Enhanced Mechanical and Thermal Properties of Waste Electric Porcelain-Based Solar Energy-Absorbing Thermal Storage Ceramics with Interwoven Mullite Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
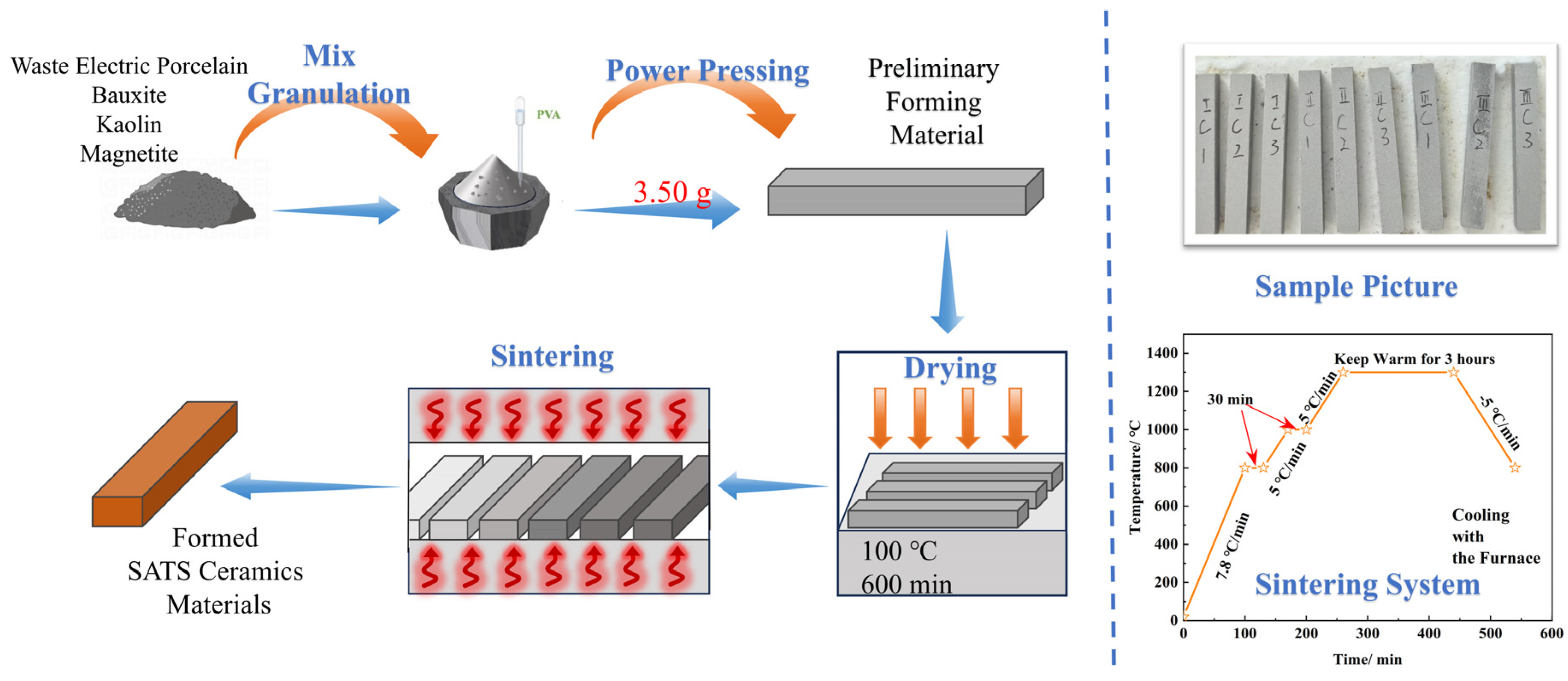
2.2. Preparation Process
2.3. Characterization
3. Results and Discussion
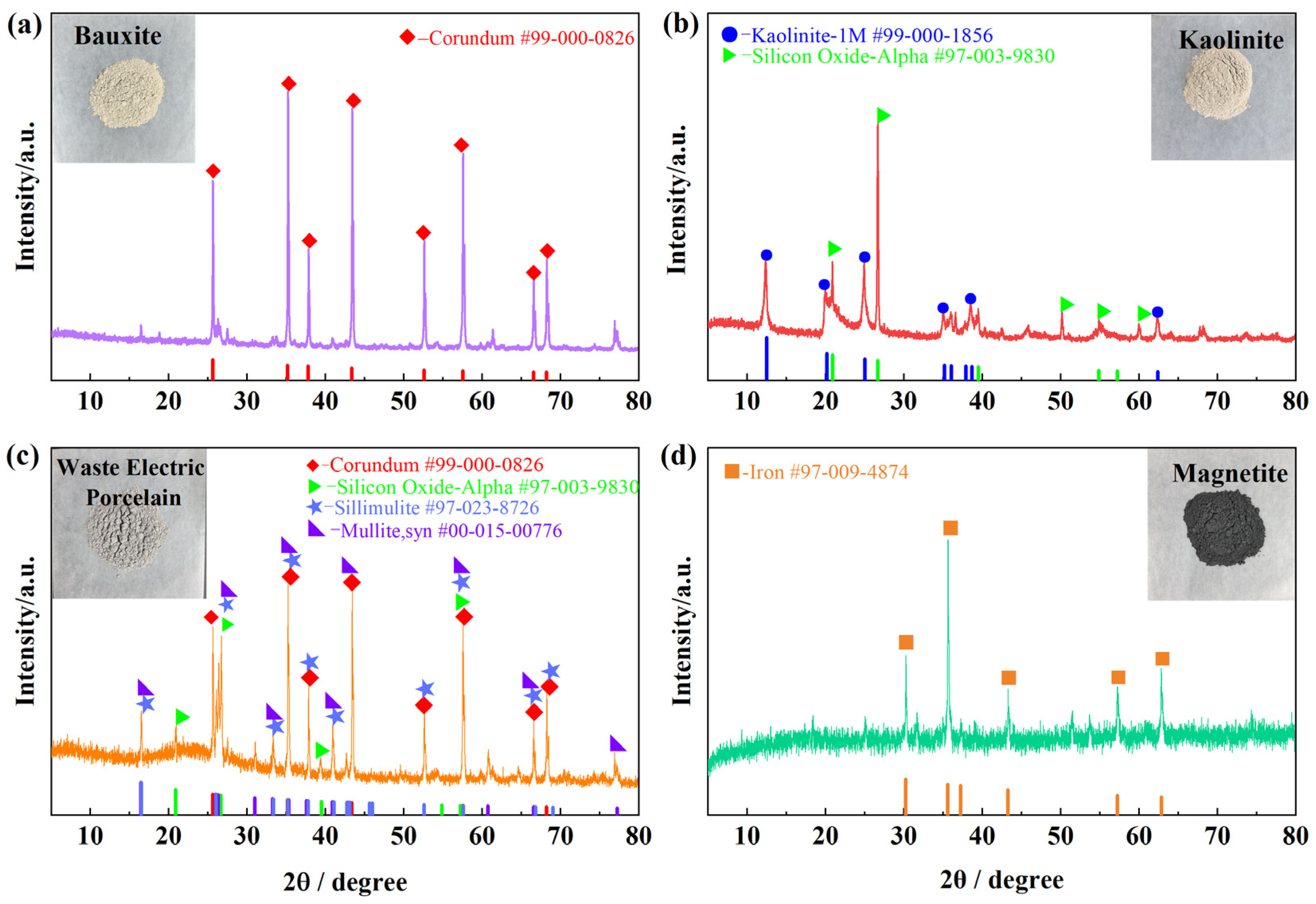
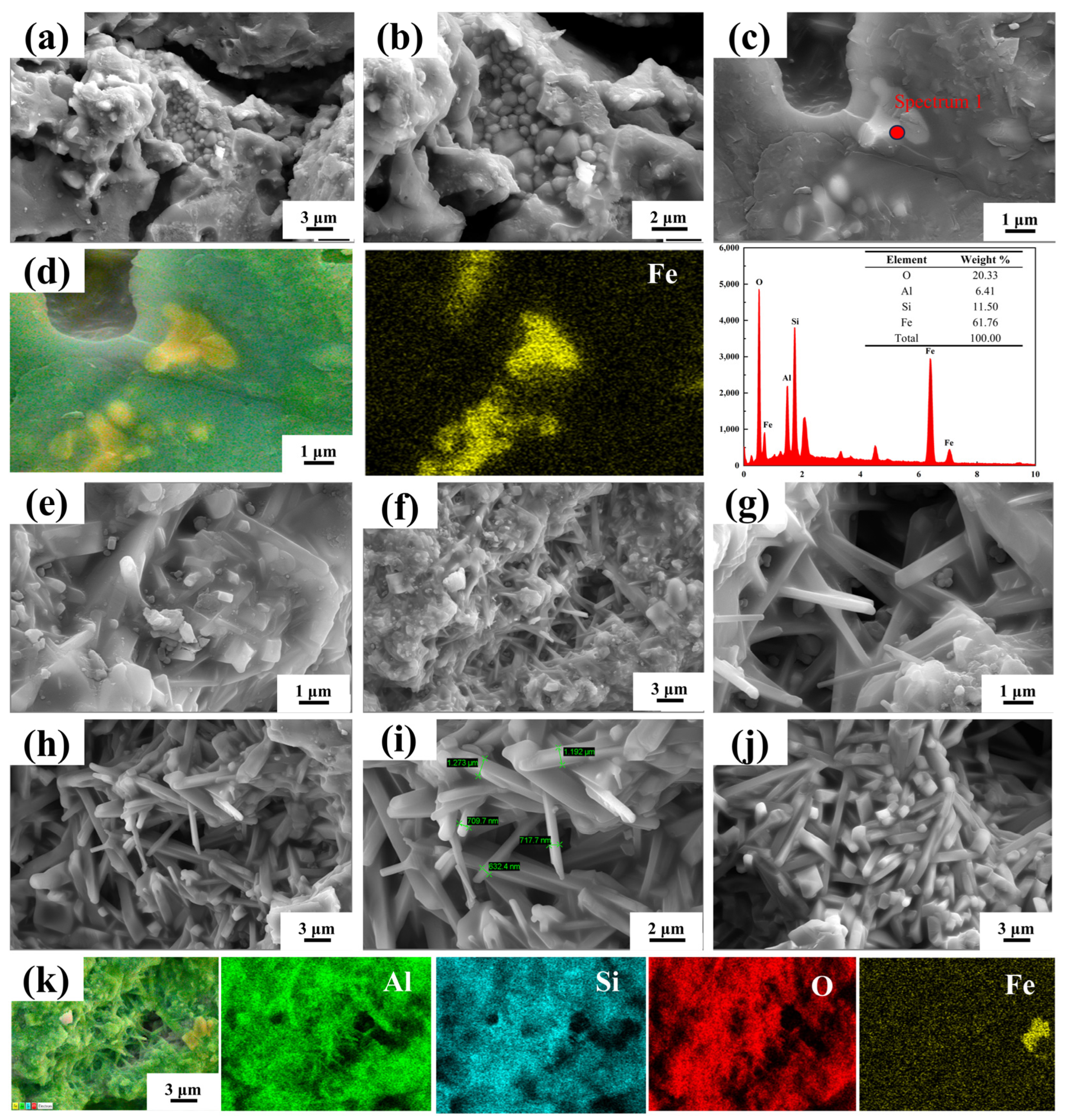
3.1. Compositional and Mineralogical Analysis of Raw Materials
