z.; Yung, L.-Y.L. Aqueous Phase Synthesis of Widely Tunable Photoluminescence Emission CdTe/CdS Core/Shell Quantum Dots under a Totally Ambient Atmosphere. J. Mater. Chem. 2012, 22, 16336–16345. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Feng, Y.; Zhong, X. Scalable Single-Step Noninjection Synthesis of High-Quality Core/Shell Quantum Dots with Emission Tunable from Violet to Near Infrared. ACS Nano 2012, 6, 11066–11073. [Google Scholar] [CrossRef]
- Ulusoy, M.; Walter, J.-G.; Lavrentieva, A.; Kretschmer, I.; Sandiford, L.; Marois, A.L.; Bongartz, R.; Aliuos, P.; Suhling, K.; Stahl, F.; et al. One-Pot Aqueous Synthesis of Highly Strained CdTe/CdS/ZnS Nanocrystals and Their Interactions with Cells. RSC Adv. 2014, 5, 7485–7494. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, G.; Wang, J.; Ye, B.-C.; Zhong, X. Design and Synthesis of Highly Luminescent Near-Infrared-Emitting Water-Soluble CdTe/CdSe/ZnS Core/Shell/Shell Quantum Dots. Inorg. Chem. 2009, 48, 9723–9731. [Google Scholar] [CrossRef]
- Lin, Q.; Song, B.; Wang, H.; Zhang, F.; Chen, F.; Wang, L.; Li, L.S.; Guo, F.; Shen, H. High-Efficiency Deep-Red Quantum-Dot Light-Emitting Diodes with Type-II CdTe/CdSe Core/Shell Quantum Dots as Emissive Layers. J. Mater. Chem. C 2016, 4, 7223–7229. [Google Scholar] [CrossRef]
- Li, F.; You, L.; Nie, C.; Zhang, Q.; Jin, X.; Li, H.; Gu, X.; Huang, Y.; Li, Q. Quantum Dot White Light Emitting Diodes with High Scotopic/Photopic Ratios. Opt. Express OE 2017, 25, 21901–21913. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Xie, R.; Zhang, Y.; Basché, T.; Knoll, W. High-Quality Violet- to Red-Emitting ZnSe/CdSe Core/Shell Nanocrystals. Chem. Mater. 2005, 17, 4038–4042. [Google Scholar] [CrossRef]
- Srivastava, B.B.; Jana, S.; Pradhan, N. Doping Cu in Semiconductor Nanocrystals: Some Old and Some New Physical Insights. J. Am. Chem. Soc. 2011, 133, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Di, Q.; Zhu, X.; Liu, J.; Zhang, X.; Shang, H.; Chen, W.; Liu, J.; Rong, H.; Xu, M.; Zhang, J. High-Performance Quantum Dots with Synergistic Doping and Oxide Shell Protection Synthesized by Cation Exchange Conversion of Ternary-Composition Nanoparticles. J. Phys. Chem. Lett. 2019, 10, 2606–2615. [Google Scholar] [CrossRef] [PubMed]
- Polovitsyn, A.; Khan, A.H.; Angeloni, I.; Grim, J.Q.; Planelles, J.; Climente, J.I.; Moreels, I. Synthesis of Anisotropic CdSe/CdS Dot-in-Giant-Rod Nanocrystals with Persistent Blue-Shifted Biexciton Emission. ACS Photonics 2018, 5, 4561–4568. [Google Scholar] [CrossRef]
- Jin, G.; Zeng, Y.; Liu, X.; Wang, Q.; Wei, J.; Liu, F.; Li, H. Synthesis and Optical Properties of CdSeTe/CdZnS/ZnS Core/Shell Nanorods. Nanomaterials 2024, 14, 989. [Google Scholar] [CrossRef]
- Rossinelli, A.A.; Riedinger, A.; Marqués-Gallego, P.; Knüsel, P.N.; Antolinez, F.V.; Norris, D.J. High-Temperature Growth of Thick-Shell CdSe/CdS Core/Shell Nanoplatelets. Chem. Commun. 2017, 53, 9938–9941. [Google Scholar] [CrossRef]
- Rossinelli, A.A.; Rojo, H.; Mule, A.S.; Aellen, M.; Cocina, A.; De Leo, E.; Schäublin, R.; Norris, D.J. Compositional Grading for Efficient and Narrowband Emission in CdSe-Based Core/Shell Nanoplatelets. Chem. Mater. 2019, 31, 9567–9578. [Google Scholar] [CrossRef]
- Hu, S.; Shabani, F.; Liu, B.; Zhang, L.; Guo, M.; Lu, G.; Zhou, Z.; Wang, J.; Huang, J.C.; Min, Y.; et al. High-Performance Deep Red Colloidal Quantum Well Light-Emitting Diodes Enabled by the Understanding of Charge Dynamics. ACS Nano 2022, 16, 10840–10851. [Google Scholar] [CrossRef]
