Investigations of Some Disordered Quaternary Compounds in the Systems Ag/Pb/Sb/Se and Ag/Pb/Sb/Te
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Powder X-ray Diffraction and Rietveld Refinements
2.3. Single-Crystal X-ray Diffraction
2.4. Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
2.5. Transport Properties
3. Results and Discussion
3.1. Ag1/3Pb1/3Sb1/3Se
3.2. Ag0.61Pb1.79Sb2.61Se6
3.3. Ag0.38Pb0.25Sb2.38Te4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsu, K.F.; Loo, S.; Guo, F.; Chen, W.; Dyck, J.S.; Uher, C.; Hogan, T.; Polychroniadis, E.K.; Kanatzidis, M.G. Cubic AgPbmSbTe2+m: Bulk Thermoelectric Materials with High Figure of Merit. Science 2004, 303, 818–821. [Google Scholar] [CrossRef]
- Quarez, E.; Hsu, K.-F.; Pcionek, R.; Frangis, N.; Polychroniadis, E.K.; Kanatzidis, M.G. Nanostructuring, Compositional Fluctuations, and Atomic Ordering in the Thermoelectric Materials AgPbmSbTe2+m. The myth of solid solutions. J. Am. Chem. Soc. 2005, 127, 9177–9190. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Božin, E.S.; Billinge, S.J.L.; Quarez, E.; Kanatzidis, M.G. Nanoscale clusters in the high performance thermoelectric AgPbmSbTem+2. Phys. Rev. B 2005, 72, 174113. [Google Scholar] [CrossRef]
- Su, T.; Zhou, Y.; Li, H.; Li, S.; Li, X.; Deng, L.; Su, Y.; Liu, J.; Sui, Y.; Ma, H.; et al. Enhanced thermoelectric performance of Ag0.8Pb18SbTe20 alloyed with Se. Phys. Status Solidi A 2012, 209, 1124–1127. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Fan, Y.; Tang, X.; Tan, G. Strategies for boosting thermoelectric performance of PbSe: A review. J. Chem. Eng. 2022, 431, 133699. [Google Scholar] [CrossRef]
- Zhong, Y.; Tang, J.; Liu, H.; Chen, Z.; Lin, L.; Ren, D.; Liu, B.; Ang, R. Optimized Strategies for Advancing n-Type PbTe Thermoelectrics: A Review. ACS Appl. Mater. Interfaces 2020, 12, 49323–49334. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Singh, S.; Nozariasbmarz, A.; Li, W.; Genç, A.; Xia, Y.; Zheng, L.; Lee, S.H.; Karan, S.K.; et al. Defect-Engineering-Stabilized AgSbTe2 with High Thermoelectric Performance. Adv. Mater. 2023, 35, 2208994. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Hong, T.; Hu, L.; Ina, T.; Zhan, S.; Qin, B.; Shi, H.; Su, L.; Gao, X.; et al. Multiple valence bands convergence and strong phonon scattering lead to high thermoelectric performance in p-type PbSe. Nat. Commun. 2022, 13, 4179. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Shi, F.; Hao, S.; Zhao, L.-D.; Chi, H.; Zhang, X.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Non-equilibrium processing leads to record high thermoelectric figure of merit in PbTe-SrTe. Nat. Commun. 2016, 7, 12167. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Ghosh, T.; Arora, R.; Samanta, M.; Xie, L.; Singh, N.K.; Soni, A.; He, J.; Waghmare, U.V.; Biswas, K. Enhanced atomic ordering leads to high thermoelectric performance in AgSbTe2. Science 2021, 371, 722–727. [Google Scholar] [CrossRef]
- Soriano, R.B.; Wu, J.; Kanatzidis, M.G. Size as a Parameter to Stabilize New Phases: Rock Salt Phases of PbmSb2nSem+3n. J. Am. Chem. Soc. 2015, 137, 9937–9942. [Google Scholar] [CrossRef]
- Zhou, C.; Lee, Y.K.; Cha, J.; Yoo, B.; Cho, S.-P.; Hyeon, T.; Chung, I. Defect Engineering for High-Performance n-Type PbSe Thermoelectrics. J. Am. Chem. Soc. 2018, 140, 9282–9290. [Google Scholar] [CrossRef]
- Luo, Z.-Z.; Hao, S.; Zhang, X.; Hua, X.; Cai, S.; Tan, G.; Bailey, T.P.; Ma, R.; Uher, C.; Wolverton, C.; et al. Soft phonon modes from off-center Ge atoms lead to ultralow thermal conductivity and superior thermoelectric performance in n-type PbSe–GeSe. Energy Environ. Sci. 2018, 11, 3220–3230. [Google Scholar] [CrossRef]
