Effects of the Doping of La and Ce in the Pt/B-TiO2 Catalyst in Selective Oxidation Reaction of Glycerol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of La-B-TiO2 and Ce-B-TiO2
2.3. Preparation of Pt/La-B-TiO2 and Pt/Ce-B-TiO2
2.4. Material Characterization
3. Results
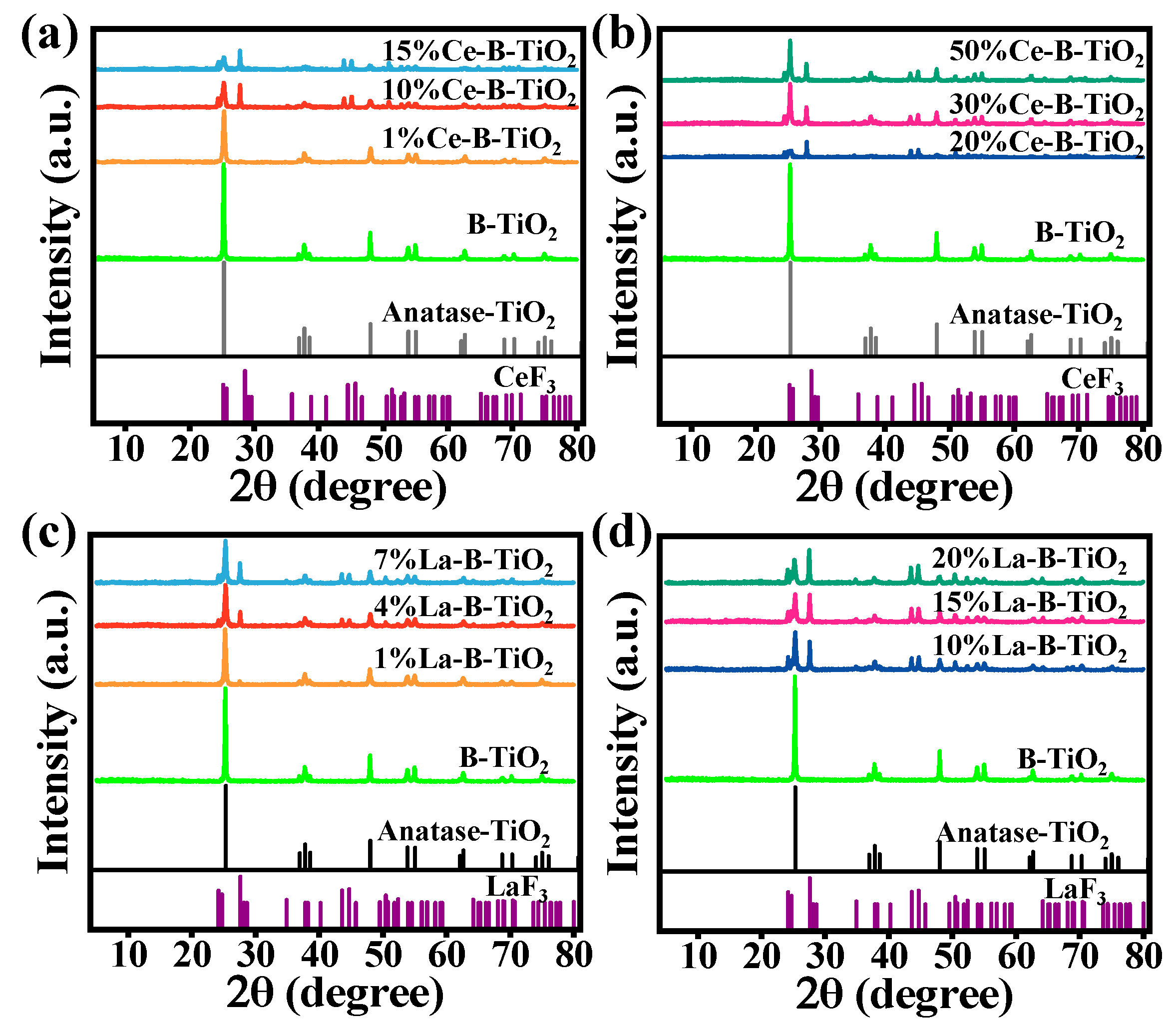
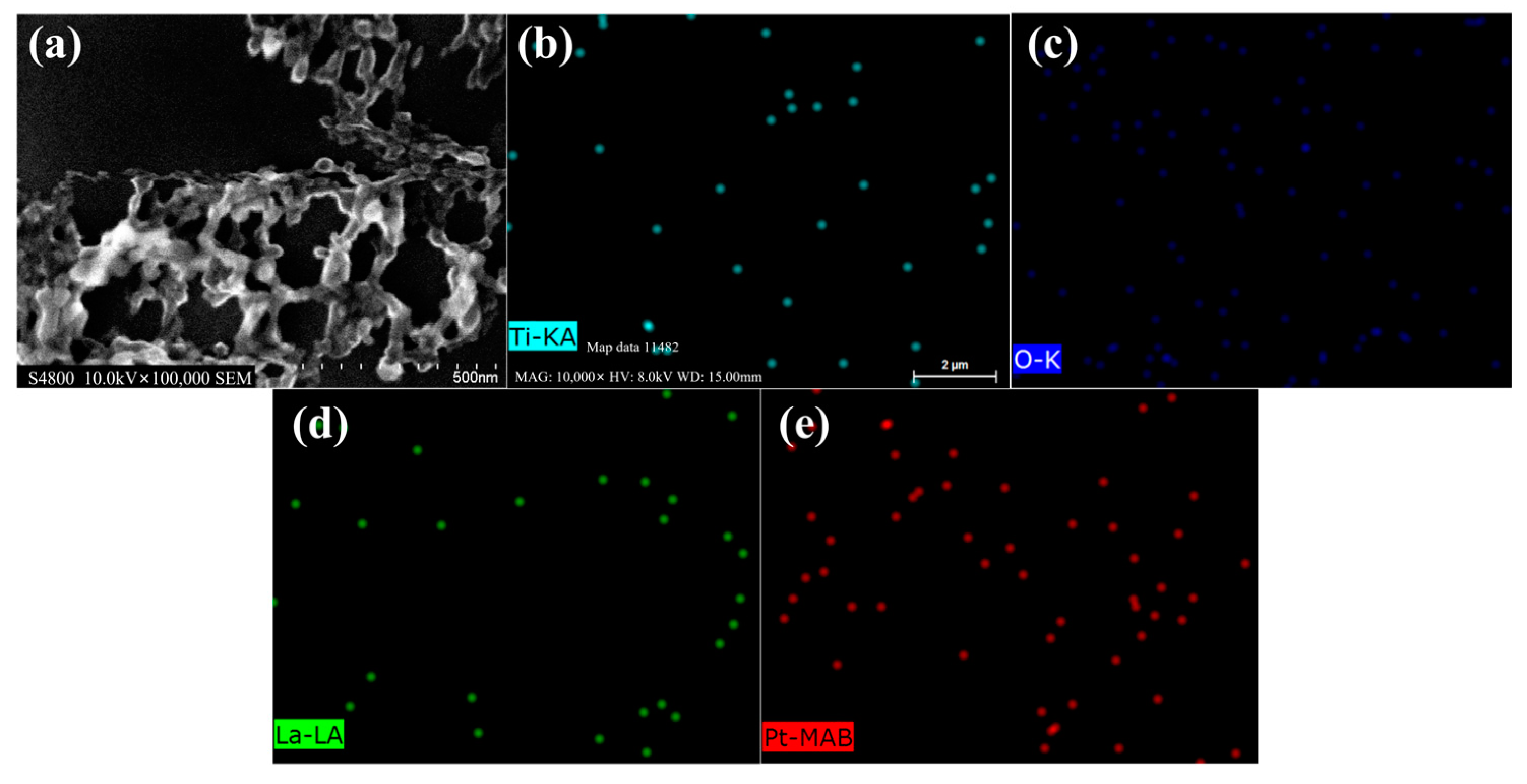
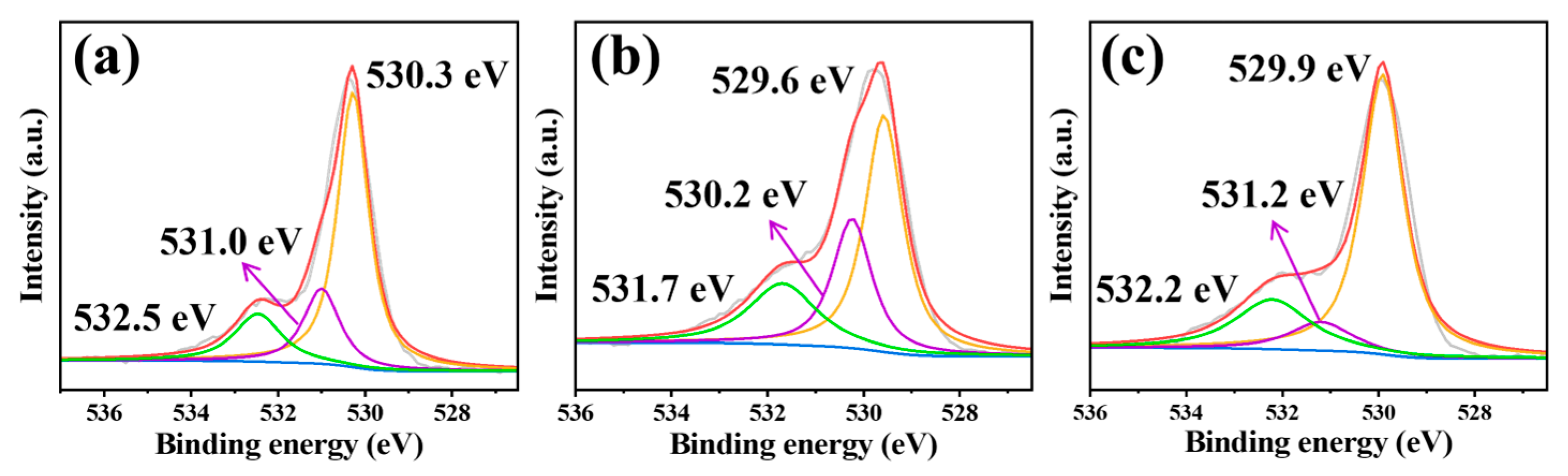
3.1. Characterization of the Materials
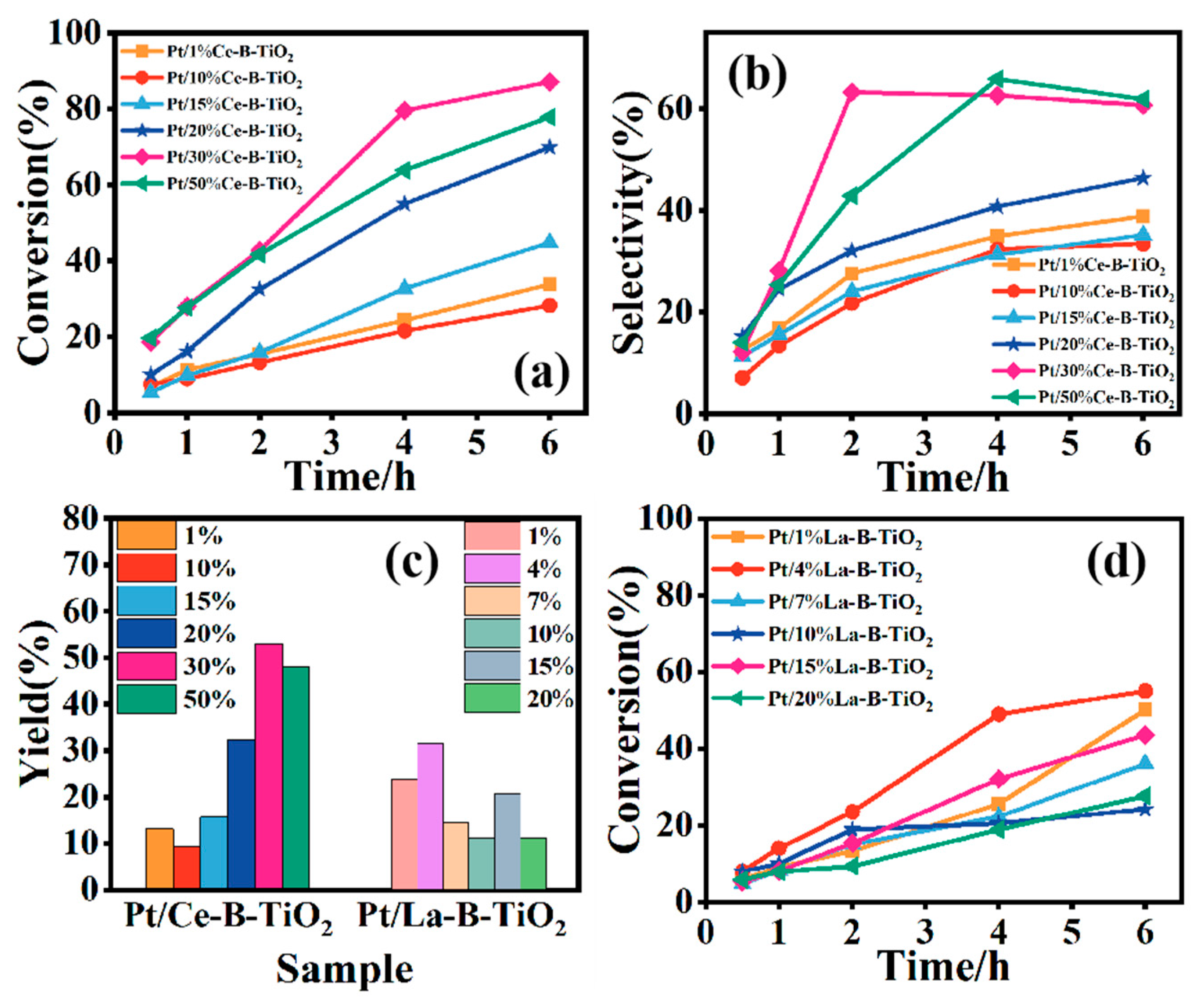
3.2. Selective Oxidation Reaction of Glycerol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasari, M.A.; Kiatsimkul, P.-P.; Sutterlin, W.R.; Suppes, G.J. Low-Pressure Hydrogenolysis of Glycerol to Propylene Glycol. Appl. Catal. Gen. 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The Potential of Glycerol as a Value-Added Commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Hu, X.; Lu, J.; Liu, Y.; Chen, L.; Zhang, X.; Wang, H. Sustainable Catalytic Oxidation of Glycerol: A Review. Environ. Chem. Lett. 2023, 21, 2825–2861. [Google Scholar] [CrossRef]
- Dodekatos, G.; Schünemann, S.; Tüysüz, H. Recent Advances in Thermo-, Photo-, and Electrocatalytic Glycerol Oxidation. ACS Catal. 2018, 8, 6301–6333. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, H.; Skrzyńska, E.; Girardon, J.-S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Mimura, N.; Paul, S.; Dumeignil, F. Selective Catalytic Oxidation of Glycerol: Perspectives for High Value Chemicals. Green Chem. 2011, 13, 1960. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Verma, V.; Singh, S.V. La-Doped TiO2 Nanoparticles for Photocatalysis: Synthesis, Activity in Terms of Degradation of Methylene Blue Dye and Regeneration of Used Nanoparticles. Arab. J. Sci. Eng. 2023, 48, 16431–16443. [Google Scholar] [CrossRef]