- Shabani, F.; Dehghanpour Baruj, H.; Yurdakul, I.; Delikanli, S.; Gheshlaghi, N.; Isik, F.; Liu, B.; Altintas, Y.; Canımkurbey, B.; Demir, H.V. Deep-Red-Emitting Colloidal Quantum Well Light-Emitting Diodes Enabled through a Complex Design of Core/Crown/Double Shell Heterostructure. Small 2022, 18, 2106115. [Google Scholar] [CrossRef]
- Chin, P.T.K.; de Mello Donegá, C.; van Bavel, S.S.; Meskers, S.C.J.; Sommerdijk, N.A.J.M.; Janssen, R.A.J. Highly Luminescent CdTe/CdSe Colloidal Heteronanocrystals with Temperature-Dependent Emission Color. J. Am. Chem. Soc. 2007, 129, 14880–14886. [Google Scholar] [CrossRef]
- Green, M.; Williamson, P.; Samalova, M.; Davis, J.; Brovelli, S.; Dobson, P.; Cacialli, F. Synthesis of Type II/Type I CdTe/CdS/ZnS Quantum Dots and Their Use in Cellular Imaging. J. Mater. Chem. 2009, 19, 8341–8346. [Google Scholar] [CrossRef]
- Mallem, K.; Prodanov, M.F.; Dezhang, C.; Marus, M.; Kang, C.; Shivarudraiah, S.B.; Vashchenko, V.V.; Halpert, J.E.; Srivastava, A.K. Solution-Processed Red, Green, and Blue Quantum Rod Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2022, 14, 18723–18735. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Olutas, M.; Yeltik, A.; Kelestemur, Y.; Sharma, A.; Delikanli, S.; Guzelturk, B.; Gungor, K.; McBride, J.R.; Demir, H.V. Understanding the Journey of Dopant Copper Ions in Atomically Flat Colloidal Nanocrystals of CdSe Nanoplatelets Using Partial Cation Exchange Reactions. Chem. Mater. 2018, 30, 3265–3275. [Google Scholar] [CrossRef]
- Khan, A.H.; Pinchetti, V.; Tanghe, I.; Dang, Z.; Martín-García, B.; Hens, Z.; Van Thourhout, D.; Geiregat, P.; Brovelli, S.; Moreels, I. Tunable and Efficient Red to Near-Infrared Photoluminescence by Synergistic Exploitation of Core and Surface Silver Doping of CdSe Nanoplatelets. Chem. Mater. 2019, 31, 1450–1459. [Google Scholar] [CrossRef]
- Najafi, A.; Sharma, M.; Delikanli, S.; Bhattacharya, A.; Murphy, J.R.; Pientka, J.; Sharma, A.; Quinn, A.P.; Erdem, O.; Kattel, S.; et al. Light-Induced Paramagnetism in Colloidal Ag+-Doped CdSe Nanoplatelets. J. Phys. Chem. Lett. 2021, 12, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.-T.; Ding, H.; Roy, I.; Law, W.-C.; Bergey, E.J.; Maitra, A.; Prasad, P.N. Imaging Pancreatic Cancer Using Bioconjugated InP Quantum Dots. ACS Nano 2009, 3, 502–510. [Google Scholar] [CrossRef]
- Liu, P.; Lou, Y.; Ding, S.; Zhang, W.; Wu, Z.; Yang, H.; Xu, B.; Wang, K.; Sun, X.W. Green InP/ZnSeS/ZnS Core Multi-Shelled Quantum Dots Synthesized with Aminophosphine for Effective Display Applications. Adv. Funct. Mater. 2021, 31, 2008453. [Google Scholar] [CrossRef]
- Shim, H.S.; Ko, M.; Nam, S.; Oh, J.H.; Jeong, S.; Yang, Y.; Park, S.M.; Do, Y.R.; Song, J.K. InP/ZnSeS/ZnS Quantum Dots with High Quantum Yield and Color Purity for Display Devices. ACS Appl. Nano Mater. 2023, 6, 1285–1294. [Google Scholar] [CrossRef]
- Micic, O.I.; Curtis, C.J.; Jones, K.M.; Sprague, J.R.; Nozik, A.J. Synthesis and Characterization of InP Quantum Dots. J. Phys. Chem. 1994, 98, 4966–4969. [Google Scholar] [CrossRef]
- Guzelian, A.A.; Katari, J.E.B.; Kadavanich, A.V.; Banin, U.; Hamad, K.; Juban, E.; Alivisatos, A.P.; Wolters, R.H.; Arnold, C.C.; Heath, J.R. Synthesis of Size-Selected, Surface-Passivated InP Nanocrystals. J. Phys. Chem. 1996, 100, 7212–7219. [Google Scholar] [CrossRef]