- Sportouch, S.; Bastea, M.; Brazis, P.; Ireland, J.; Kannewurf, C.R.; Uher, C.; Kanatzidis, M.G. Thermoelectric properties of the cubic family of compounds AgPbBiQ3 (Q = S, Se, Te). Very low thermal conductivity materials. Mat. Res. Soc. Symp. Proc. 1999, 545, 123–130. [Google Scholar] [CrossRef]
- Luo, Y.; Hao, S.; Cai, S.; Slade, T.J.; Luo, Z.Z.; Dravid, V.P.; Wolverton, C.; Yan, Q.; Kanatzidis, M.G. High Thermoelectric Performance in the New Cubic Semiconductor AgSnSbSe3 by High-Entropy Engineering. J. Am. Chem. Soc. 2020, 142, 15187–15198. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Pal, K.; Waghmare, U.V.; Biswas, K. Bonding heterogeneity and lone pair induced anharmonicity resulted in ultralow thermal conductivity and promising thermoelectric properties in n-type AgPbBiSe3. Chem. Sci. 2019, 10, 4905–4913. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ma, Z.; Li, W.; Li, C.; Yang, B.; Sun, C.; Gang, S.; Zhang, W.; Long, H.; Li, X.; et al. A New n-Type High Entropy Semiconductor AgBiPbSe2S with High Thermoelectric and Mechanical Properties. J. Adv. Funct. Mater. 2024, 34, 2313719. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, T.; Ma, Z.; Zhang, D.; Guo, Z.; Jiang, Q.; Yang, J.; Yan, Q.; Kanatzidis, M.G. Cubic AgMnSbTe3 Semiconductor with a High Thermoelectric Performance. J. Am. Chem. Soc. 2021, 143, 13990–13998. [Google Scholar] [CrossRef]
- Kheifets, O.L.; Kobelev, L.Y.; Melnikova, N.V.; Nugaeva, L.L. Electrical properties of solid electrolytes with the general formula ABCD3 (A = Ag, Cu; B = Pb, Sn; C = As, Sb; and D = S, Se). Tech. Phys. 2007, 52, 86–92. [Google Scholar] [CrossRef]
- Dittrich, H.; Stadler, A.; Topa, D.; Schimper, H.-J.; Basch, A. Progress in sulfosalt research. Phys. Status Solidi A 2009, 206, 1034–1041. [Google Scholar] [CrossRef]
- Melnikova, N.V.; Alibekov, A.G.; Saypulaeva, L.A.; Kheifets, O.L.; Babushkin, A.N.; Mollaev, A.Y.; Kallaev, S.N.; Ferzaliev, R.M. Electrical Properties of AgSnSbSe3 under Different External Effects. Phys. Solid State 2011, 53, 2476–2479. [Google Scholar] [CrossRef]
- Harker, D. The Crystal Structure of the Mineral Tetradymite, Bi2Te2S. Z. Kristallogr. 1934, 89, 175–181. [Google Scholar] [CrossRef]
- Schneider, M.N.; Seibald, M.; Lagally, P.; Oeckler, O. Ambiguities in the structure determination of antimony tellurides arising from almost homometric structure models and stacking disorder. J. Appl. Crystallogr. 2010, 43, 1012–1020. [Google Scholar] [CrossRef]
- Navrátil, J.; Klichová, I.; Karamazov, S.; Šrámková, J.; Horák, J. Behavior of Ag Admixtures in Sb2Te3 and Bi2Te3 Single Crystals. J. Solid State Chem. 1998, 140, 29–37. [Google Scholar] [CrossRef]
- Schneider, M.N.; Seibald, M.; Oeckler, O. A new series of long-range ordered metastable phases in the system M-Sb-Te (M = Ge, Ag). Dalton Trans. 2009, 2004–2011. [Google Scholar] [CrossRef]
- Shelimova, L.E.; Karpinskii, O.G.; Svechnikova, T.E.; Avilov, E.S.; Kretova, M.A.; Zemskov, V.S. Synthesis and structure of layered compounds in the PbTe−Bi2Te3 and PbTe−Sb2Te3 systems. Inorg. Mater. 2004, 40, 1264–1270. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, K.H.; Mun, H.A.; Kim, H.S.; Hwang, S.W.; Roh, J.W.; Yang, D.J.; Shin, W.H.; Li, X.S.; Lee, Y.H.; et al. Dense dislocation arrays embedded in grain boundaries for high-performance bulk thermoelectrics. Science 2015, 348, 109–114. [Google Scholar] [CrossRef]