- Gong, Z.; Li, X.; Zhang, Z.; Liu, Y.; Song, H.; Wang, Y. Anodic Oxidation of TC4 Substrate to Synthesize Ce-Doped TiO2 Nanotube Arrays with Enhanced Photocatalytic Performance. J. Electron. Mater. 2021, 50, 3276–3282. [Google Scholar] [CrossRef]
- Kim, S.; An, E.; Oh, I.; Hwang, J.B.; Seo, S.; Jung, Y.; Park, J.-C.; Choi, H.; Choi, C.H.; Lee, S. CeO2 Nanoarray Decorated Ce-Doped ZnO Nanowire Photoanode for Efficient Hydrogen Production with Glycerol as a Sacrificial Agent. Catal. Sci. Technol. 2022, 12, 5517–5523. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, T.; Bu, C.; Liu, D.; Piao, G. Investigation on the Activity of Ni Doped Ce0.8Zr0.2O2 for Solar Thermochemical Water Splitting Combined with Partial Oxidation of Methane. Int. J. Hydrogen Energy 2024, 62, 1077–1088. [Google Scholar] [CrossRef]
- Yang, Z.; Cui, Y.; Ge, P.; Chen, M.; Xu, L. CO2 Methanation over Rare Earth Doped Ni-Based Mesoporous Ce0.8Zr0.2O2 with Enhanced Low-Temperature Activity. Catalysts 2021, 11, 463. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Yan, Y.; Hao, Z.; Chen, X.; Li, F. Optimisation of the Electronic Structure by Rare Earth Doping to Enhance the Bifunctional Catalytic Activity of Perovskites. Appl. Energy 2023, 339, 120931. [Google Scholar] [CrossRef]
- Dewoolkar, K.D.; Vaidya, P.D. Tailored Ce- and Zr-Doped Ni/Hydrotalcite Materials for Superior Sorption-Enhanced Steam Methane Reforming. Int. J. Hydrogen Energy 2017, 42, 21762–21774. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, L.; Gong, Y. Ce Doped Ni(OH)2/Ni-MOF Nanosheets as an Efficient Oxygen Evolution and Urea Oxidation Reactions Electrocatalyst. Int. J. Hydrogen Energy 2024, 58, 416–425. [Google Scholar] [CrossRef]
- Duan, E.; Wang, Y.; Wang, S.; Bai, J.; Li, D.; Zhang, L.; Deng, J.; Tang, X. Revealing La Doping Activation or Inhibition of Crystal Facet Effects in Co3O4 for Catalytic Combustion and Water Resistance Improvement. Chem. Eng. J. 2024, 496, 153839. [Google Scholar] [CrossRef]