- Mićić, O.I.; Sprague, J.; Lu, Z.; Nozik, A.J. Highly Efficient Band-Edge Emission from InP Quantum Dots. Appl. Phys. Lett. 1996, 68, 3150–3152. [Google Scholar] [CrossRef]
- Adam, S.; Talapin, D.V.; Borchert, H.; Lobo, A.; McGinley, C.; De Castro, A.R.B.; Haase, M.; Weller, H.; Möller, T. The Effect of Nanocrystal Surface Structure on the Luminescence Properties: Photoemission Study of HF-Etched InP Nanocrystals. J. Chem. Phys. 2005, 123, 084706. [Google Scholar] [CrossRef]
- Chen, B.; Li, D.; Wang, F. InP Quantum Dots: Synthesis and Lighting Applications. Small 2020, 16, 2002454. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wang, S.; Wang, F.; Wu, Q.; Zhao, D.; Yang, X. A Layer-by-Layer Growth Strategy for Large-Size InP/ZnSe/ZnS Core–Shell Quantum Dots Enabling High-Efficiency Light-Emitting Diodes. Chem. Mater. 2018, 30, 8002–8007. [Google Scholar] [CrossRef]
- Sun, Z.; Hou, Q.; Kong, J.; Wang, K.; Zhang, R.; Liu, F.; Ning, J.; Tang, J.; Du, Z. Surface Passivation toward Multiple Inherent Dangling Bonds in Indium Phosphide Quantum Dots. Inorg. Chem. 2024, 63, 6396–6407. [Google Scholar] [CrossRef]
- Kim, T.-G.; Zherebetskyy, D.; Bekenstein, Y.; Oh, M.H.; Wang, L.-W.; Jang, E.; Alivisatos, A.P. Trap Passivation in Indium-Based Quantum Dots through Surface Fluorination: Mechanism and Applications. ACS Nano 2018, 12, 11529–11540. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Gaponik, N.; Borchert, H.; Rogach, A.L.; Haase, M.; Weller, H. Etching of Colloidal InP Nanocrystals with Fluorides: Photochemical Nature of the Process Resulting in High Photoluminescence Efficiency. J. Phys. Chem. B 2002, 106, 12659–12663. [Google Scholar] [CrossRef]
- Stone, D.; Li, X.; Naor, T.; Dai, J.; Remennik, S.; Banin, U. Size and Emission Control of Wurtzite InP Nanocrystals Synthesized from Cu3−xP by Cation Exchange. Chem. Mater. 2023, 35, 10594–10605. [Google Scholar] [CrossRef]
- Yadav, R.; Kwon, Y.; Rivaux, C.; Saint-Pierre, C.; Ling, W.L.; Reiss, P. Narrow Near-Infrared Emission from InP QDs Synthesized with Indium(I) Halides and Aminophosphine. J. Am. Chem. Soc. 2023, 145, 5970–5981. [Google Scholar] [CrossRef]
- Baek, J.; Shen, Y.; Lignos, I.; Bawendi, M.G.; Jensen, K.F. Multistage Microfluidic Platform for the Continuous Synthesis of III–V Core/Shell Quantum Dots. Angew. Chem. Int. Ed. 2018, 57, 10915–10918. [Google Scholar] [CrossRef] [PubMed]
- Achorn, O.B.; Franke, D.; Bawendi, M.G. Seedless Continuous Injection Synthesis of Indium Phosphide Quantum Dots as a Route to Large Size and Low Size Dispersity. Chem. Mater. 2020, 32, 6532–6539. [Google Scholar] [CrossRef]
- Huang, P.; Liu, X.; Jin, G.; Liu, F.; Shen, H.; Li, H. Deep-Red InP Core-Multishell Quantum Dots for Highly Bright and Efficient Light-Emitting Diodes. Adv. Opt. Mater. 2023, 11, 2300612. [Google Scholar] [CrossRef]
- Soheyli, E.; Biçer, A.; Ozel, S.S.; Sahin Tiras, K.; Mutlugun, E. Tuning the Shades of Red Emission in InP/ZnSe/ZnS Nanocrystals with Narrow Full Width for Fabrication of Light-Emitting Diodes. ACS Omega 2023, 8, 39690–39698. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Yadav, R.; Rivaux, C.; Aldakov, D.; Reiss, P. Water-Soluble Alumina-Coated Indium Phosphide Core–Shell Quantum Dots with Efficient Deep-Red Emission Beyond 700 Nm. Small 2024, 2404426. [Google Scholar] [CrossRef]