- Heremans, J.; Cava, R.; Samarth, N. Tetradymites as thermoelectrics and topological insulators. Nat. Rev. Mater. 2017, 2, 17049. [Google Scholar] [CrossRef]
- Srikrishnan, T.; Nowacki, W. A redetermination of the crystal structure of cosalite, Pb2Bi2S5. Z. Kristallogr.-Cryst. Mater. 1974, 140, 114–136. [Google Scholar] [CrossRef]
- Skowron, A.; Brown, I.D. Structure of antimony lead selenide, Pb4Sb4Se10, a selenium analogue of cosalite. Acta Crystallogr. Sect. C 1990, 46, 2287–2291. [Google Scholar] [CrossRef]
- Derakhshan, S.; Assoud, A.; Taylor, N.J.; Kleinke, H. Crystal and electronic structures and physical properties of two semiconductors: Pb4Sb6Se13 and Pb6Sb6Se17. Intermetallics 2006, 14, 198–207. [Google Scholar] [CrossRef]
- Emirdag-Eanes, M.; Kolis, J.W. Structural Characterization of Pb6Sb6Se17. Z. Anorg. Allg. Chem. 2002, 628, 10–11. [Google Scholar] [CrossRef]
- Skowron, A.; Brown, I.D. Refinement of the structure of robinsonite, Pb4Sb6S13. Acta Crystallogr. Sect. C 1990, 46, 527–531. [Google Scholar] [CrossRef]
- Orlandi, P.; Meerschaut, A.; Palvadeau, P.; Merlino, S. Lead-antimony sulfosalts from Tuscany (Italy). V. Definition and crystal structure of moeloite, Pb6Sb6S14(S3), a new mineral from the Ceragiola marble quarry. Eur. J. Mineral. 2002, 14, 599–606. [Google Scholar] [CrossRef]
- Skowron, A.; Boswell, F.W.; Corbett, J.M.; Taylor, N.J. Structure Determination of PbSb2Se4. J. Solid State Chem. 1994, 112, 251–254. [Google Scholar] [CrossRef]
- Moëlo, Y.; Makovicky, E.; Mozgova, N.N.; Jambor, J.L.; Cook, N.; Pring, A.; Paar, W.; Nickel, E.H.; Graeser, S.; Karup-Møller, S.; et al. Sulfosalt systematics: A review. Report of the sulfosalt sub-committee of the IMA Commission on Ore Mineralogy. Eur. J. Mineral. 2008, 20, 7–62. [Google Scholar] [CrossRef]
- Takagi, J.; Takéuchi, Y. The crystal structure of lillianite. Acta Crystallogr. Sect. B 1972, 28, 649–651. [Google Scholar] [CrossRef]
- Ohsumi, K.; Tsutsui, K.; Takéuchi, Y.; Tokonami, M. Reinvestigation of lillianite structure with synchrotron radiation. Acta Crystallogr. Sect. A 1984, 40, 255–256. [Google Scholar] [CrossRef]
- Makovicky, E.; Karup-Møller, S. Chemistry and crystallography of the lillianite homologous series. Part I. General properties and definitions. Neues Jahrb. Mineral. Abh. 1977, 130, 264–287. [Google Scholar]
- Makovicky, E.; Karup-Møller, S. Chemistry and crystallography of the lillianite homologous series. Part II. Definition of new minerals: Eskimoite, vikingite, ourayite and treasurite. Redefinition of schirmerite and new data on the lillianite-gustavite solid-solution series. Neues Jahrb. Mineral. Abh. 1977, 131, 56–82. [Google Scholar]
- Makovicky, E. Chemistry and crystallography of the lillianite homologous series. Part III. Crystal chemistry of lillianite homologues. Related phases. Neues Jahrb. Mineral. Abh. 1977, 131, 187–207. [Google Scholar]
- Olvera, A.; Shi, G.; Djieutedjeu, H.; Page, A.; Uher, C.; Kioupakis, E.; Poudeu, P.F.P. Pb7Bi4Se13: A Lillianite Homologue with Promising Thermoelectric Properties. Inorg. Chem. 2015, 54, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Fang, Y.-W.; Qin, F.; Cao, X.; Zhao, X.; Luo, Y.; Repaka, D.V.M.; Luo, W.; Suwardi, A.; Soldi, T.; et al. High thermoelectric performance enabled by convergence of nested conduction bands in Pb7Bi4Se13 with low thermal conductivity. Nat. Commun. 2021, 12, 4793. [Google Scholar] [CrossRef]
- TOPAS, version 5; Bruker AXS GmbH: Karlsruhe, Germany, 2015.