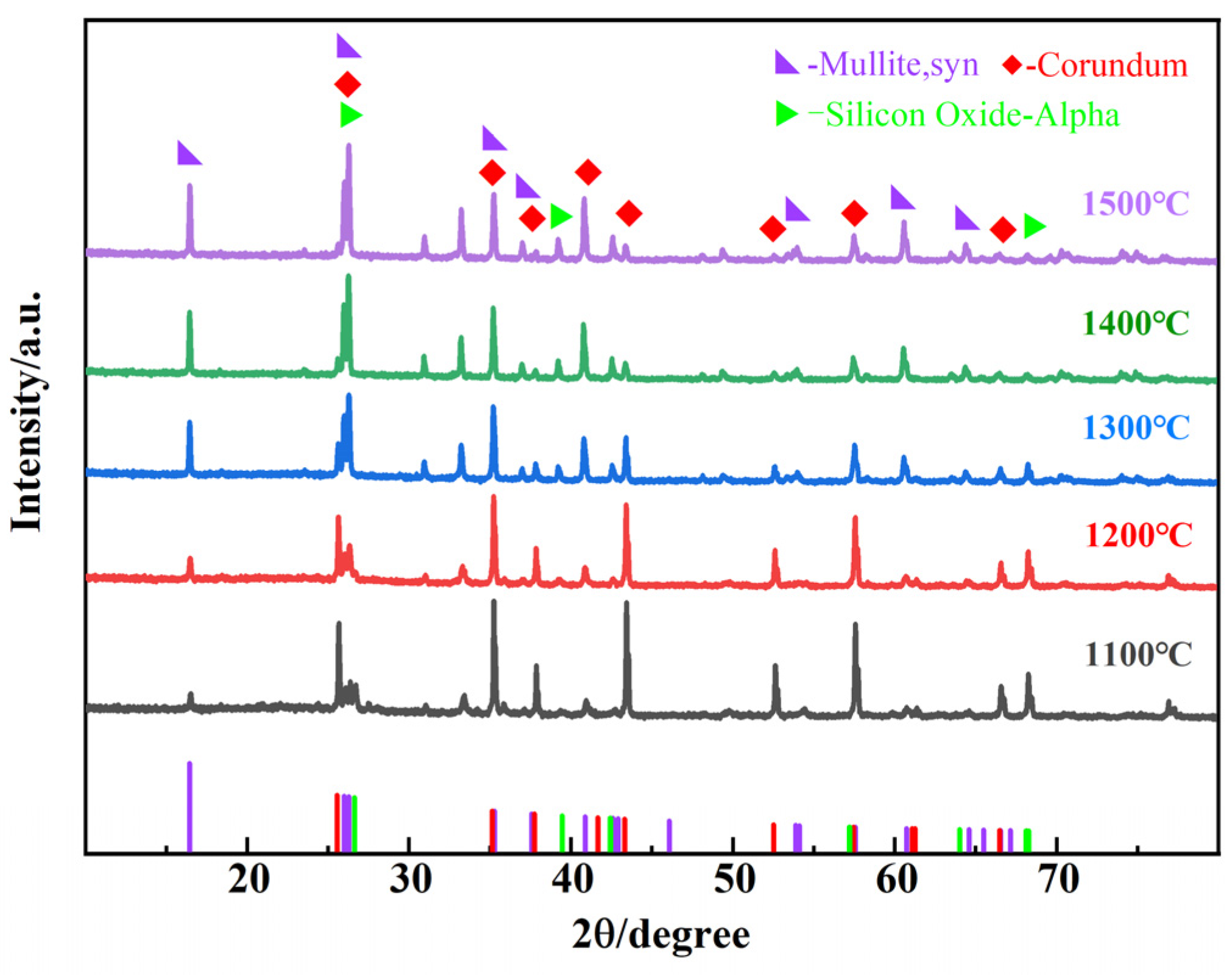
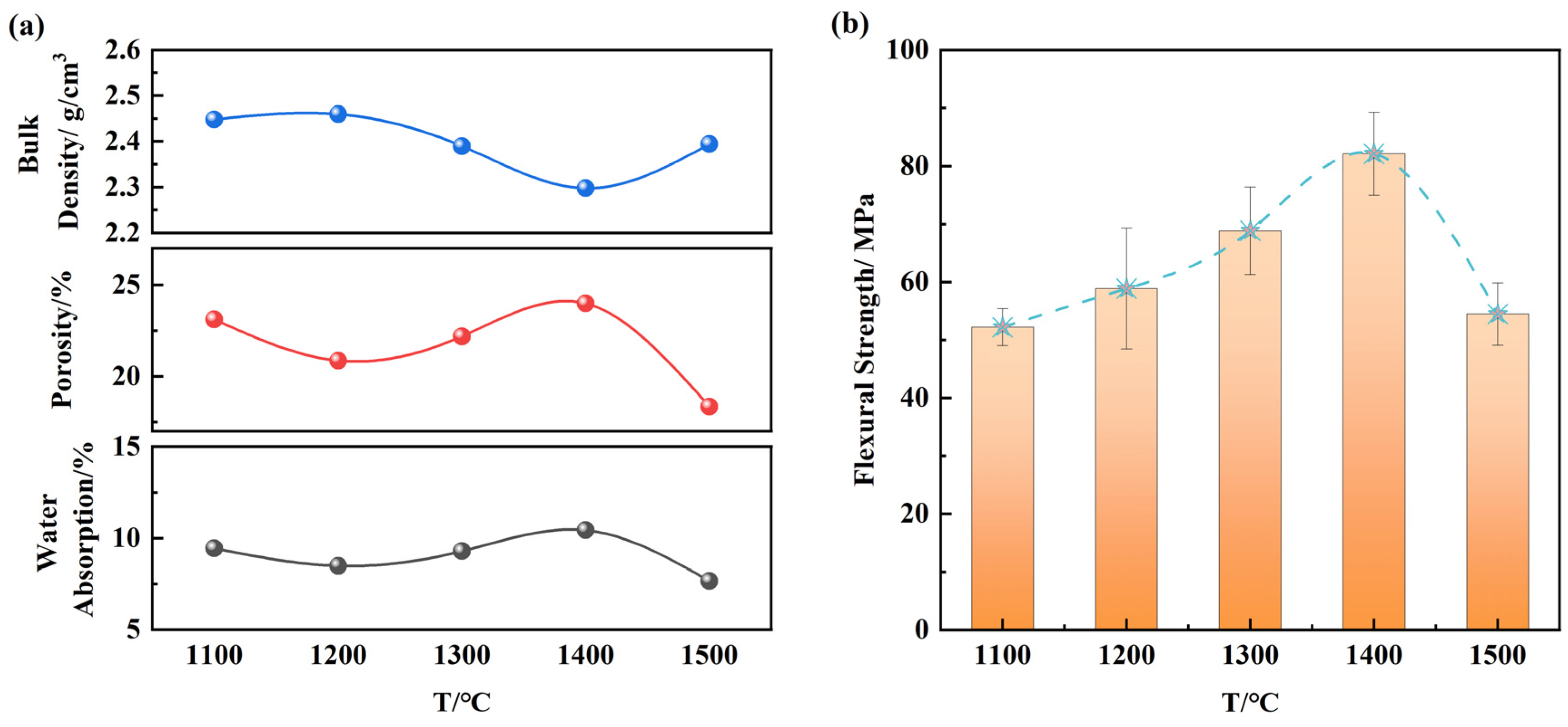
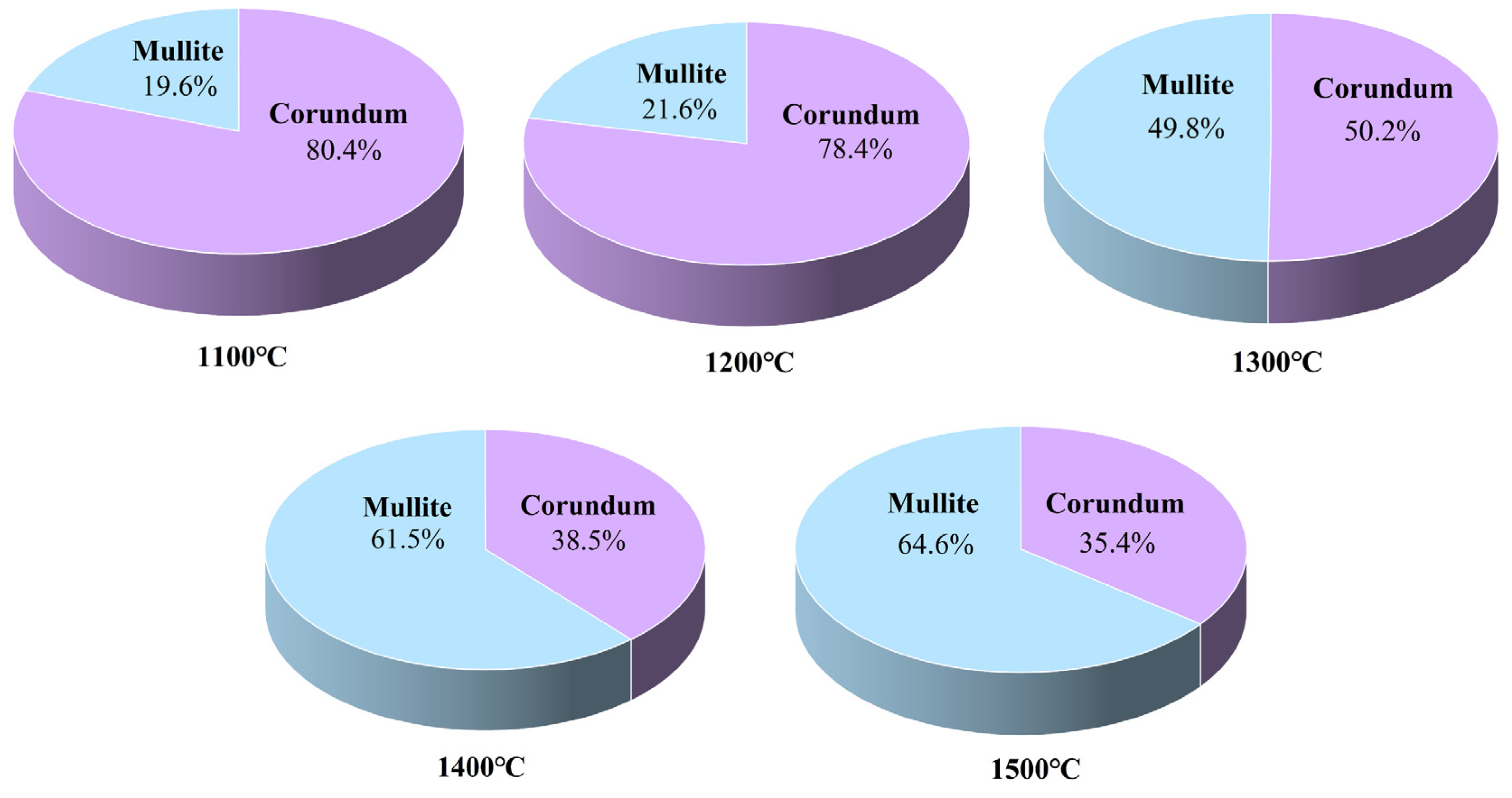
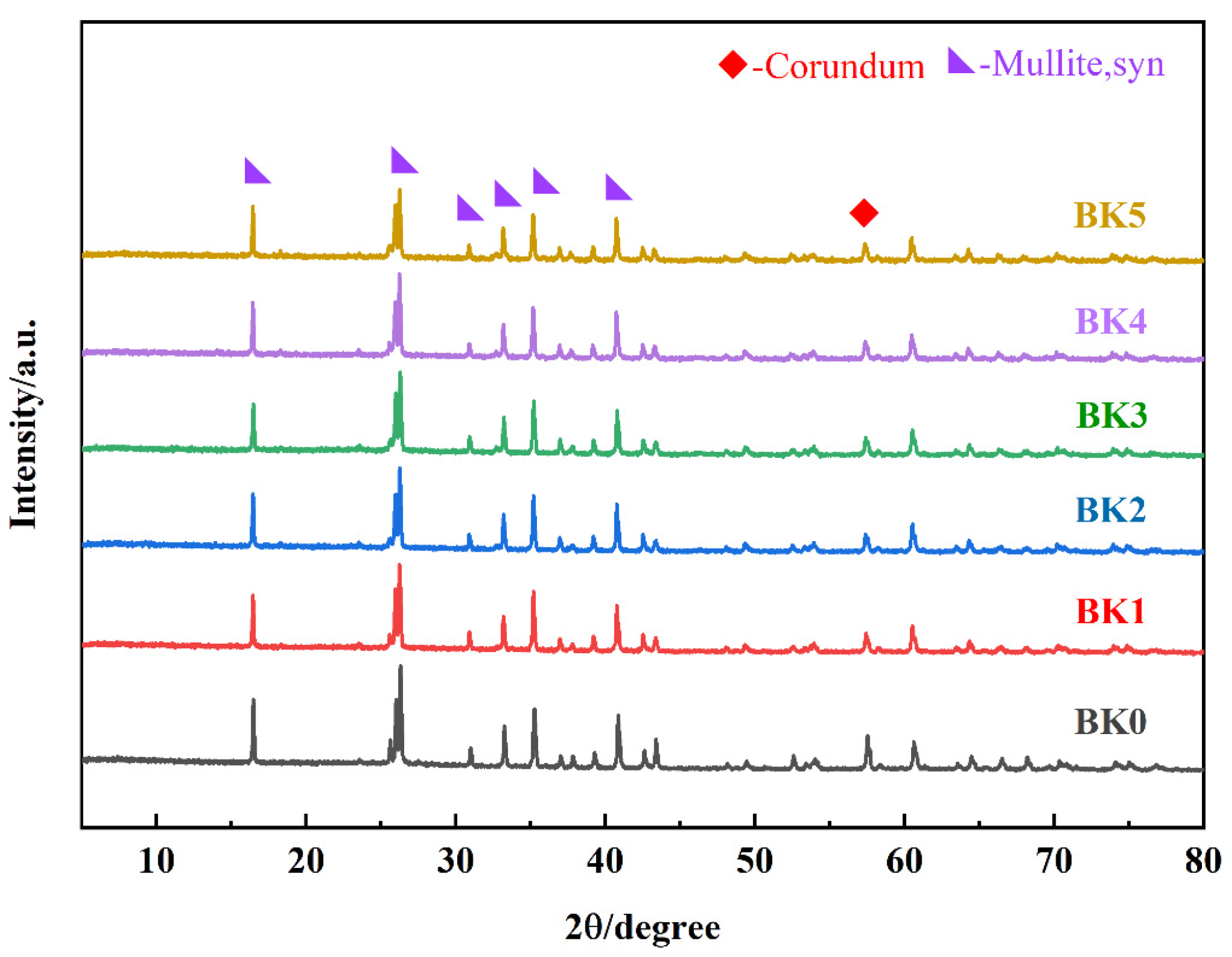
3.2. Effect of Sintering Temperature on Sample Properties
