Combining Cocatalyst and Oxygen Vacancy to Synergistically Improve Fe2O3 Photoelectrochemical Water Oxidation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fe2O3, FeOOH-Fe2O3, VO-Fe2O3, and Vo-FeOOH-Fe2O3 Photoanodes
2.2. Structural Characterization
2.3. Photoelectrochemical Measurements
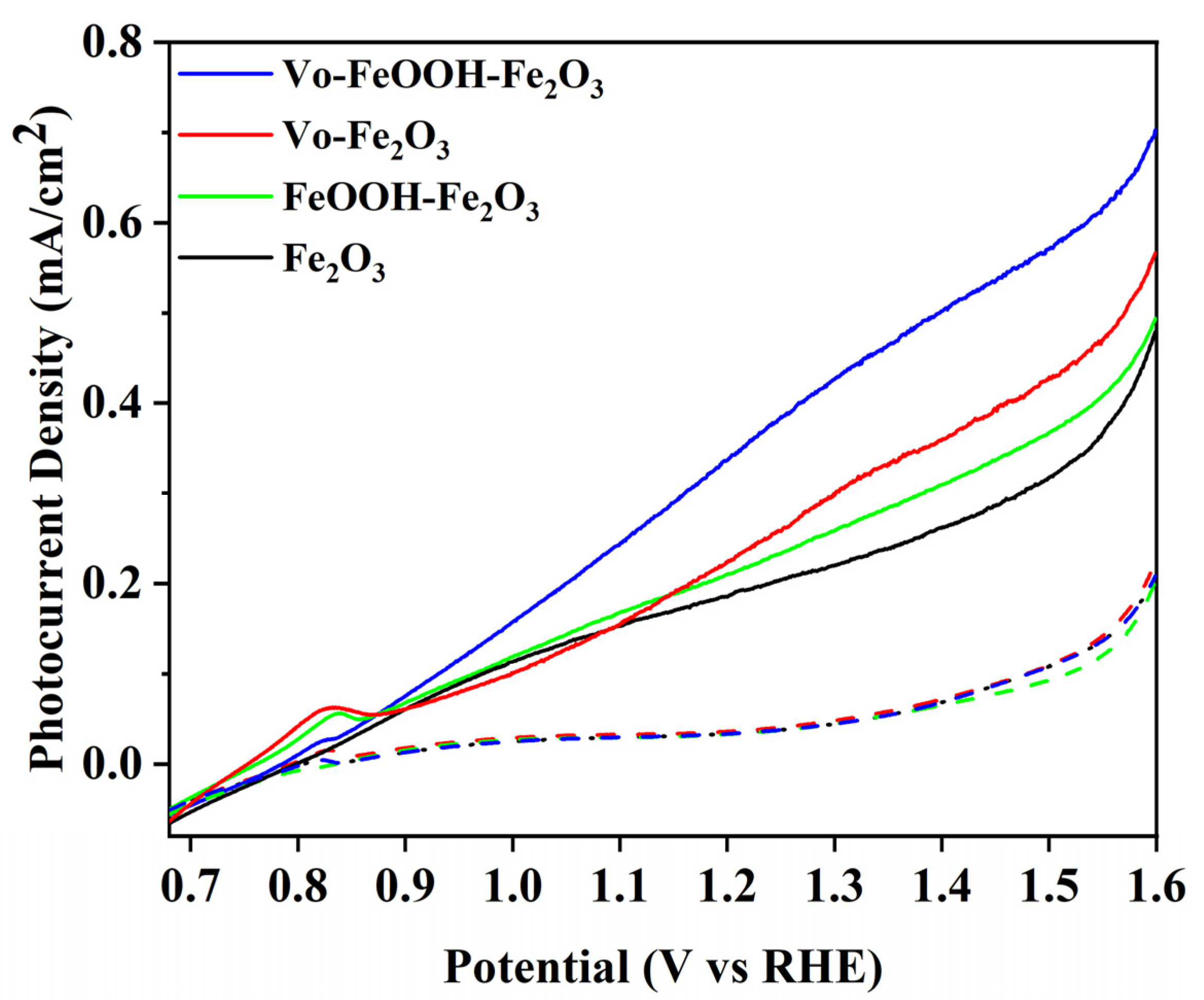
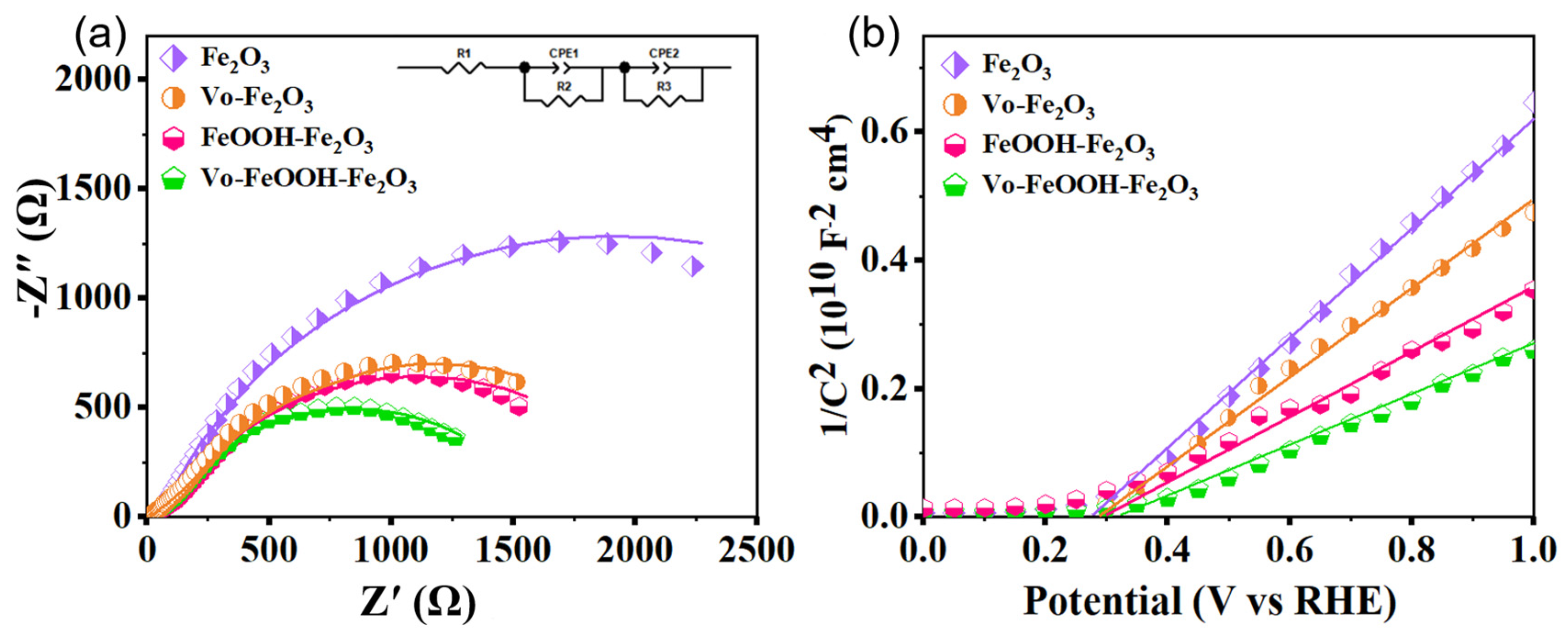
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Wang, D.; Li, F.; Liu, Y.; Shen, S.; Meyer, T.J. Hybrid Photoelectrochemical Water Splitting Systems: From Interface Design to System Assembly. Adv. Energy Mater. 2020, 10, 1900399. [Google Scholar] [CrossRef]
- He, Y.; Hamann, T.; Wang, D. Thin film photoelectrodes for solar water splitting. Chem. Soc. Rev. 2019, 48, 2182–2215. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Wang, C.; Zhang, M.; Huang, L.; Lai, J.; Wang, L.; Li, J.; Jin, X.; Yang, W. Construction of a TiO2 heterostructure nanowire with a sulfurized shell via a simple sulfurization process for enhanced photoelectrochemical water oxidation. J. Alloys Compd. 2021, 858, 158375. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, J.; Chen, S.; Bai, J.; Li, J.; Zhang, Y.; Li, L.; Xia, L.; Rahim, M.; Xu, Q.; et al. Bird-nest structured ZnO/TiO2 as a direct Z-scheme photoanode with enhanced light harvesting and carriers kinetics for highly efficient and stable photoelectrochemical water splitting. Appl. Catal. B Environ. 2020, 267, 118599–118610. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Seo, H. Directing WO3 crystal growth towards artificial photosynthesis favorable {002} plane via aluminum incorporation in the lattice for enhanced water splitting. J. Alloys Compd. 2021, 864, 158186. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, X.; Zhang, Y.; Lu, G.; Chou, L.; Bi, Y. Unveiling the Activity and Stability Origin of BiVO4 Photoanodes with FeNi Oxyhydroxides for Oxygen Evolution. Angew. Chem. Int. Ed. 2020, 59, 18990–18995. [Google Scholar] [CrossRef]