- X-Area, version 1.82; Stoe & Cie GmbH: Darmstadt, Germany, 2018.
- Blessing, R.H. An empirical correction for absorption anisotropy. Acta Crystallogr. Sect. A 1995, 51, 33–38. [Google Scholar] [CrossRef]
- CrysAlisPro Software System, version 1.171.41.113a; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2021.
- SADABS, version 2.05; Bruker AXS Inc.: Madison, WI, USA, 2016.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond, version 3.2k; Crystal Impact GbR: Bonn, Germany, 2014.
- Wills, A.S. VaList—Bond Valence Calculation and Listing, version 4.0.7; Wills Group: London, UK, 2010.
- INCA Suite, version 4.09; Oxford Instruments Analytical Limited: Abingdon, UK, 2007.
- EDAX Ametek, version 4.6.2001.0293; AMETEK, Inc.: Cassatt Road Berwyn, PA, USA, 2010.
- JEMS, version 3.8326 U2012; CIME-EPFL: Lausanne, Switzerland, 2012.
- Stadelmann, P.A. EMS—A software package for electron diffraction analysis and HREM image simulation in materials science. Ultramicroscopy 1987, 21, 131–146. [Google Scholar] [CrossRef]
- Digital Micrograph (TM), version 3.6.5; Gatan Inc.: Pleasanton, CA, USA, 1999.
- Dusza, L. Combined solution of the simultaneous heat loss and finite pulse time corrections with the laser flash method. High Temp.-High Press. 1995, 27, 467–473. [Google Scholar] [CrossRef]
- Liu, Y.; Cadavid, D.; Ibáñez, M.; De Roo, J.; Ortega, S.; Dobrozhan, O.; Kovalenko, M.V.; Cabot, A. Colloidal AgSbSe2 nanocrystals: Surface analysis, electronic doping and processing into thermoelectric nanomaterials. J. Mater. Chem. C 2016, 4, 4756–4762. [Google Scholar] [CrossRef]
- Kim, H.-S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]
- Ito, T.; Nowacki, W. The crystal structure of freieslebenite, PbAgSbS3. Z. Kristallogr. 1974, 139, 85–102. [Google Scholar] [CrossRef]
- Yu, J.; Yun, H. Reinvestigation of the low-temperature form of Ag2Se (naumannite) based on single-crystal data. Acta Crystallogr. Sect. E 2011, 67, 45. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Masumoto, K.; Ohba, S.; Saito, Y.; Toriumi, K.; Iwata, Y.; Shibuya, I. Temperature dependence of atomic thermal parameters of lead chalcogenides, PbS, PbSe and PbTe. Acta Crystallogr. Sect. C 1987, 43, 1443–1445. [Google Scholar] [CrossRef]
- Kyono, A.; Hayakawa, A.; Horiki, M. Selenium substitution effect on crystal structure of stibnite (Sb2S3). Phys. Chem. Miner. 2015, 42, 475–490. [Google Scholar] [CrossRef]
- Goldsmid, H.J.; Sharp, J.W. Estimation of the thermal band gap of a semiconductor from Seebeck measurements. J. Electron. Mater. 1999, 28, 869–872. [Google Scholar] [CrossRef]
- Brown, I.D. Chemical and steric constraints in inorganic solids. Acta Crystallogr. Sect. B 1992, 48, 553–572. [Google Scholar] [CrossRef]
- Brown, I.D. Influence of Chemical and Spatial Constraints on the Structures of Inorganic Compounds. Acta Crystallogr. Sect. B 1997, 53, 381–393. [Google Scholar] [CrossRef]
- Elcoro, L.; Perez-Mato, J.M.; Friese, K.; Petříček, V.; Balić-Žunić, T.; Olsen, L.A. Modular crystals as modulated structures: The case of the lillianite homologous series. Acta Crystallogr. Sect. B 2008, 64, 684–701. [Google Scholar] [CrossRef]
- Heinke, F.; Urban, P.; Werwein, A.; Fraunhofer, C.; Rosenthal, T.; Schwarzmüller, S.; Souchay, D.; Fahrnbauer, F.; Dyadkin, V.; Wagner, G.; et al. Cornucopia of Structures in the Pseudobinary System (SnSe)xBi2Se3: A Crystal-Chemical Copycat. Inorg. Chem. 2018, 57, 4427–4440. [Google Scholar] [CrossRef]
- Makovicky, E.; Topa, D. Lillianites and andorites: New life for the oldest homologous series of sulfosalts. Mineral. Mag. 2014, 78, 387–414. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Pearson’s Crystal Data, Crystal Structure Database for Inorganic Compounds, version 2.6; Release 2022/23; Crystal Impact GbR: Bonn, Germany, 2023.