- Saeboe, A.M.; Nikiforov, A.Y.; Toufanian, R.; Kays, J.C.; Chern, M.; Casas, J.P.; Han, K.; Piryatinski, A.; Jones, D.; Dennis, A.M. Extending the Near-Infrared Emission Range of Indium Phosphide Quantum Dots for Multiplexed In Vivo Imaging. Nano Lett. 2021, 21, 3271–3279. [Google Scholar] [CrossRef]
- Xie, R.; Peng, X. Synthesis of Cu-Doped InP Nanocrystals (d-Dots) with ZnSe Diffusion Barrier as Efficient and Color-Tunable NIR Emitters. J. Am. Chem. Soc. 2009, 131, 10645–10651. [Google Scholar] [CrossRef]
- Lim, M.; Lee, W.; Bang, G.; Lee, W.J.; Park, Y.; Kwon, Y.; Jung, Y.; Kim, S.; Bang, J. Synthesis of Far-Red- and near-Infrared-Emitting Cu-Doped InP/ZnS (Core/Shell) Quantum Dots with Controlled Doping Steps and Their Surface Functionalization for Bioconjugation. Nanoscale 2019, 11, 10463–10471. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.S.; Wedel, A.; Yoon, S.-Y.; Jo, J.-H.; Kim, H.-M.; Han, C.-J.; Song, H.-J.; Yi, J.-M.; Jang, J.-S.; et al. Highly Luminescent Near-Infrared Cu-Doped InP Quantum Dots with a Zn–Cu–In–S/ZnS Double Shell Scheme. J. Mater. Chem. C 2021, 9, 4330–4337. [Google Scholar] [CrossRef]
- Eren, G.O.; Onal, A.; Karatum, O.; Jahangiri, H.; Ozer, M.S.; Eroglu, Z.; Sundu, B.; Kaya, L.; Burcak, A.B.; Aydemir, U.; et al. Ruthenium-Doped InP Quantum Dots for Efficient Red Emission. ACS Appl. Nano Mater. 2023, 6, 10044–10053. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, D.; Leck, K.S.; Tan, S.T.; Tang, Y.X.; Zhao, J.; Demir, H.V.; Sun, X.W. Full Visible Range Covering InP/ZnS Nanocrystals with High Photometric Performance and Their Application to White Quantum Dot Light-Emitting Diodes. Adv. Mater. 2012, 24, 4180–4185. [Google Scholar] [CrossRef] [PubMed]
- Buffard, A.; Dreyfuss, S.; Nadal, B.; Heuclin, H.; Xu, X.; Patriarche, G.; Mézailles, N.; Dubertret, B. Mechanistic Insight and Optimization of InP Nanocrystals Synthesized with Aminophosphines. Chem. Mater. 2016, 28, 5925–5934. [Google Scholar] [CrossRef]
- Van Avermaet, H.; Schiettecatte, P.; Hinz, S.; Giordano, L.; Ferrari, F.; Nayral, C.; Delpech, F.; Maultzsch, J.; Lange, H.; Hens, Z. Full-Spectrum InP-Based Quantum Dots with Near-Unity Photoluminescence Quantum Efficiency. ACS Nano 2022, 16, 9701–9712. [Google Scholar] [CrossRef]
- Won, Y.-H.; Cho, O.; Kim, T.; Chung, D.-Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly Efficient and Stable InP/ZnSe/ZnS Quantum Dot Light-Emitting Diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jo, J.-H.; Yoon, S.-Y.; Jo, D.-Y.; Kim, H.-S.; Park, B.; Yang, H. Emission Enhancement of Cu-Doped InP Quantum Dots through Double Shelling Scheme. Materials 2019, 12, 2267. [Google Scholar] [CrossRef] [PubMed]
- Aboulaich, A.; Michalska, M.; Schneider, R.; Potdevin, A.; Deschamps, J.; Deloncle, R.; Chadeyron, G.; Mahiou, R. Ce-Doped YAG Nanophosphor and Red Emitting CuInS2/ZnS Core/Shell Quantum Dots for Warm White Light-Emitting Diode with High Color Rendering Index. ACS Appl. Mater. Interfaces 2014, 6, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Huang, Y.; Pan, Q.; Tao, T.; Li, F.; Zhang, Q.; Jin, X.; Li, Q. The Role of Deep-Red Emission CuInS2/ZnS QDs in White Light Emitting Diodes. Semicond. Sci. Technol. 2019, 34, 035025. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, R.; Chen, B.; Zhang, J.; Zhang, X.; Rogach, A.L. Template Synthesis of Silver Indium Sulfide Based Nanocrystals Performed through Cation Exchange in Organic and Aqueous Media. Nano Res. 2021, 14, 2321–2329. [Google Scholar] [CrossRef]