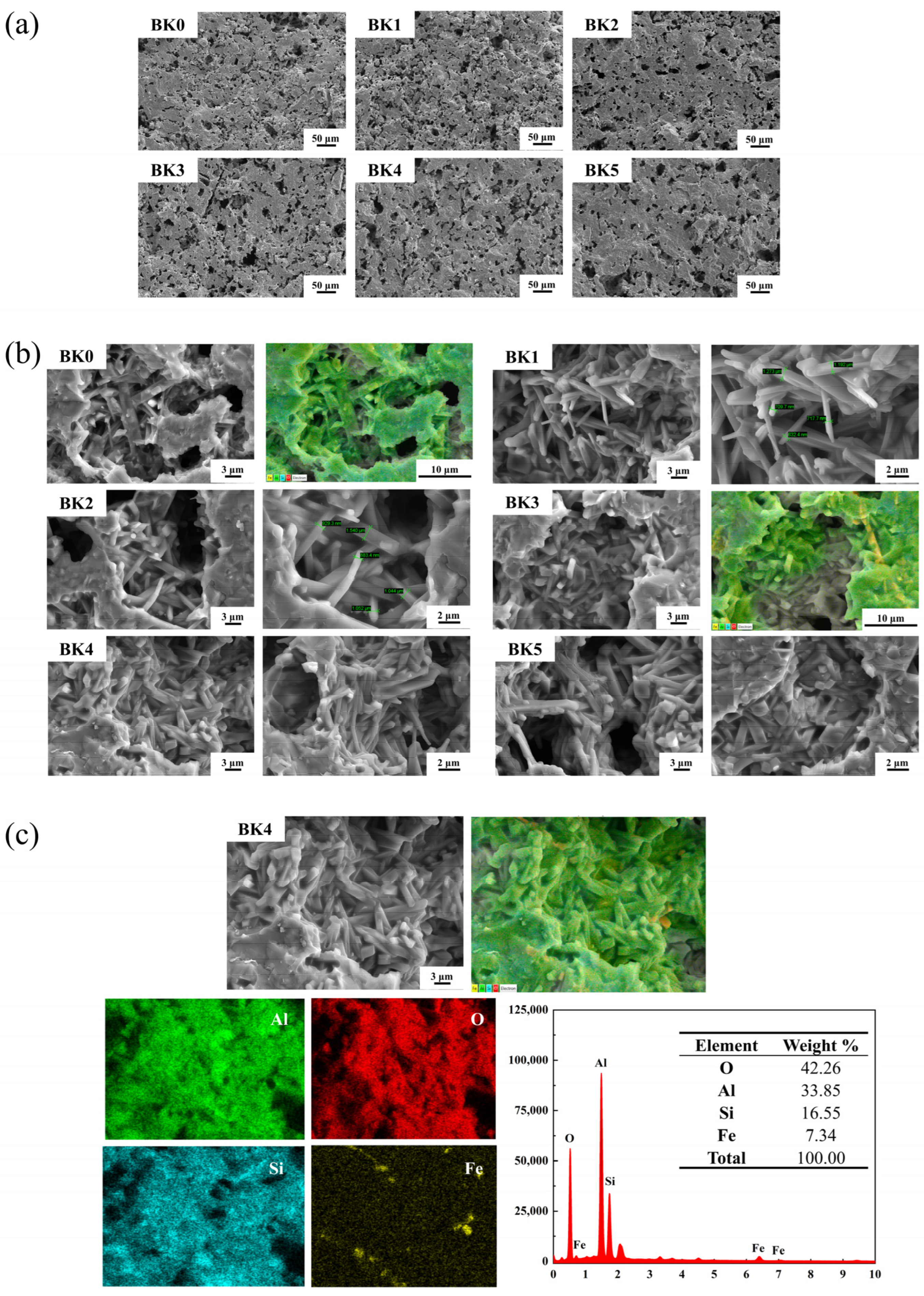
3.3. Effect of Magnetite Content on Sample Properties
4. Conclusions
- (1)
- At a sintering temperature of 1400 °C and a magnetite content of 11 wt.%, the material formed an optimal short-cluster, ordered interwoven columnar mullite structure, resulting in a flexural strength of 96.05 MPa and a bulk density of 2.35 g/cm3, which significantly enhanced the material’s properties.