- Bae, J.; Shin, D.; Jeong, H.; Kim, B.-S.; Han, J.W.; Lee, H. Highly Water-Resistant La-Doped Co3O4 Catalyst for CO Oxidation. ACS Catal. 2019, 9, 10093–10100. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Song, J.; Qu, H.; Yu, S. High-Pressure Hydrothermal Dope Ce into MoVTeNbOx for One-Step Oxidation of Propylene to Acrylic Acid. Catal. Commun. 2024, 187, 106849. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, G.; Pan, T.; Wang, Y.; Xu, Y.; Pang, H. Design of Fe-Doped Ni-Based Bimetallic Oxide Hierarchical Assemblies Boost Urea Oxidation Reaction. Int. J. Hydrogen Energy 2024, 93, 338–345. [Google Scholar] [CrossRef]
- Xie, K.; Jia, Q.; Wang, Y.; Zhang, W.; Xu, J. The Electronic Structure and Optical Properties of Anatase TiO2 with Rare Earth Metal Dopants from First-Principles Calculations. Materials 2018, 11, 179. [Google Scholar] [CrossRef]
- Wakhare, S.Y.; Deshpande, M.D. Rare Earth Metal Element Doped G-GaN Monolayer: Study of Structural, Electronic, Magnetic, and Optical Properties by First-Principle Calculations. Phys. B Condens. Matter 2022, 647, 414367. [Google Scholar] [CrossRef]
- Ducut, M.R.D.; Rojas, K.I.M.; Bautista, R.V.; Arboleda, N.B. Structural, Electronic, and Optical Properties of Copper Doped Monolayer Molybdenum Disulfide: A Density Functional Theory Study. Mater. Sci. Semicond. Process. 2025, 185, 108971. [Google Scholar] [CrossRef]
- Zhong, J.; Xu, Z.; Lu, J.; Li, Y.Y. Precise Electronic Structures of Amorphous Solids: Unraveling the Color Origin and Photocatalysis of Black Titania. J. Phys. Chem. C 2023, 127, 7268–7274. [Google Scholar] [CrossRef]