- Lim, L.J.; Zhao, X.; Tan, Z. Non-Toxic CuInS2/ZnS Colloidal Quantum Dots for Near-Infrared Light-Emitting Diodes. Adv. Mater. 2023, 35, 2301887. [Google Scholar] [CrossRef]
- Deng, D.; Chen, Y.; Cao, J.; Tian, J.; Qian, Z.; Achilefu, S.; Gu, Y. High-Quality CuInS2/ZnS Quantum Dots for In Vitro and In Vivo Bioimaging. Chem. Mater. 2012, 24, 3029–3037. [Google Scholar] [CrossRef]
- Pons, T.; Pic, E.; Lequeux, N.; Cassette, E.; Bezdetnaya, L.; Guillemin, F.; Marchal, F.; Dubertret, B. Cadmium-Free CuInS2/ZnS Quantum Dots for Sentinel Lymph Node Imaging with Reduced Toxicity. ACS Nano 2010, 4, 2531–2538. [Google Scholar] [CrossRef]
- Zhong, H.; Bai, Z.; Zou, B. Tuning the Luminescence Properties of Colloidal I–III–VI Semiconductor Nanocrystals for Optoelectronics and Biotechnology Applications. J. Phys. Chem. Lett. 2012, 3, 3167–3175. [Google Scholar] [CrossRef]
- Castro, S.L.; Bailey, S.G.; Raffaelle, R.P.; Banger, K.K.; Hepp, A.F. Synthesis and Characterization of Colloidal CuInS2 Nanoparticles from a Molecular Single-Source Precursor. J. Phys. Chem. B 2004, 108, 12429–12435. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Xin, W.; Yao, D.; Liu, Y.; Zhang, L.; Liu, W.; Zhang, W.; Zheng, W.; Yang, B.; et al. Facile Synthesis of Cu–In–S/ZnS Core/Shell Quantum Dots in 1-Dodecanethiol for Efficient Light-Emitting Diodes with an External Quantum Efficiency of 7.8%. Chem. Mater. 2018, 30, 8939–8947. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, R.; Yang, W. A Simple Route for Highly Luminescent Quaternary Cu-Zn-In-S Nanocrystal Emitters. Chem. Mater. 2011, 23, 3357–3361. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, L.; Zhou, T.; Xie, R.-J. Achieving Deep-Red-to-near-Infrared Emissions in Sn-Doped Cu–In–S/ZnS Quantum Dots for Red-Enhanced White LEDs and near-Infrared LEDs. Nanoscale 2018, 10, 9788–9795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lou, Q.; Ji, W.; Zhao, J.; Zhong, X. Color-Tunable Highly Bright Photoluminescence of Cadmium-Free Cu-Doped Zn–In–S Nanocrystals and Electroluminescence. Chem. Mater. 2014, 26, 1204–1212. [Google Scholar] [CrossRef]
- Tan, L.; Liu, S.; Li, X.; Chronakis, I.S.; Shen, Y. A New Strategy for Synthesizing AgInS2 Quantum Dots Emitting Brightly in Near-Infrared Window for in Vivo Imaging. Colloids Surf. B Biointerfaces 2015, 125, 222–229. [Google Scholar] [CrossRef]
- Tang, X.; Ho, W.B.A.; Xue, J.M. Synthesis of Zn-Doped AgInS2 Nanocrystals and Their Fluorescence Properties. J. Phys. Chem. C 2012, 116, 9769–9773. [Google Scholar] [CrossRef]
- Huong, T.T.T.; Loan, N.T.; Van Long, L.; Phong, T.D.; Ung Thi Dieu, T.; Liem, N.Q. Highly Luminescent Air-Stable AgInS2/ZnS Core/Shell Nanocrystals for Grow Lights. Opt. Mater. 2022, 130, 112564. [Google Scholar] [CrossRef]
- Branzi, L.; Liang, J.; Dee, G.; Kavanagh, A.; Gun’ko, Y.K. Multishell Silver Indium Selenide-Based Quantum Dots and Their Poly(Methyl Methacrylate) Composites for Application in Red-Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2024, 16, 37017–37027. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Y.; Liu, Z.; Wei, J.; Zhu, Q.; Jiang, G.; Jin, X.; Li, Q.; Li, F. White Light-Emitting Diodes Based on Quaternary Ag–In-Ga-S Quantum Dots and Their Influences on Melatonin Suppression Index. J. Lumin. 2021, 233, 117903. [Google Scholar] [CrossRef]
- Xie, R.; Rutherford, M.; Peng, X. Formation of High-Quality I–III–VI Semiconductor Nanocrystals by Tuning Relative Reactivity of Cationic Precursors. J. Am. Chem. Soc. 2009, 131, 5691–5697. [Google Scholar] [CrossRef]