- Heinke, F.; Nietschke, F.; Fraunhofer, C.; Dovgaliuk, I.; Schiller, J.; Oeckler, O. Structure and thermoelectric properties of the silver lead bismuth selenides Ag5Pb9Bi19Se40 and AgPb3Bi7Se14. Dalton Trans. 2018, 47, 12431–12438. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.W.; Jaulmes, S.; Flahaut, J. Système As–Ge–Te. J. Solid State Chem. 1988, 74, 277–286. [Google Scholar] [CrossRef]
- Karpinsky, O.G.; Shelimova, L.E.; Kretova, M.A.; Fleurial, J.-P. An X-ray study of the mixed-layered compounds of (GeTe)n(Sb2Te3)mhomologous series. J. Alloys Compd. 1998, 268, 112–117. [Google Scholar] [CrossRef]
- Matsunaga, T.; Yamada, N.; Kubota, Y. Structures of stable and metastable Ge2Sb2Te5, an intermetallic compound in GeTe-Sb2Te3 pseudobinary systems. Acta Crystallogr. Sect. B 2004, 60, 685–691. [Google Scholar] [CrossRef]
- Poudeu, P.F.P.; Kanatzidis, M.G. Design in solid state chemistry based on phase homologies. Sb4Te3 and Sb8Te9 as new members of the series (Sb2Te3)m∙(Sb2)n. Chem. Commun. 2005, 2672–2674. [Google Scholar] [CrossRef]