- Naik, K.M.; Hamada, T.; Higuchi, E.; Inoue, H. Defect-Rich Black Titanium Dioxide Nanosheet-Supported Palladium Nanoparticle Electrocatalyst for Oxygen Reduction and Glycerol Oxidation Reactions in Alkaline Medium. ACS Appl. Energy Mater. 2021, 4, 12391–12402. [Google Scholar] [CrossRef]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-Earth Doping in Nanostructured Inorganic Materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tian, X.; Ren, L.; Su, Y.; Su, Q. Understanding of Lanthanide-Doped Core–Shell Structure at the Nanoscale Level. Nanomaterials 2024, 14, 1063. [Google Scholar] [CrossRef]
- Bian, T.; Zhou, T.; Zhang, Y. Preparation and Applications of Rare-Earth-Doped Ferroelectric Oxides. Energies 2022, 15, 8442. [Google Scholar] [CrossRef]
- Dinamarca, R.; Garcia, X.; Jimenez, R.; Fierro, J.L.G.; Pecchi, G. Effect of A-Site Deficiency in LaMn0.9Co0.1O3 Perovskites on Their Catalytic Performance for Soot Combustion. Mater. Res. Bull. 2016, 81, 134–141. [Google Scholar] [CrossRef]
- Li, G.; Li, X.; Hao, X.; Li, Q.; Zhang, M.; Jia, H. Ti3+/Ti4+ and Co2+/Co3+ Redox Couples in Ce-Doped Co-Ce/TiO2 for Enhancing Photothermocatalytic Toluene Oxidation. J. Environ. Sci. 2025, 149, 164–176. [Google Scholar] [CrossRef]
- Nain, P.; Pawar, M.; Rani, S.; Sharma, B.; Kumar, S.; Majeed Khan, M.A. (Ce, Nd) Co-Doped TiO2 NPs via Hydrothermal Route:Structural, Optical, Photocatalytic and Thermal Behavior. Mater. Sci. Eng. B 2024, 309, 117648. [Google Scholar] [CrossRef]
- Li, Z.; Wang, E.; Zhang, Y.; Luo, R.; Gai, Y.; Ouyang, H.; Deng, Y.; Zhou, X.; Li, Z.; Feng, H. Antibacterial Ability of Black Titania in Dark: Via Oxygen Vacancies Mediated Electron Transfer. Nano Today 2023, 50, 101826. [Google Scholar] [CrossRef]
- Gulina, L.B.; Tolstoy, V.P.; Kasatkin, I.A.; Kolesnikov, I.E.; Danilov, D.V. Formation of Oriented LaF3 and LaF3: Eu3+ Nanocrystals at the Gas−Solution Interface. J. Fluor. Chem. 2017, 200, 18–23. [Google Scholar] [CrossRef]