- (2)
- Thermophysical analyses showed that the specific heat capacity (Cp) of the ceramics increased with increasing temperature, and that sample BK4 had the highest Cp value of 0.6415 J/(g* °C) at 300 °C, indicating excellent thermophysical properties.
- (3)
- XRD and SEM analyses confirmed the massive formation of mullite phase and grain growth under the optimized conditions, while physical property tests revealed the trends of porosity, bulk density, and water absorption, which affect the thermal stability and mechanical strength of the materials.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Lv, Z.; Zhang, K.; Lan, C.; Fan, M.; Lu, X. Waste electric porcelain-based refractory bricks with significantly enhanced mechanical properties: Preparation, characterization and mechanism. Ceram. Int. 2024, 50, 49698–49711. [Google Scholar] [CrossRef]
- Qiu, Z.; Sun, Y.; Cui, Y.; Zhang, Z.; Liu, Y.; Chen, X. Optimized Design of Shed Parameters for Polluted Hollow Porcelain Insulators at High Altitude. IEEE Access 2023, 11, 63451–63462. [Google Scholar] [CrossRef]
- Lv, Z.; Cao, Y.; Lan, C.; Fan, M.; Ke, Y.; Guo, W.; Yang, Y. Property dependence on particle size and sintering temperature of waste porcelain high-temperature resistant material. Int. J. Appl. Ceram. Technol. 2025, 22, e14890. [Google Scholar] [CrossRef]
- Dai, S.; Li, M.; Wu, X.; Wu, Y.; Li, X.; Hao, Y.; Luo, B. Combinatorial optimization of perovskite-based ferroelectric ceramics for energy storage applications. J. Adv. Ceram. 2024, 13, 877–910. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, Z.; Mostofinejad, D. Effects of high-temperature exposure on concrete containing waste porcelain coarse aggregates and steel chips. J. Build. Eng. 2020, 29, 101211. [Google Scholar] [CrossRef]
- Gautam, L.; Kalla, P.; Jain, J.K.; Choudhary, R.; Jain, A. Robustness of self-compacting concrete incorporating bone china ceramic waste powder along with granite cutting waste for sustainable development. J. Clean. Prod. 2022, 367, 132969. [Google Scholar] [CrossRef]
- Shen, X.; Zhu, L.; Lv, Z.; Shi, T.; Yin, Z.; Min, X. The Behavior of Slag Resistance of MgO-C Refractory Prepared by Sucrose as Binder. IOP Conf. Ser. Mater. Sci. Eng. 2019, 678, 012091. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Wei, H.; Xu, Y.; Zhou, R.; Huang, Z.; Zhan, H. Study on phase behavior and mechanical properties of high temperature resistant materials prepared from waste electric porcelain. Ceram. Int. 2023, 49, 11537–11543. [Google Scholar] [CrossRef]
- Pivák, A.; Pavlíková, M.; Záleská, M.; Lojka, M.; Lauermannová, A.-M.; Jankovský, O.; Pavlík, Z. Low-Carbon Composite Based on MOC, Silica Sand and Ground Porcelain Insulator Waste. Processes 2020, 8, 829. [Google Scholar] [CrossRef]
- López-Perales, J.F.; Sánchez-Rodríguez, R.; Suárez-Suárez, D.D.; Rodríguez, E.A. Fired electrical porcelain scrap (chamotte waste) recycling and reuse as an alternative raw material for sustainable porcelain stoneware production. J. Clean. Prod. 2024, 434, 140385. [Google Scholar] [CrossRef]
- Talaei, M.; Mostofinejad, D. Mechanical properties of fiber-reinforced concrete containing waste porcelain aggregates under elevated temperatures. Constr. Build. Mater. 2021, 289, 122854. [Google Scholar] [CrossRef]
- Xu, X.; Shen, Y.; Wu, J.; Zhang, Z.; Yu, J.; Qiu, S. The thermal shock resistance of in-situ synthesized mullite absorption and storage integrated ceramics for solar thermal power generation. Ceram. Int. 2024, 50, 7843–7852. [Google Scholar] [CrossRef]
- Xu, X.; Song, J.; Wu, J.; Zhang, Y.; Zhou, Y.; Zhang, Q. Preparation and Thermal Shock Resistance of Mullite and Corundum Co-bonded SiC Ceramics for Solar Thermal Storage. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2020, 35, 16–25. [Google Scholar] [CrossRef]
- Xu, X.; Liu, S.; Liu, Y.; Wu, J.; Yu, J.; Shen, Y. Preparation and thermal shock resistance of solar thermal absorbing corundum ceramics for concentrated solar power. Int. J. Appl. Ceram. Technol. 2024, 21, 2102–2113. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Z.; Li, L.; Wu, D.; Wang, Q. Synergistically enhancing the piezoelectric activity and Curie temperature of CaBi4Ti4O15 ceramics via co-doping Gd/Mn at the A/B-site. J. Adv. Ceram. 2024, 13, 1482–1497. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.; Liu, Q.; Li, J. Research progress and prospects of colored zirconia ceramics: A review. J. Adv. Ceram. 2024, 13, 1505–1522. [Google Scholar] [CrossRef]
- Huan, Y.; Gui, D.; Li, C.; Wei, T.; Wu, L. Simultaneously enhanced energy storage performance and luminance resistance in (K0.5Na0.5) NbO3-based ceramics via synergistic optimization strategy. J. Adv. Ceram. 2024, 13, 34–43. [Google Scholar] [CrossRef]