- Anderson, T.L.; Krause, H.B. Refinement of the Sb2Te3 and Sb2Te2Se structures and their relationship to nonstoichiometric Sb2Te3−ySeycompounds. Acta Crystallogr. Sect. B 1974, 30, 1307–1310. [Google Scholar] [CrossRef]
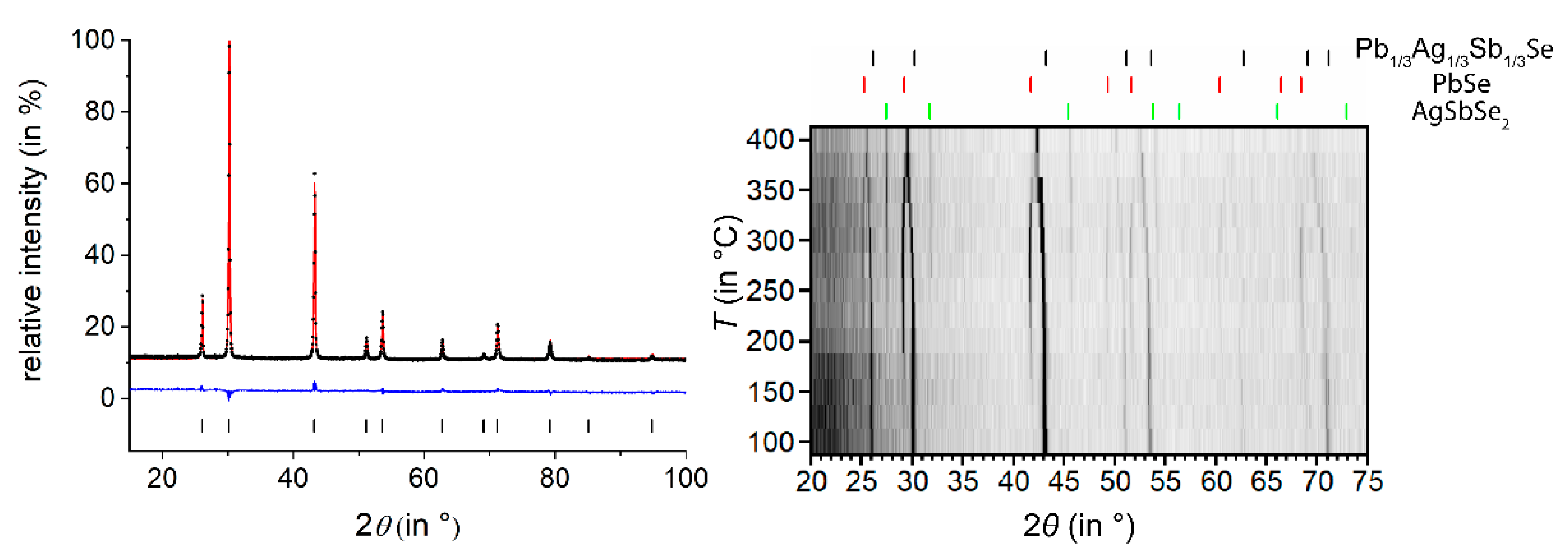

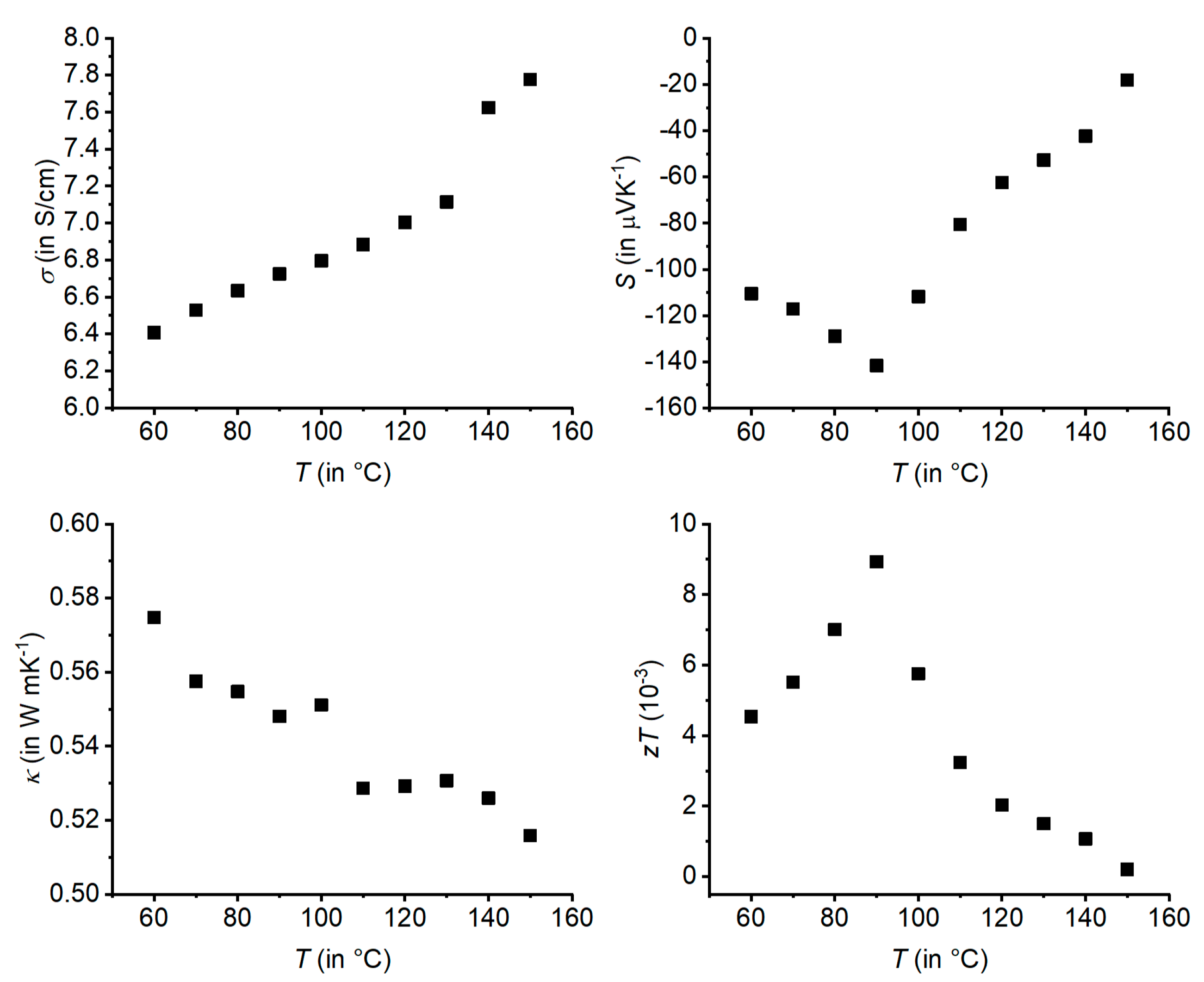
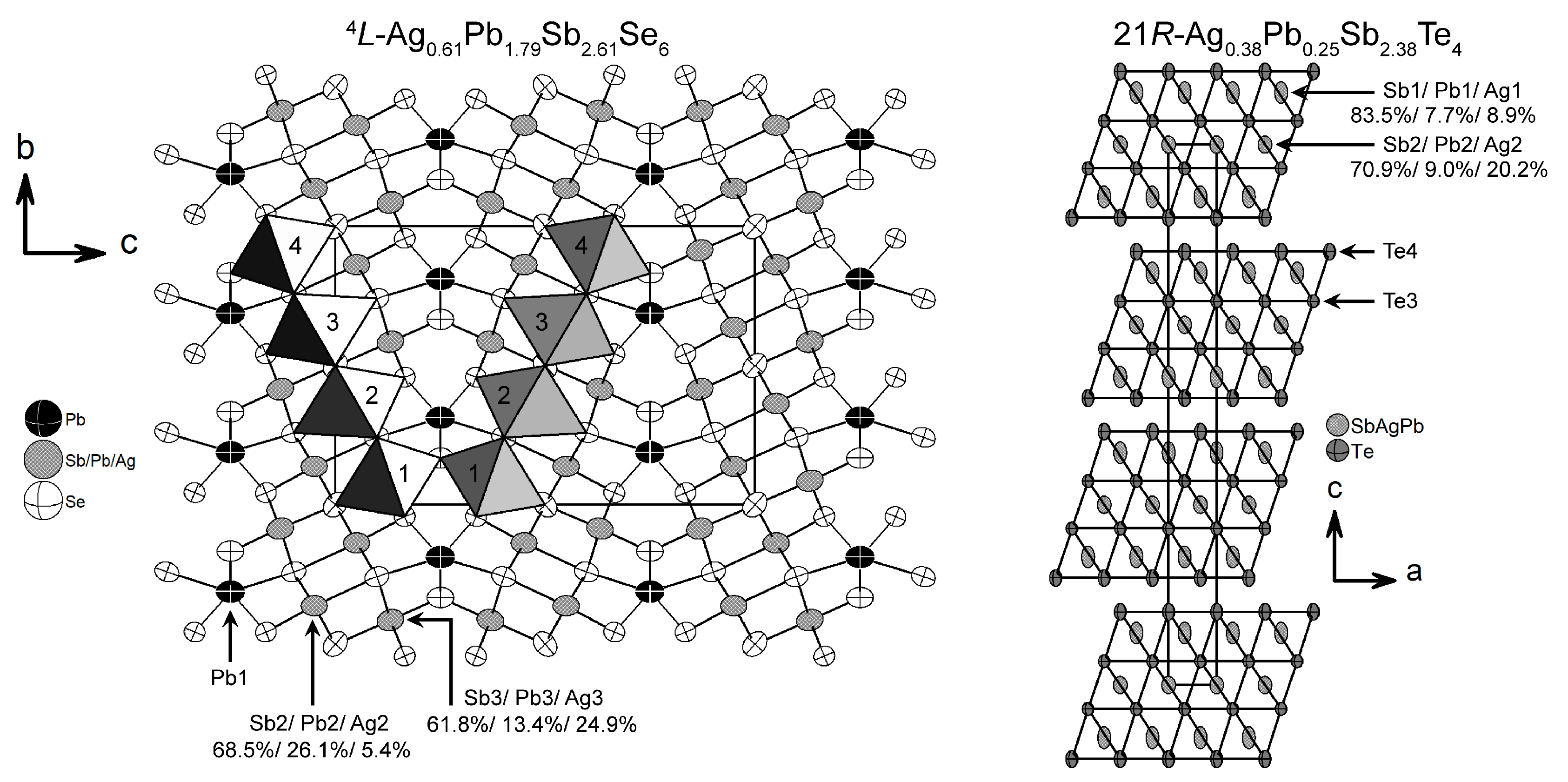
| Empirical Formula | Ag1/3Pb1/3Sb1/3Se | Ag0.38Pb0.25Sb2.38Te4 | Ag0.61Pb1.79Sb2.61Se6 |
|---|---|---|---|
| M (in g∙mol−1) | 224.56 | 892.19 | 1226.96 |
| crystal system/space group | cubic/Fmm (№ 225) | trigonal/Rm (№ 166) | orthorhombic/Cmcm (№ 63) |
| cell parameters (in Å) | a = 5.9268(7) | a = 4.2887(1) c = 41.544(1) | a = 4.2118(1) b = 13.859(1) c = 20.862(1) |
| V (in ų) | 208.19(7) | 661.74(3) | 1217.74(11) |
| X-ray density (in g∙cm−3) | 7.165 | 6.716 | 6.692 |
| Z (per unit cell) | 4 | 3 | 4 |
| F(000) | 376 | 1102 | 2048 |
| radiation | Ag-Kα1 | synchrotron | |
| λ (in Å)/E (in keV) | 0.56086/22.106 | 0.500/24.800 | |
| absorption correction | semiempirical (LANA) [45] | semiempirical (SADABS) [48] | |