- Han, T.; Wu, L.; Wang, P.; Wang, T.; Yang, Z.; Tian, K.; Jin, J. Room chemical bath temperature deposition of Mn:FeOOH on BiVO4 photoanode to enhance water oxidation. J. Alloys Compd. 2022, 894, 162571. [Google Scholar] [CrossRef]
- Peng, Y.; Ruan, Q.; Lam, C.H.; Meng, F.; Guan, C.-Y.; Santoso, S.P.; Zou, X.; Yu, E.T.; Chu, P.K.; Hsu, H.-Y. Plasma-implanted Ti-doped hematite photoanodes with enhanced photoelectrochemical water oxidation performance. J. Alloys Compd. 2021, 870, 159376. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, Q.; Lv, X.; Xu, H.; Ma, D.; Zhong, J. Understanding Photoelectrochemical Water Oxidation with X-ray Absorption Spectroscopy. ACS Energy Lett. 2020, 5, 975–993. [Google Scholar] [CrossRef]
- Hu, W.; Quang, N.D.; Majumder, S.; Jeong, M.J.; Park, J.H.; Cho, Y.J.; Kim, S.B.; Lee, K.; Kim, D.; Chang, H.S. Three-dimensional nanoporous SnO2/CdS heterojunction for high-performance photoelectrochemical water splitting. Appl. Surf. Sci. 2021, 560, 149904. [Google Scholar] [CrossRef]
- Majumder, S.; Quang, N.D.; Hien, T.T.; Chinh, N.D.; Yang, H.; Kim, C.; Kim, D. Nanostructured β-Bi2O3/PbS heterojunction as np-junction photoanode for enhanced photoelectrochemical performance. J. Alloys Compd. 2021, 870, 159545. [Google Scholar] [CrossRef]
- Majumder, S.; Gu, M.; Kim, K.H. Effect of annealing of β-Bi2O3 over enhanced photoelectrochemical performance. Mater. Sci. Semicon. Proc. 2022, 141, 106439. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, L.; Lou, X.W.D. Construction of Heterostructured Fe2O3-TiO2 Microdumbbells for Photoelectrochemical Water Oxidation. Angew. Chem. Int. Ed. 2018, 57, 15076–15080. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Zhang, G.; Gong, Z.; Wu, B.; Wang, T.; Gong, J. Tandem Cells for Unbiased Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2023, 52, 4644–4671. [Google Scholar]