- Ning, Y.; Pu, Y.; Wu, C.; Chen, Z.; Zhang, X.; Zhang, L.; Wang, B. Design strategy of high-entropy perovskite energy-storage ceramics: A review. J. Eur. Ceram. Soc. 2024, 44, 4831–4843. [Google Scholar] [CrossRef]
- Xu, X.; Shen, Y.; Zhang, Z.; Wu, J.; Yu, J.; Zhou, Y. Influence of Fe2O3 on the absorptivity of in situ synthesized solar high-temperature absorbing and storing integrated mullite-based ceramics. Ceram. Int. 2024, 50, 27339–27348. [Google Scholar] [CrossRef]
- Lao, X.; Xu, X.; Wu, J.; Xu, X. High-temperature alloy/honeycomb ceramic composite materials for solar thermal storage applications: Preparation and stability evaluation. Ceram. Int. 2017, 43, 4583–4593. [Google Scholar] [CrossRef]
- Hu, C.; Wu, J.; Xu, X.; Chen, P. Investigating the effect of andalusite on mechanical strength and thermal shock resistance of cordierite-spodumene composite ceramics. Ceram. Int. 2018, 44, 3240–3247. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Wu, J.; Liu, S.; Ma, S.; Cheng, T. Preparation and characterization of solar absorption and thermal storage integrated ceramics from calcium and iron-rich steel slag. Ceram. Int. 2023, 49, 8381–8389. [Google Scholar] [CrossRef]
- Xu, X.; Li, M.; Wang, Y.; Wu, J.; Liu, S.; Ma, S.; Cheng, T. Preparation and thermal shock resistance of solar thermal storage ceramics from high calcium and high iron steel slag. Ceram. Int. 2024, 50, 8099–8108. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Wu, J.; Tang, Z.; Li, Y.; Lu, C. Preparation and thermal shock resistance of corundum-mullite composite ceramics from andalusite. Ceram. Int. 2017, 43, 1762–1767. [Google Scholar] [CrossRef]
- Lao, X.; Xu, X.; Cheng, H.; Liu, H.; Liang, L. Effect of rare-earth oxides on microstructure and thermal shock resistance of Al2O3-SiCw composite ceramics for solar thermal storage. Ceram. Int. 2019, 45, 2003–2011. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Zhou, R.; Wei, H.; Zhang, W.; Zhan, H.; Ma, S.; Yang, C.; Huang, Z. Study on Preparation of Porous Light Refractory from Waste Electric Porcelain. ChemistrySelect 2024, 9, e202303743. [Google Scholar] [CrossRef]
- Gautam, L.; Kumar Jain, J.; Jain, A.; Kalla, P. Valorization of bone-china ceramic powder waste along with granite waste in self-compacting concrete. Constr. Build. Mater. 2022, 315, 125730. [Google Scholar] [CrossRef]
- Wu, J.; Hao, S.; Xu, X.; Ma, S.; Li, P.; Shi, X. Preparation of MgAl2O4 solar thermal storage ceramics from different magnesium sources. Int. J. Appl. Ceram. Technol. 2023, 20, 3223–3236. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, Y.; Chen, M.; Peng, X.; Wang, B.; Shang, J. Design strategies of perovskite energy-storage dielectrics for next-generation capacitors. J. Eur. Ceram. Soc. 2023, 43, 5713–5747. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, Y.; Zhu, Q. Multi-mode luminescent ceramic glaze for advanced anti-counterfeiting. Opt. Mater. 2024, 157, 116152. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Ghica, M.E.; Durães, L. An overview on alumina-silica-based aerogels. Adv. Colloid Interface Sci. 2020, 282, 102189. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, G.J.; Kan, Y.M. Pressureless densification and mechanical properties of hafnium diboride doped with B4C: From solid state sintering to liquid phase sintering. J. Eur. Ceram. Soc. 2010, 30, 2699–2705. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behaviour. Fuel 2007, 86, 1490–1512. [Google Scholar] [CrossRef]
- Elsayed, A.H.; Sayed, M.A.; Dawood, O.M.; Daoush, W.M. Effect of Transition Metals Oxides on the Physical and Mechanical Properties of Sintered Tungsten Heavy Alloys. Crystals 2020, 10, 825. [Google Scholar] [CrossRef]
- Jia, J.; Wu, D.; Ren, Y.; Lin, J. Nanoarchitectonics of Illite-Based Materials: Effect of Metal Oxides Intercalation on the Mechanical Properties. Nanomaterials 2022, 12, 997. [Google Scholar] [CrossRef]
- Hou, Z.; Cui, B.; Liu, L.; Liu, Q. Effect of the different additives on the fabrication of porous kaolin-based mullite ceramics. Ceram. Int. 2016, 42, 17254–17258. [Google Scholar] [CrossRef]
- Feng, M.; Wu, Y.-Q.; Ji, G.-R.; Zhou, Y.; Wang, X.-J. Sintering mechanism and properties of corundum-mullite duplex ceramic with MnO2 addition. Ceram. Int. 2022, 48, 14237–14245. [Google Scholar] [CrossRef]
- Lv, Z.; Lan, C.; Cao, Y.; Fan, M.; Ke, Y.; Guo, W.; Yang, Y.; Shen, X. One-step preparation of N-doped porous carbon materials with excellent microwave absorption properties based on methylene blue saturated wood-based activated carbon. Carbon Lett. 2024. [Google Scholar] [CrossRef]