| μ (in mm−1) | 27.775 | 9.902 | 19.408 |
| resolution (in Å) | 0.60 | 0.71 | 0.65 |
| parameters/constraints | 4/0 | 19/3 | 43/3 |
| weighting scheme P = [Max(0, Fo2) + 2Fc2]/3 | w = 1/(σ2(Fo2) + (0.0152 P)2) | w = 1/(σ2(Fo2) + (0.0361 P)2 + 3.0996 P) | w = 1/(σ2(Fo2) + (0.0313 P)2 + 4.0 P) |
| ∆ρmax/∆ρmin (in e/Å3) | 0.494/−0.434 | 2.126/−2.081 | 1.746/−2.632 |
| θmin/θmax | 4.701/27.619 | 2.069/20.586 | 2.179/22.618 |
| reflections collected | 1342 | 3975 | 9589 |
| independent reflections | 40 | 294 | 1325 |
| Rint/Rσ | 0.0378/0.0075 | 0.0278/0.0139 | 0.0558/0.0434 |
| R1/wR2 [observed] | 0.0133/0.0269 | 0.0203/0.0599 | 0.0360/0.0965 |
| R1/wR2 [all data] | 0.0133/0.0269 | 0.0208/0.0605 | 0.0413/0.0991 |
| GooF [all data] | 1.201 | 1.128 | 1.348 |
| Ag0.38Pb0.25Sb2.38Te4 | Ag0.61Pb1.79Sb2.61Se6 | ||||
|---|---|---|---|---|---|
| Ag1/Pb1/Sb1 | Ag2/Pb2/Sb2 | Pb1 | Ag2/Pb2/Sb2 | Ag3/Pb3/Sb3 | |
| Ag | 1.248 | 1.302 | - | 0.909 | 1.048 |
| Pb | 3.045 | 3.180 | 2.058 | 2.902 | 3.349 |