- Zhang, Z.-H.; Yang, T.; Wang, Z.-Y.; Wang, H.-C.; Yue, X.-Z.; Yi, S.-S. Extremely low onset potential of modified Fe2O3 photoanode for water oxidation. Appl. Surf. Sci. 2024, 642, 158597. [Google Scholar] [CrossRef]
- Xia, W.W.; Zhang, R.; Chai, Z.C.; Pu, J.Y.; Kang, R.; Wu, G.Q.; Zeng, X.H. Synergies of Zn/P-co-doped α-Fe2O3 photoanode for improving photoelectrochemical water splitting performance. Int. J. Hydrog. Energy 2024, 59, 22–29. [Google Scholar] [CrossRef]
- Ye, K.H.; Wang, Z.; Li, H.; Yuan, Y.; Mai, W. A novel CoOOH/(Ti, C)-Fe2O3 nanorod photoanode for photoelectrochemical water splitting. Sci. China Mater. 2018, 61, 887–894. [Google Scholar] [CrossRef]
- Zhou, D.; Fan, K. Recent strategies to enhance the efficiency of hematite photoanodes in photoelectrochemical water splitting. Chin. J. Catal. 2021, 42, 904–919. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Reddy, J.; Wang, C.; Zhang, J.Z.; Li, Y. The influence of oxygen content on the thermal activation of hematite nanowires. Angew. Chem. Int. Ed. 2012, 51, 4074–4079. [Google Scholar] [CrossRef]
- Yuan, Y.; Gu, J.; Ye, K.H.; Chai, Z.; Yu, X.; Chen, X.; Zhao, C.; Zhang, Y.; Mai, W. Combining Bulk/Surface Engineering of Hematite to Synergistically Improve Its Photoelectrochemical Water Splitting Performance. ACS Appl. Mater. Interfaces 2016, 8, 16071–16077. [Google Scholar] [CrossRef] [PubMed]
- Pu, A.; Deng, J.; Li, M.; Gao, J.; Zhang, H.; Hao, Y.; Zhong, J.; Sun, X. Coupling Ti-Doping and Oxygen Vacancies in Hematite Nanostructures for Solar Water Oxidation with High Efficiency. J. Mater. Chem. A 2014, 2, 2491–2497. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.X.; Zhang, W.D. Photoelectrochemical properties of Ni-doped Fe2O3 thin films prepared by electrodeposition. Electrochim. Acta 2012, 59, 121–127. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Wheeler, D.A.; Zhang, J.Z.; Li, Y. Sn-doped hematite nanostructures for photoelectrochemical water splitting. Nano Lett. 2011, 11, 2119–2125. [Google Scholar] [CrossRef]