- Xiang, W.-D.; Yang, H.-L.; Liang, X.-J.; Zhong, J.-S.; Wang, J.; Luo, L.; Xie, C.-P. Direct Synthesis of Highly Luminescent Cu–Zn–In–S Quaternary Nanocrystals with Tunable Photoluminescence Spectra and Decay Times. J. Mater. Chem. C 2013, 1, 2014–2020. [Google Scholar] [CrossRef]
- Chung, W.; Jung, H.; Lee, C.H.; Kim, S.H. Extremely High Color Rendering White Light from Surface Passivated Carbon Dots and Zn-Doped AgInS2 Nanocrystals. J. Mater. Chem. C 2014, 2, 4227–4232. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Xia, T.; Su, X.; Ma, Q. Aqueous Synthesis of High Quality Multicolor Cu-Zn-In-S Quantum Dots. J. Lumin. 2017, 188, 162–167. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, L.; Han, D.; Sun, G.; Zhu, C.; Wang, Y.; Wang, Q.; Artemyev, M.; Tang, J. Synthesis and Optical Properties of In2S3-Hosted Colloidal Zn–Cu–In–S Nanoplatelets. ACS Omega 2021, 6, 18939–18947. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Lin, Q.; Chen, F.; Wang, L.; Du, Z. Highly Efficient Near-Infrared Light-Emitting Diodes Based on Zn:CuInSe2/ZnS//ZnS Quantum Dots with Double Shell Engineering. Opt. Express OE 2022, 30, 29449–29460. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, F.; Lin, H.; Lin, Z.; Xu, J.; Wang, Y. Inorganic Halide Perovskite Quantum Dot Modified YAG-Based White LEDs with Superior Performance. J. Mater. Chem. C 2016, 4, 7601–7606. [Google Scholar] [CrossRef]
- Imran, M.; Caligiuri, V.; Wang, M.; Goldoni, L.; Prato, M.; Krahne, R.; De Trizio, L.; Manna, L. Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. [Google Scholar] [CrossRef]
- Pan, J.; Shang, Y.; Yin, J.; De Bastiani, M.; Peng, W.; Dursun, I.; Sinatra, L.; El-Zohry, A.M.; Hedhili, M.N.; Emwas, A.-H.; et al. Bidentate Ligand-Passivated CsPbI3 Perovskite Nanocrystals for Stable Near-Unity Photoluminescence Quantum Yield and Efficient Red Light-Emitting Diodes. J. Am. Chem. Soc. 2018, 140, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, S.; Zhang, X.; Wang, H.; Zhang, X.; Wang, C.; Gao, Y.; Shi, Z.; Yu, W.W.; Zhang, Y. Enhancing the Efficiency of CsPbX3 (X = Cl, Br, I) Nanocrystals via Simultaneous Surface Peeling and Surface Passivation. Nanoscale 2019, 11, 11464–11469. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Kumar, S.; Bär, J.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Grotevent, M.; Shorubalko, I.; Bodnarchuk, M.I.; et al. Dismantling the “Red Wall” of Colloidal Perovskites: Highly Luminescent Formamidinium and Formamidinium–Cesium Lead Iodide Nanocrystals. ACS Nano 2017, 11, 3119–3134. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Kershaw, S.V.; Li, T.; Wang, C.; Zhang, X.; Wang, W.; Li, D.; Wang, Y.; Lu, M.; et al. Zn-Alloyed CsPbI3 Nanocrystals for Highly Efficient Perovskite Light-Emitting Devices. Nano Lett. 2019, 19, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lu, M.; Zhang, X.; Sun, S.; Han, C.; Zhang, Y.; Yang, X.; Kershaw, S.V.; Zheng, W.; Rogach, A.L. Highly Stable and Efficient Light-Emitting Diodes Based on Orthorhombic γ-CsPbI3 Nanocrystals. ACS Nano 2023, 17, 9290–9301. [Google Scholar] [CrossRef]