| Sb | 2.589 | 2.706 | - | 2.378 | 2.626 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grauer, M.; Benndorf, C.; Rohr, V.; Paulmann, C.; Oeckler, O. Investigations of Some Disordered Quaternary Compounds in the Systems Ag/Pb/Sb/Se and Ag/Pb/Sb/Te. Crystals 2024, 14, 789. https://doi.org/10.3390/cryst14090789
Grauer M, Benndorf C, Rohr V, Paulmann C, Oeckler O. Investigations of Some Disordered Quaternary Compounds in the Systems Ag/Pb/Sb/Se and Ag/Pb/Sb/Te. Crystals. 2024; 14(9):789. https://doi.org/10.3390/cryst14090789
Chicago/Turabian StyleGrauer, Maxim, Christopher Benndorf, Valentin Rohr, Carsten Paulmann, and Oliver Oeckler. 2024. "Investigations of Some Disordered Quaternary Compounds in the Systems Ag/Pb/Sb/Se and Ag/Pb/Sb/Te" Crystals 14, no. 9: 789. https://doi.org/10.3390/cryst14090789
APA StyleGrauer, M., Benndorf, C., Rohr, V., Paulmann, C., & Oeckler, O. (2024). Investigations of Some Disordered Quaternary Compounds in the Systems Ag/Pb/Sb/Se and Ag/Pb/Sb/Te. Crystals, 14(9), 789. https://doi.org/10.3390/cryst14090789