- Wang, L.; Lee, C.Y.; Mazare, A.; Lee, K.; Müller, J.; Spiecher, E.; Schmuki, P. Enhancing the Water Splitting Efficiency of Sn-Doped Hematite Nanoflakes by Flame Annealing. Chem. Eur. J. 2014, 20, 77–82. [Google Scholar] [CrossRef]
- Feng, C.; Bi, Y.; Zhan, F.; Bi, Y. Boosting Interfacial Bonding between FeOOH Catalysts and Fe2O3 Photoanodes toward Efficient Water Oxidation. J. Mater. Chem. A 2024, 12, 16361–16366. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Li, Y. Oxygen-Deficient Metal Oxide Nanostructures for Photoelectrochemical Water Oxidation and other Applications. Nanoscale 2012, 4, 6682–6691. [Google Scholar] [CrossRef]
- Yang, T.Y.; Kang, H.Y.; Sim, U.; Lee, Y.J.; Lee, J.H.; Koo, B.; Nam, K.T.; Joo, Y.C. A New Hematite Photoanode Doping Strategy for Solar Water Splitting: Oxygen Vacancy Generation. Phys. Chem. Chem. Phys. 2013, 15, 2117–2124. [Google Scholar] [CrossRef]
- Zhu, C.; Li, C.; Zheng, M.; Delaunay, J.J. Plasma-Induced Oxygen Vacancies in Ultrathin Hematite Nanoflakes Promoting Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 22355–22363. [Google Scholar]
- Wang, L.; Zhu, J.; Liu, X. Oxygen-Vacancy-Dominated Cocatalyst/Hematite Interface for Boosting Solar Water Splitting. ACS Appl. Mater. Interfaces 2019, 11, 22272–22277. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Chen, S.; Huang, Y.; Sum, T.C.; Chen, Z. New insight into the roles of oxygen vacancies in hematite for solar water splitting. Phys. Chem. Chem. Phys. 2017, 19, 1074–1082. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Gong, D.; Chen, Y.; Xu, S.; Qiu, P. Solar water splitting with nanostructured hematite: The role of oxygen vacancy. J. Mater. Sci. 2022, 57, 19716–19729. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. Visible light-driven α-Fe2O3 nanorod/graphene/BiV(1 − x)MoxO4 core/shell heterojunction array for efficient photoelectrochemical water splitting. Nano Lett. 2012, 12, 6464–6473. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Lindley, S.A.; Chen, X.; Zhang, J.Z. Hematite Heterostructures for Photoelectrochemical Water Splitting: Rational Materials Design and Charge Carrier Dynamics. Energy Environ. Sci. 2016, 9, 2744–2775. [Google Scholar] [CrossRef]
- Sang, Y.; Cao, X.; Ding, G.; Guo, Z.; Xue, Y.; Li, G.; Yu, R. Constructing oxygen vacancy-enriched Fe2O3@NiO heterojunctions for highly efficient electrocatalytic alkaline water splitting. Cryst. Eng. Comm. 2022, 24, 199–207. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Ding, R.; Fu, Q.; Bi, L.L.; Zhou, X.; Yan, W.; Xia, W.; Luo, Z. A highly efficient MoOx/Fe2O3 photoanode with rich vacancies for photoelectrochemical O2 evolution from water splitting. New J. Chem. 2024, 48, 1587–1595. [Google Scholar] [CrossRef]
- Makimizu, Y.; Yoo, J.E.; Poornajar, M.; Nguyen, N.T.; Ahn, H.J.; Hwang, I.; Kment, S.; Schmuki, P. Effects of low oxygen annealing on the photoelectrochemical water splitting properties of α-Fe2O3. J. Mater. Chem. A 2020, 8, 1315–1325. [Google Scholar] [CrossRef]
- Noh, M.F.M.; Ullah, H.; Arzaee, N.A.; Halim, A.A.; Rahim, M.A.F.A.; Mohamed, N.A.; Safaei, J.; Nasir, S.N.F.M.; Wang, G.; Teridi, M.A.M. Rapid fabrication of oxygen defective α-Fe2O3 for enhanced photoelectrochemical activities. Dalton Trans. 2020, 49, 12037–12048. [Google Scholar]
- Shi, J.; Zhao, X.; Li, C. Surface Passivation Engineering for Photoelectrochemical Water Splitting. Catalysts 2023, 13, 217. [Google Scholar] [CrossRef]
- Yu, Q.; Meng, X.; Wang, T.; Li, P.; Ye, J. Hematite Films Decorated with Nanostructured Ferric Oxyhydroxide as Photoanodes for Efficient and Stable Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2015, 25, 2686–2692. [Google Scholar] [CrossRef]
- Quang, N.D.; Van, P.C.; Majumder, S.; Jeong, J.-R.; Kim, D.; Kim, C. Rational construction of S-doped FeOOH onto Fe2O3 nanorods for enhanced water oxidation. J. Colloid. Interface Sci. 2022, 616, 749–758. [Google Scholar] [CrossRef]