- Shen, X.; Lv, Z.; Ichikawa, K.; Sun, H.; Huang, Z.; Koide, S.; Liao, M. Stress effect on the resonance properties of single-crystal diamond cantilever resonators for microscopy applications. Ultramicroscopy 2022, 234, 113464. [Google Scholar] [CrossRef]
- Alves, M.F.R.P.; Ribeiro, S.; Suzuki, P.A.; Strecker, K.; Santos, C.D. Effect of Fe2O3 Addition and Sintering Temperature on mechanical Properties and Translucence of Zirconia Dental Ceramics with Different Y2O3 Content. Mater. Res. 2021, 24, e20200402. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, Z.; Han, B.; Peng, H.; Dai, K.; Xu, Z.; Fu, Z.; Hu, Z.; Wang, G. Designing silver niobate-based relaxor antiferroelectrics for ultrahigh energy storage performance. J. Adv. Ceram. 2024, 13, 1282–1290. [Google Scholar] [CrossRef]
- Tian, Y.; Xue, F.; Li, L.; Qiu, L.; Xie, Y.; Du, G. High energy storage performance of Bi0.5Na0.5TiO3-Ba0.65Sr0.35TiO3-AgNbO3 relaxor ferroelectrics with excellent temperature and frequency stability. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2024, 309, 117600. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, H.; Zhang, J.; Da Silva, L.L.; Sheng, Z.; Yao, Y.; Wang, G.; Hinterstein, M.; Zhang, S.; Chen, J. Ultrahigh Energy-Storage in Dual-Phase Relaxor Ferroelectric Ceramics. Adv. Mater. 2024, 36, 2410088. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Liu, H.; Zhang, X.; Shen, X.; Liu, S.; Wu, X.; Fang, M.; Liu, Y.; Min, X.; Huang, Z. Solvothermally synthesized highly porous W18O49 nanowires for UV, humidity and pH detection. Phys. E: Low-Dimens. Syst. Nanostructures 2020, 117, 113719. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Xu, X.; Ma, S.; Li, P.; Shi, X. Preparation and Thermal Shock Resistance of Mullite Ceramics for High Temperature Solar Thermal Storage. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2023, 38, 743–752. [Google Scholar] [CrossRef]
- Wu, J.; Yu, J.; Xu, X.; Liu, Y.; Zhang, Z.; Wei, P. Preparation and thermal shock resistance of anorthite solar thermal energy storage ceramics from magnesium slag. Ceram. Int. 2022, 48, 33604–33614. [Google Scholar] [CrossRef]




| Raw Materials Used in This Experiment | Price/ ¥/Tonne | Raw Materials Used by Xu et al. [24] | Price/ ¥/Tonne |
|---|---|---|---|
| Waste Electric Porcelain (45 wt.%) | 100 | Bauxite (50 wt.%) | 1800 |
| Bauxite (45 wt.%) | 1800 | Kaolin (50 wt.%) | 2500 |
| Kaolin (10 wt.%) | 2500 | Iron Oxide (11 wt.%) | 7000 |
| Magnetite (9 wt.%) | 880 | ||
| Total Price | 1184.2 | Total Price | 2920 |
| Ingredient | Waste Electric Porcelain | Bauxite | Kaolin (Clay) | Magnetite (Fe3O4, Addition) | PVA (Addition) |
|---|---|---|---|---|---|
| BK0 | 45 | 45 | 10 | 0 | 5 |
| BK1 | 5 | ||||
| BK2 | 7 | ||||
| BK3 | 9 | ||||
| BK4 | 11 | ||||
| BK5 | 13 |
| Raw Materials | Al2O3 | SiO2 | FeO | K2O | CaO | TiO2 | MgO | MoO3 | i.t. |
|---|---|---|---|---|---|---|---|---|---|
| Waste Electric Porcelain | 50.203 | 29.155 | 5.373 | 6.045 | 1.775 | 2.578 | 1.11 | 1.468 | 2.289 |
| Bauxite | 79.192 | 2.782 | - | 1.431 | 2.112 | 6.854 | 1.25 | 1.088 | 5.289 |
| Kaolin | 49.147 | 35.086 | 6.527 | 0.738 | 0.868 | 2.548 | 0.91 | 1.589 | 2.591 |
| Magnetite | 2.099 | 4.323 | 85.28 | 1.28 | 2.199 | 0.993 | 0.15 | 0.62 | 3.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Lv, Z.; Weng, J.; Fan, M.; Fan, F.; Wang, X.; Chen, X.; Shi, S.; Shen, X. Enhanced Mechanical and Thermal Properties of Waste Electric Porcelain-Based Solar Energy-Absorbing Thermal Storage Ceramics with Interwoven Mullite Structure. Crystals 2025, 15, 90. https://doi.org/10.3390/cryst15010090
Zhang X, Lv Z, Weng J, Fan M, Fan F, Wang X, Chen X, Shi S, Shen X. Enhanced Mechanical and Thermal Properties of Waste Electric Porcelain-Based Solar Energy-Absorbing Thermal Storage Ceramics with Interwoven Mullite Structure. Crystals. 2025; 15(1):90. https://doi.org/10.3390/cryst15010090
Chicago/Turabian StyleZhang, Xuejia, Zhenfei Lv, Junchi Weng, Mengke Fan, Feiyu Fan, Xin Wang, Xuyi Chen, Siqi Shi, and Xiulin Shen. 2025. "Enhanced Mechanical and Thermal Properties of Waste Electric Porcelain-Based Solar Energy-Absorbing Thermal Storage Ceramics with Interwoven Mullite Structure" Crystals 15, no. 1: 90. https://doi.org/10.3390/cryst15010090
APA StyleZhang, X., Lv, Z., Weng, J., Fan, M., Fan, F., Wang, X., Chen, X., Shi, S., & Shen, X. (2025). Enhanced Mechanical and Thermal Properties of Waste Electric Porcelain-Based Solar Energy-Absorbing Thermal Storage Ceramics with Interwoven Mullite Structure. Crystals, 15(1), 90. https://doi.org/10.3390/cryst15010090