- Gulina, L.B.; Schäfer, M.; Privalov, A.F.; Tolstoy, V.P.; Murin, I.V. Synthesis of LaF3 Nanosheets with High Fluorine Mobility Investigated by NMR Relaxometry and Diffusometry. J. Chem. Phys. 2015, 143, 234702. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, S.; Kajihara, K.; Kanamura, K. Synthesis of Nanocrystalline LaF3 Doped Silica Glasses by Hydrofluoric Acid Catalyzed Sol–Gel Process. Mater. Sci. Eng. B 2012, 177, 510–514. [Google Scholar] [CrossRef]
- Stewart, L.; Lu, W.; Wei, Z.-W.; Ila, D.; Padilla, C.; Zhou, H.-C. A Zirconium Metal–Organic Framework with an Exceptionally High Volumetric Surface Area. Dalton Trans. 2017, 46, 14270–14276. [Google Scholar] [CrossRef]
- Hu, M.; Cao, Y.; Li, Z.; Yang, S.; Xing, Z. Ti3+ Self-Doped Mesoporous Black TiO2/SiO2 Nanocomposite as Remarkable Visible Light Photocatalyst. Appl. Surf. Sci. 2017, 426, 734–744. [Google Scholar] [CrossRef]
- Hong, Z.; Kang, M.; Chen, X.; Zhou, K.; Huang, Z.; Wei, M. Synthesis of Mesoporous Co2+-Doped TiO2 Nanodisks Derived from Metal Organic Frameworks with Improved Sodium Storage Performance. ACS Appl. Mater. Interfaces 2017, 9, 32071–32079. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Zhang, K.; Wang, J.; Sun, B.; Li, H.; Qiao, P.; Wang, L.; Zhou, W. Ti3+ Self-Doped Black TiO2 Nanotubes with Mesoporous Nanosheet Architecture as Efficient Solar-Driven Hydrogen Evolution Photocatalysts. ACS Sustain. Chem. Eng. 2017, 5, 6894–6901. [Google Scholar] [CrossRef]
- Shi, X.; Guo, J.; Shen, T.; Fan, A.; Liu, Y.; Yuan, S. Improvement of NH3-SCR Activity and Resistance to SO2 and H2O by Ce Modified La-Mn Perovskite Catalyst. J. Taiwan Inst. Chem. Eng. 2021, 126, 102–111. [Google Scholar] [CrossRef]
- Liang, D.; Gao, J.; Sun, H.; Chen, P.; Hou, Z.; Zheng, X. Selective oxidation of glycerol with oxygen in a base-free aqueous solution over MWNTs supported Pt catalysts. Appl. Catal. B Environ. 2011, 106, 423–432. [Google Scholar] [CrossRef]