- Lan, H.; Wei, A.; Zheng, H.; Sun, X.; Zhong, J. Boron-passivated surface Fe(IV) defects in hematite for highly efficient water oxidation. Nanoscale 2018, 10, 7033–7039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, M.K.; Hou, J.X.; Niu, Y.K.; Ni, D.W.; Shen, H.Y.; Niu, P.; Ma, Y. Introduction of oxygen vacancies into hematite in local reducing atmosphere for solar water oxidation. Sol. Energy 2019, 179, 99–105. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Zhang, Y.; Rui, Q.; Zhang, B.; Shen, Z.; Bi, Y. One-Dimensional Hematite Photoanodes with Spatially Separated Pt and FeOOH Nanolayers for Efficient Solar Water Splitting. J. Mater. Chem. A 2017, 5, 17056–17063. [Google Scholar] [CrossRef]
- Faria, D.L.A.D.; Silva, S.V.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Wang, J.; Leng, X.; Kan, S.; Cui, Y.; Bai, J.; Xu, L. Oxygen vacancies boosted photoelectrochemical performance of α-Fe2O3 photoanode via butane flame annealing. J. Alloy. Compd. 2024, 976, 172990. [Google Scholar] [CrossRef]
- Chemelewski, W.D.; Lee, H.C.; Lin, J.F.; Bard, A.J.; Mullins, C.B. Amorphous FeOOH oxygen evolution reaction catalyst for photoelectrochemical water splitting. J. Am. Chem. Soc. 2014, 136, 2843–2850. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Zetaruk, D.G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Wang, H.; Yang, Y.; Li, Y. Low-Temperature Activation of Hematite Nanowires for Photoelectrochemical Water Oxidation. ChemSusChem 2014, 7, 848–853. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, L.; Guo, X.; Xu, J.; Shi, S.; Li, L. Surface oxygen vacancies on WO3 nanoplate arrays induced by Ar plasma treatment for efficient photoelectrochemical water oxidation. J. Phys. Chem. Solids 2021, 149, 109823. [Google Scholar] [CrossRef]