- Vighnesh, K.; Sergeev, A.A.; Hassan, M.S.; Portniagin, A.S.; Sokolova, A.V.; Zhu, D.; Sergeeva, K.A.; Kershaw, S.V.; Wong, K.S.; Rogach, A.L. Red-Emitting CsPbI3/ZnSe Colloidal Nanoheterostructures with Enhanced Optical Properties and Stability. Small 2024, 2400745. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Meggiolaro, D.; Dang, Z.; De Angelis, F.; Manna, L. Fluorescent Alloy CsPbxMn1–xI3 Perovskite Nanocrystals with High Structural and Optical Stability. ACS Energy Lett. 2017, 2, 2183–2186. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, L.; Zhang, H.; Ding, L.; Shao, G.; Liang, X.; Xiang, W. Novel Red-Emitting CsPb1−xTixI3 Perovskite QDs@glasses with Ambient Stability for High Efficiency White LEDs and Plant Growth LEDs. Chem. Eng. J. 2019, 378, 122125. [Google Scholar] [CrossRef]
- Jiang, H.; Zang, C.; Dong, Y.; Lai, M.; Xu, S.; Xu, J. One-Step Preparation of Ion-Doped Cesium Lead Halide Perovskite Nanocrystals by Ultrasonication. Part. Part. Syst. Charact. 2023, 40, 2300032. [Google Scholar] [CrossRef]
- Jia, C.; Li, H.; Meng, X.; Li, H. CsPbX3/Cs4PbX6 Core/Shell Perovskite Nanocrystals. Chem. Commun. 2018, 54, 6300–6303. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.K.; Saikia, S.; Yadav, S.; Nawale, V.V.; Nag, A. CsPbBr3/ZnS Core/Shell Type Nanocrystals for Enhancing Luminescence Lifetime and Water Stability. ACS Energy Lett. 2020, 5, 1794–1796. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Yu, S.; Li, L.; Xu, J.; Gong, X.; Ding, R.; Zhang, J.; Yin, H. Epitaxial Growth of Highly Stable Perovskite CsPbBr3/nZnS/Al Core/Multi-Shell Quantum Dots with Aluminium Self-Passivation. Nanotechnology 2020, 31, 375703. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Li, X.; Yuan, M.; Turyanska, L.; Yang, X. Core/Shell Perovskite Nanocrystals: Synthesis of Highly Efficient and Environmentally Stable FAPbBr3/CsPbBr3 for LED Applications. Adv. Funct. Mater. 2020, 30, 1910582. [Google Scholar] [CrossRef]
- Gao, F.; Wu, J.; Zhao, Y.; Song, T.; Deng, Z.; Wang, P.; Wang, Y.; Li, H. A General Approach to Realizing Perovskite Nanocrystals with Insulating Metal Sulfate Shells. Nanoscale 2021, 13, 10329–10334. [Google Scholar] [CrossRef]
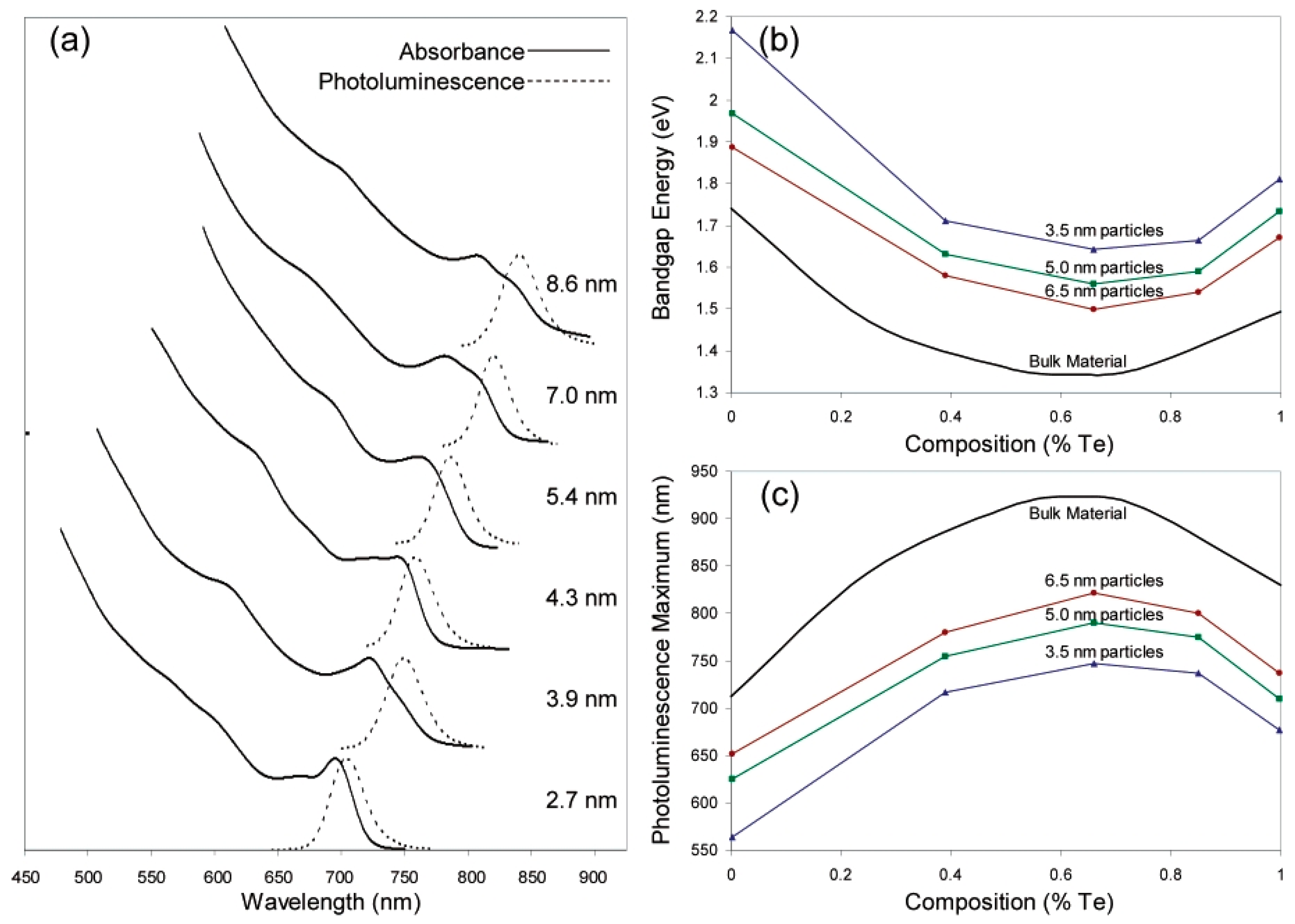
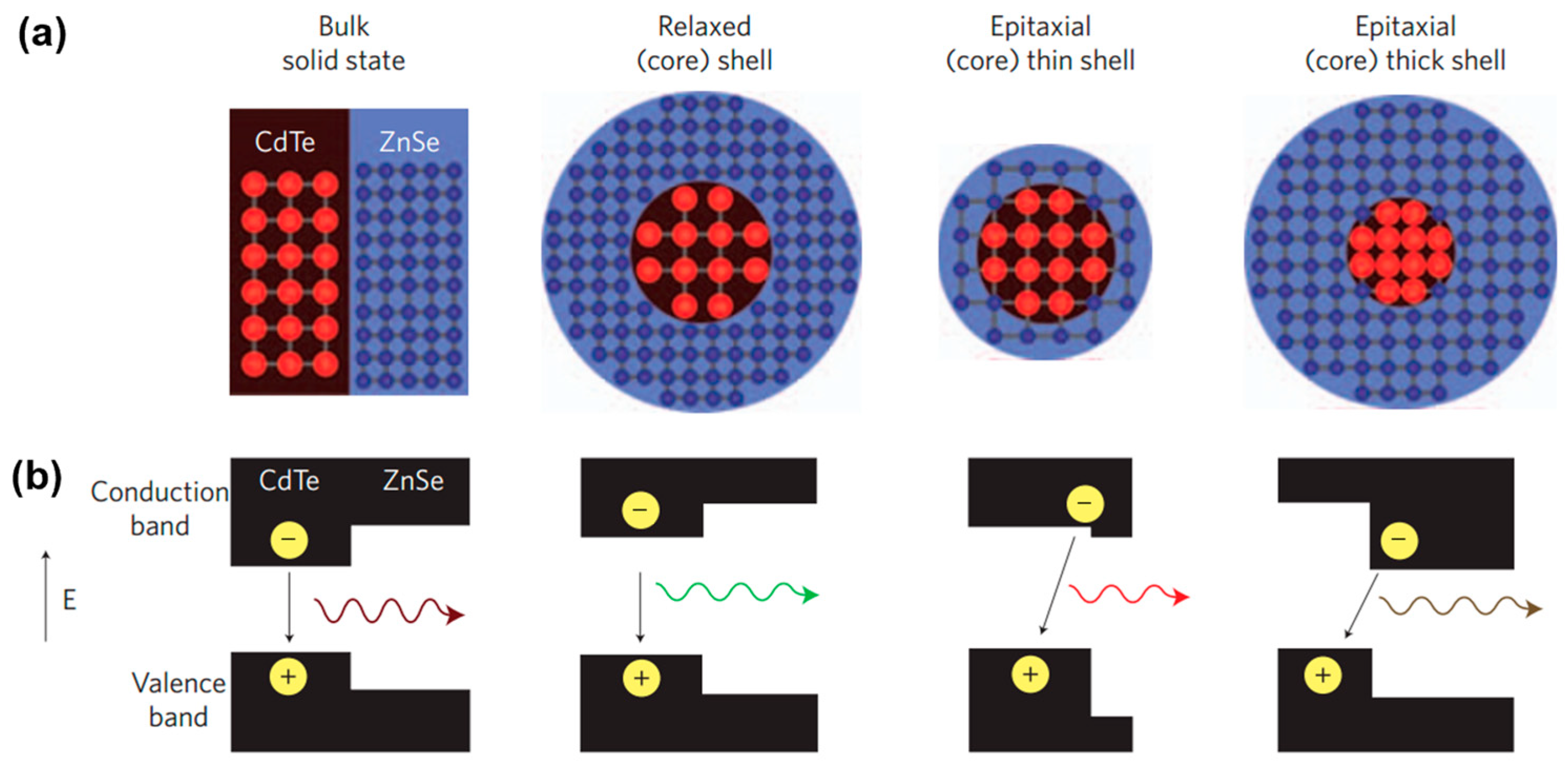
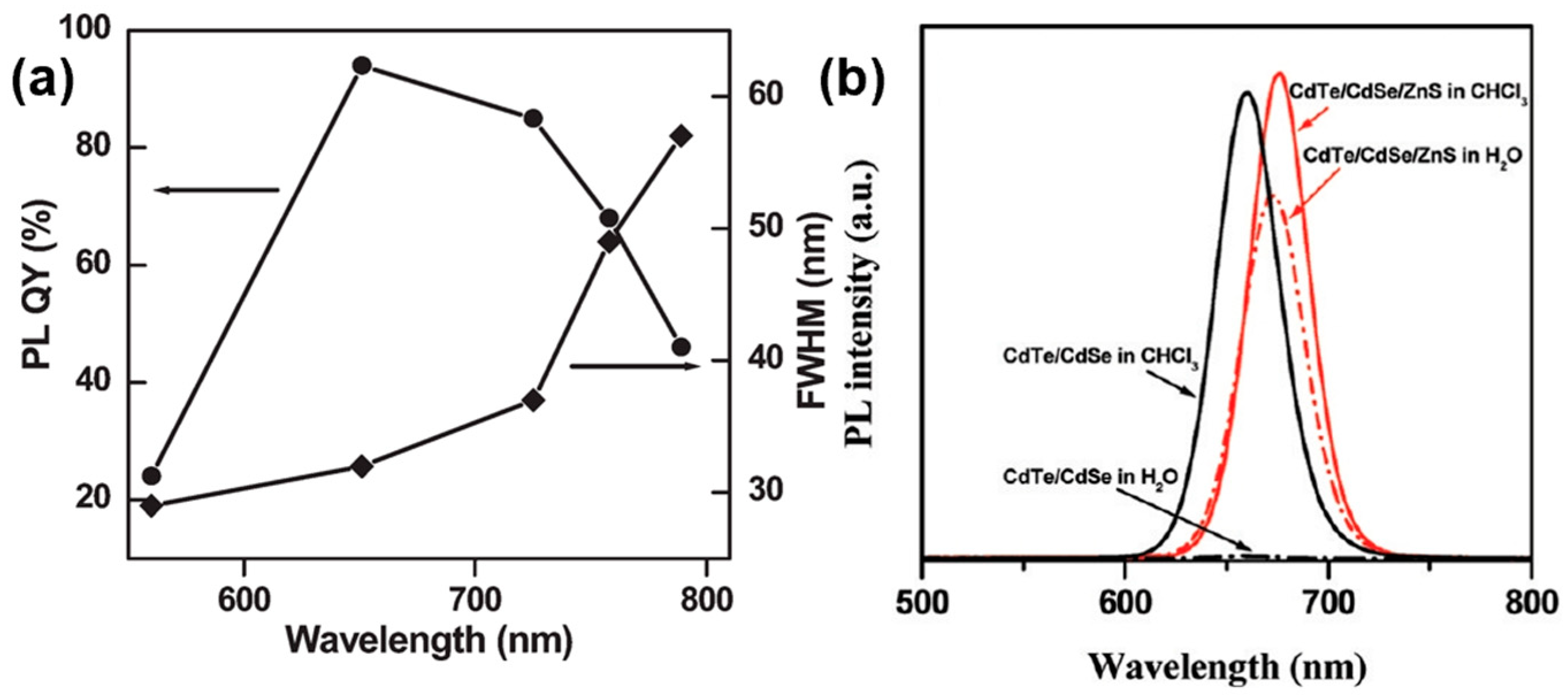

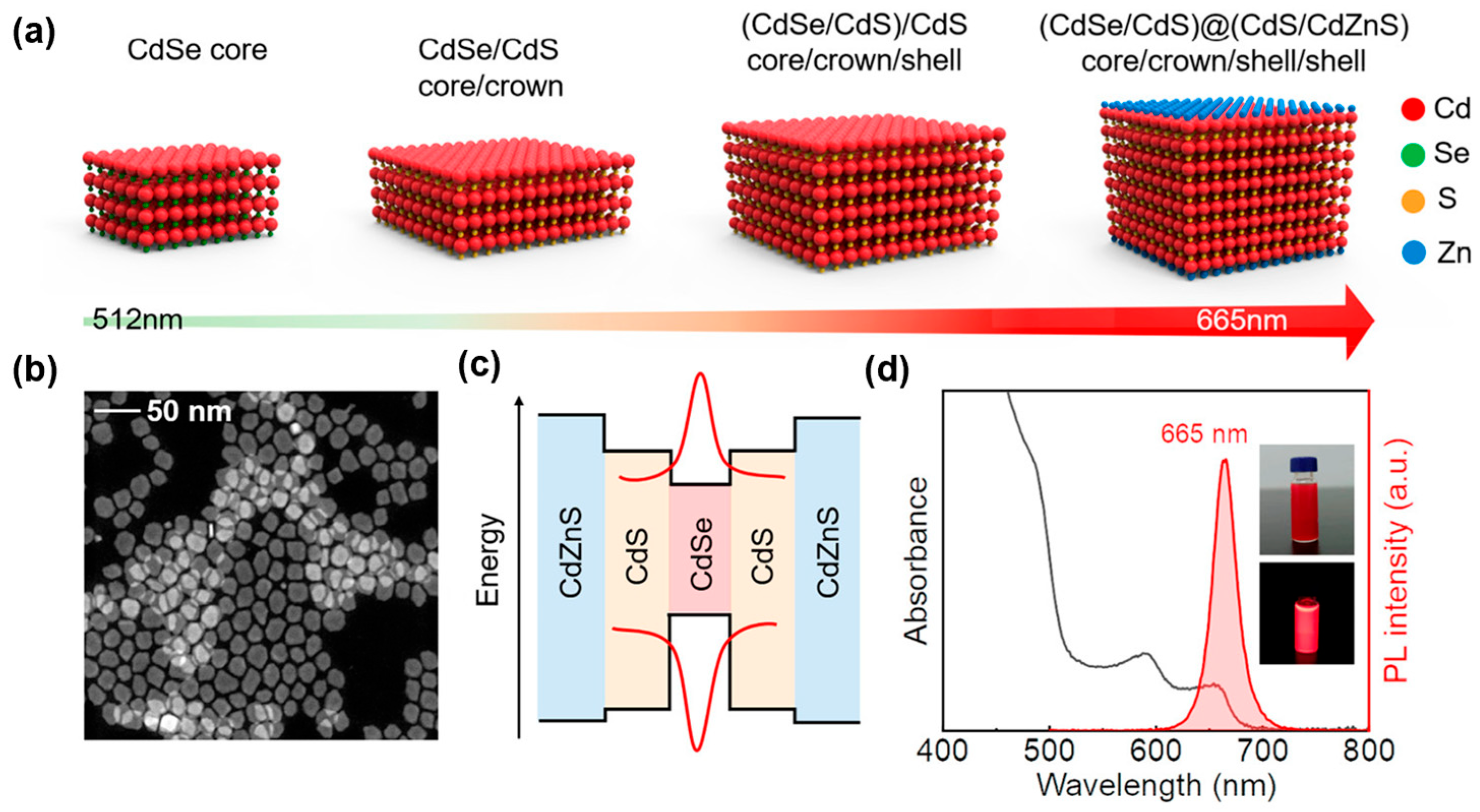



| # | Material | λ (nm) | FWHM (nm) | PLQY | Synthesis | Year | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | CdSeTe/CdS | 580–750 | 40–50 | 30–50% |
| 2006 | [26] |
| 2 | CdTeSe/CdZnS | 650–800 | - | 60–80% |
| 2009 | [30] |
| 3 | CdSeTe/ZnS | 650–750 | - | 40–70% |
| 2010 | [31] |
| 4 | CdSTe (homogeneous) | 690–745 | - | 6–7% |
| 2008 | [29] |
| CdSTe (gradient) | 670–695 | - | 4% |
| |||
| 5 | CdTeS/ZnS | 550–750 | - | 35% |
| 2018 | [32] |
| 6 | CdTe0.12S0.88/CdxZn1−xS | 694 | >150 | 56% |
| 2024 | [33] |
| 7 | CdTe/ZnSe | 500–1050 | 40–90 | 25–60% |
| 2009 | [34] |
| 8 | CdTe/CdS | 652–795 | 62–94 | 45–62% |
| 2009 | [35] |
| 9 | CdTe/CdS | 600–815 | 75–80 | >40% |
| 2012 | [36] |
| 10 | CdTe/CdZnS | 650–820 | 33–60 | 30–65% |
| 2012 | [37] |
| 11 | CdTe/CdS/ZnS | 660 | - | 64% |
| 2014 | [38] |
| 13 | CdTe/CdSe/ZnS | 540–825 | ~40 | ~90% |
| 2009 | [39] |
| 15 | CdTe/CdSe | 664,689 | 40 | 63–68% |
| 2016 | [40] |
| 16 | CdZnS/ZnSe | 520–680 | ~35 | 50–60% |
| 2017 | [41] |
| 17 | ZnSe/CdSe | 418–674 | ~30 | 25–85% |
| 2005 | [42] |
| 18 | ZnCdSe/ZnSeS | ~650 | 24.2 | >90% |
| 2023 | [16] |
| 19 | ZnS/Zn1−xCdxS:Cu | 520–730 | ~100–200 | 20–30% |
| 2011 | [43] |
| 20 | CdS:Cu/ZnS | 630–710 | - | 40–50% |
| 2012 | [6] |
| 21 | CdS/ZnS:Cu | 657–678 | - | 40% |
| 2015 | [5] |
| 22 | CdS:Ag@Sb2O3 | 687 | 132 | 66.50% |
| 2019 | [44] |