- Gao, J.; Liang, D.; Chen, P.; Hou, Z.; Zheng, X. Oxidation of glycerol with oxygen in a base-free aqueous solution over Pt/AC and Pt/MWNTs catalysts. Catal. Lett. 2009, 130, 185–191. [Google Scholar] [CrossRef]
- Ke, Y.; Xu, H.; Qin, H. Low-temperature catalytic oxidation of glycerol into glyceric acid over Zr@MCM-41-supported Pt catalyst. Chem. Lett. 2024, 53, upad021. [Google Scholar] [CrossRef]
- Chen, X.; Shen, L.; Yin, H. Selective conversion of glycerin to glyceric acid under mild and base-free conditions using layered double hydroxides-supported Pt nanoparticle catalysts. J. Chem. Technol. Biotechnol. 2023, 98, 2915–2924. [Google Scholar] [CrossRef]
- Liang, D.; Gao, J.; Wang, J.; Chen, P.; Wei, Y.; Hou, Z. Bimetallic Pt-Cu catalysts for glycerol oxidation with oxygen in a base-free aqueous solution. Catal. Commun. 2011, 12, 1059–1062. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; He, Y.; Feng, J.; Li, D. Reaction pathway investigation using in situ Fourier transform infrared technique over Pt/CuO and Pt/TiO2 for selective glycerol oxidation. Appl. Catal. B Environ. 2021, 291, 120061. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Miura, H.; Nakatani, N.; Hada, M.; Shishido, T.; Yan, H. Experimental and theoretical investigation of the role of bismuth in promoting the selective oxidation of glycerol over supported Pt-Bi catalyst under mild conditions. ACS Catal. 2020, 10, 6071–6083. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, D.; Wang, X.; Zhou, J.; Li, J.; Zhang, M.; Shen, Y.; Chu, H.; Qu, Y.; Yan, H. Overcoming the deactivation of Pt/CNT by introducing CeO2 for selective base-free glycerol-to-glyceric acid oxidation. ACS Catal. 2020, 10, 3832–3837. [Google Scholar] [CrossRef]
- Yan, H.; Qin, H.; Feng, X.; Jin, X.; Liang, W.; Sheng, N.; Zhu, C.; Wang, H.; Yin, B.; Liu, Y.; et al. Synergistic Pt/MgO/SBA-15 nanocatalysts for glycerol oxidation in base-free medium: Catalyst design and mechanistic study. J. Catal. 2019, 370, 434–446. [Google Scholar] [CrossRef]