- Zhang, J.; Eslava, S. Understanding charge transfer, defects and surface states at hematite photoanodes. Sustain. Energy Fuels 2019, 3, 1351–1364. [Google Scholar] [CrossRef]
- Mouchani, N.; Farahmand-Dashtarjandi, A.H.; Yourdkhani, A.; Poursalehi, R.; Simhachalam, N.B. Oxygen vacancy modulation of hematite thin films using annealing in graphite bed for photoelectrochemical applications. Surf. Interfaces 2023, 42, 103456. [Google Scholar] [CrossRef]



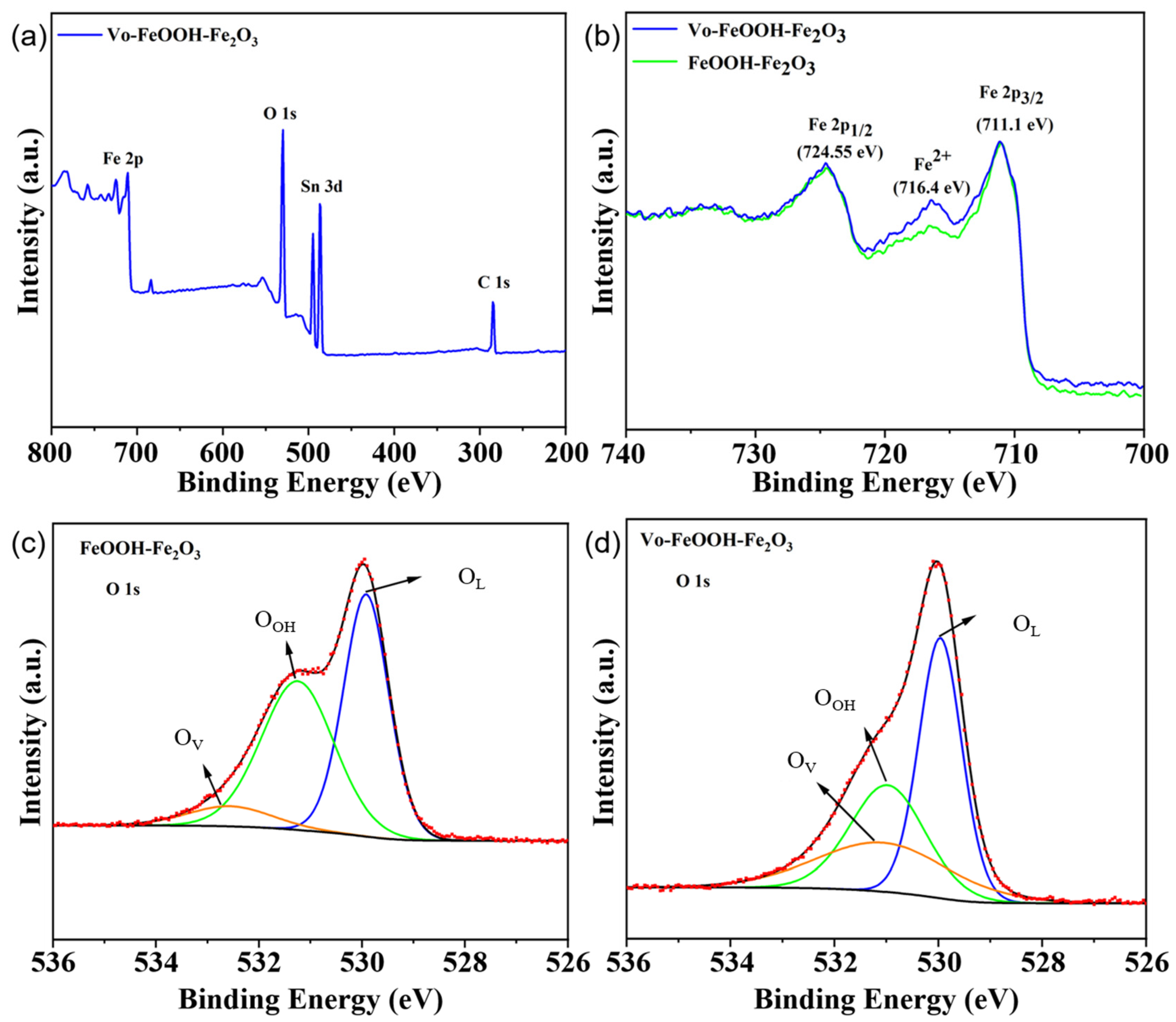
| Sample | OL (Proportion) | -OH (Proportion) | OV (Proportion) |
|---|---|---|---|
| FeOOH-Fe2O3 | 47% | 46% | 7% |
| Vo- FeOOH-Fe2O3 | 44% | 31% | 25% |
| Sample | R1 (Ω) | R2 (Ω) | R3 (Ω) | CPE1 (F) | CPE2 (F) |
|---|---|---|---|---|---|
| Fe2O3 | 12.35 | 44.35 | 3732 | 3.4006 × 10−5 | 2.9527 × 10−6 |
| Vo-Fe2O3 | 8.01 | 61.31 | 2312 | 5.4402 × 10−5 | 6.6265 × 10−6 |
| FeOOH-Fe2O3 | 12.38 | 81.98 | 2035 | 4.9873 × 10−6 | 3.3932 × 10−6 |
| Vo- FeOOH-Fe2O3 | 13.69 | 7.14 | 471.9 | 8.9288 × 10−6 | 3.3378 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Li, J.; Zhang, W.; Zhu, C. Combining Cocatalyst and Oxygen Vacancy to Synergistically Improve Fe2O3 Photoelectrochemical Water Oxidation Performance. Crystals 2025, 15, 85. https://doi.org/10.3390/cryst15010085
Liu C, Li J, Zhang W, Zhu C. Combining Cocatalyst and Oxygen Vacancy to Synergistically Improve Fe2O3 Photoelectrochemical Water Oxidation Performance. Crystals. 2025; 15(1):85. https://doi.org/10.3390/cryst15010085
Chicago/Turabian StyleLiu, Chen, Jiajuan Li, Wenyao Zhang, and Changqing Zhu. 2025. "Combining Cocatalyst and Oxygen Vacancy to Synergistically Improve Fe2O3 Photoelectrochemical Water Oxidation Performance" Crystals 15, no. 1: 85. https://doi.org/10.3390/cryst15010085
APA StyleLiu, C., Li, J., Zhang, W., & Zhu, C. (2025). Combining Cocatalyst and Oxygen Vacancy to Synergistically Improve Fe2O3 Photoelectrochemical Water Oxidation Performance. Crystals, 15(1), 85. https://doi.org/10.3390/cryst15010085