| 23 | CdSe/CdS NR (giant rod) | 653 | 32 | 10% |
| 2018 | [45] |
| 24 | CdSeTe/CdZnS/ZnS NR | 670 | 61 | 45% |
| 2024 | [46] |
| 25 | CdSe/CdS NPL | 670 | 20 | 50–60% |
| 2017 | [47] |
| 26 | CdSe (4 ML)/CdxZn1−xS NPL | 655 | 20 | 88% |
| 2019 | [48] |
| 27 | CdSe (6 ML)/CdxZn1–xS NPL | 692 | 22 | 92% | |||
| 28 | CdSe/ZnSe NPL | 684 | 22 | 47% |
| ||
| 29 | (CdSe/CdS)@(CdS/CdZnS) NPL | 665 | 30 | 81% |
| 2022 | [49] |
| 30 | (CdSe/CdS)@(1–4 CdS/CdZnS) NPL | 701 | 26 | 88% | 2022 | [50] |
| # | Material | λ (nm) | FWHM (nm) | PLQY | Synthesis | Year | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | InP (HF treated) | 575–730 | - | 30% |
| 1996 | [61] |
| 2 | InP (HF treated) | 522–752 | - | 20~40% |
| 2002 | [67] |
| 3 | InP (Wurtzite) | 600–810 | - | >30% |
| 2023 | [68] |
| 4 | InP; InP/ZnS | 507–728 | 43–75 | 40–79% |
| 2023 | [69] |
| 5 | InP/ZnS | 554–681 | 187 meV | 40% |
| 2018 | [70] |
| InP/CdS | 608–768 | 215 meV | 50% | ||||
| 6 | InP/ZnSe | 655 | 53 | - |
| 2020 | [71] |
| 7 | InP/ZnSe/ZnSeS/ZnS | 680 | 66 | 95% |
| 2023 | [72] |
| 8 | InP/ZnSe/ZnS | 626–670 | 44–52 | 60–74% |
| 2023 | [73] |
| 9 | InP/ZnSe/ZnS/Al2O3 | 720 | 45 | 43% |
| 2024 | [74] |
| 10 | ZnSe/InP/ZnS | 515–845 | >120 | 38% |
| 2021 | [75] |
| 11 | InP:Cu/ZnSe | 630–1100 | 240 meV | 35–40% |
| 2009 | [76] |
| 12 | InP/ZnS:Cu | 680–900 | 190 | 40% |
| 2019 | [77] |
| 13 | InP:Cu/ZnCuInS/ZnS | 694–850 | - | 71.5–82.4% |
| 2021 | [78] |
| 14 | InP:Ru/ZnS | 655 | - | 77.6% |
| 2023 | [79] |
| # | Material | λ (nm) | FWHM (nm) | PLQY | Synthesis | Year | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | CuInS2 | ~663 | 100 | <5% |
| 2004 | [92] |
| 2 | CuInS2/ZnS | 550–800 | 90–120 | >50% |
| 2012 | [89] |
| 3 | CuInS2/ZnS | 530–710 | 128 | 5–80% |
| 2018 | [93] |
| 4 | Cu–Zn–In–S | 580–750 | - | ~70% |
| 2011 | [94] |
| 5 | Cu-Sn-In-S/ZnS | 628–785 | >120 | 75% |
| 2018 | [95] |
| 6 | Cu:Zn–In–S/ZnS | 450–810 | - | 70–80% |
| 2014 | [96] |
| 7 | AgInS2 | 689–817 | ~98 | ~35% |
| 2015 | [97] |
| 8 | AgInS2:Zn | 520–680 | - | 41% |
| 2012 | [98] |
| 9 | AgInS2/ZnS | 600–700 | 140–150 | 60% |
| 2022 | [99] |
| 10 | AgInSe/ZnSe/ZnS | 670 | 148 | 46% |
| 2024 | [100] |
| 11 | AgInGaS | 550–670 | 160 | 55% |
| 2022 | [101] |
| # | Material | λ (nm) | FWHM (nm) | PLQY | Synthesis | Year | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | CsPbI3 | 650–700 | ~42 | 60–90% |
| 2015 | [108] |
| 2 | CsPbBr3−xIx | 514–695 | 20–42 | 56–78% |
| 2016 | [109] |
| 3 | CsPbI3 | 691 | 32 | 58% |
| 2018 | [110] |
| 4 | CsPbI3 | 688 | 33 | 95% |
| 2018 | [111] |
| 5 | CsPbI3 | 687 | - | 98% |
| 2019 | [112] |
| 6 | FA0.1Cs0.9PbI3 | 690 | 45 | >70% |
| 2017 | [113] |
| 7 | CsPb0.64Zn0.36I3 | 682 | - | 98.5% |
| 2019 | [114] |
| 8 | CaIx/γ-CsPbI3/CaI2 | 684 | 31 | 98% |
| 2023 | [115] |
| 9 | CsPbI3/ZnSe | 675 | - | 96% |
| 2024 | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, G.; Liu, F.; Wei, J.; Li, H. A Review of Deep-Red (650–700 nm)-Emitting Semiconductor Nanocrystals. Crystals 2024, 14, 788. https://doi.org/10.3390/cryst14090788
Jin G, Liu F, Wei J, Li H. A Review of Deep-Red (650–700 nm)-Emitting Semiconductor Nanocrystals. Crystals. 2024; 14(9):788. https://doi.org/10.3390/cryst14090788
Chicago/Turabian StyleJin, Geyu, Fangze Liu, Jing Wei, and Hongbo Li. 2024. "A Review of Deep-Red (650–700 nm)-Emitting Semiconductor Nanocrystals" Crystals 14, no. 9: 788. https://doi.org/10.3390/cryst14090788