- Panda, A.B.; Mahapatra, S.K.; Barhai, P.K.; Das, A.K.; Banerjee, I. Understanding of Gas Phase Deposition of Reactive Magnetron Sputtered TiO2 Thin Films and Its Correlation with Bactericidal Efficiency. Appl. Surf. Sci. 2012, 258, 9824–9831. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Sahdan, M.Z.; Nafarizal, N.; Saim, H.; Embong, Z.; Cik Rohaida, C.H.; Adriyanto, F. Influence of Substrate Annealing on Inducing Ti3+ and Oxygen Vacancy in TiO2 Thin Films Deposited via RF Magnetron Sputtering. Appl. Surf. Sci. 2018, 462, 575–582. [Google Scholar] [CrossRef]
- Tereshchuk, P.; Chaves, A.S.; Da Silva, J.L. Glycerol adsorption on platinum surfaces: A density functional theory investigation with van der Waals corrections. J. Phys. Chem. C 2014, 118, 15251–15259. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, X.; Hai, B.; Zhang, H.; Ding, L. Effects of the Doping of La and Ce in the Pt/B-TiO2 Catalyst in Selective Oxidation Reaction of Glycerol. Crystals 2025, 15, 301. https://doi.org/10.3390/cryst15040301
Wang Z, Zhang X, Hai B, Zhang H, Ding L. Effects of the Doping of La and Ce in the Pt/B-TiO2 Catalyst in Selective Oxidation Reaction of Glycerol. Crystals. 2025; 15(4):301. https://doi.org/10.3390/cryst15040301
Chicago/Turabian StyleWang, Zhihui, Xueqiong Zhang, Bo Hai, Hao Zhang, and Lijun Ding. 2025. "Effects of the Doping of La and Ce in the Pt/B-TiO2 Catalyst in Selective Oxidation Reaction of Glycerol" Crystals 15, no. 4: 301. https://doi.org/10.3390/cryst15040301
APA StyleWang, Z., Zhang, X., Hai, B., Zhang, H., & Ding, L. (2025). Effects of the Doping of La and Ce in the Pt/B-TiO2 Catalyst in Selective Oxidation Reaction of Glycerol. Crystals, 15(4), 301. https://doi.org/10.3390/cryst15040301





