Enhanced Fenton-like Catalytic Activation of Peroxymonosulfate over Macroporous LaFeO3 for Water Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Carbon Microsphere Preparation
2.2.2. Macroporous LaFeO3 Preparation
2.3. Methods of Characterization
2.4. Catalytic Reaction for Degradation of TCH
3. Results and Discussion
3.1. Characterization of the Samples
3.1.1. Carbon Spheres, CS
3.1.2. XRD of LaFeO3 and LFO-CS-X (LaFeO3, Synthesized in Presence of CS-X)
3.1.3. Morphology of Pure LaFeO3 and Macroporous LFO-CS-X
3.1.4. Optical Properties of LaFeO3 and Macroporous LFO-CS-X (LaFeO3 Synthesized in CS Presence)
3.2. Catalytic Experiments
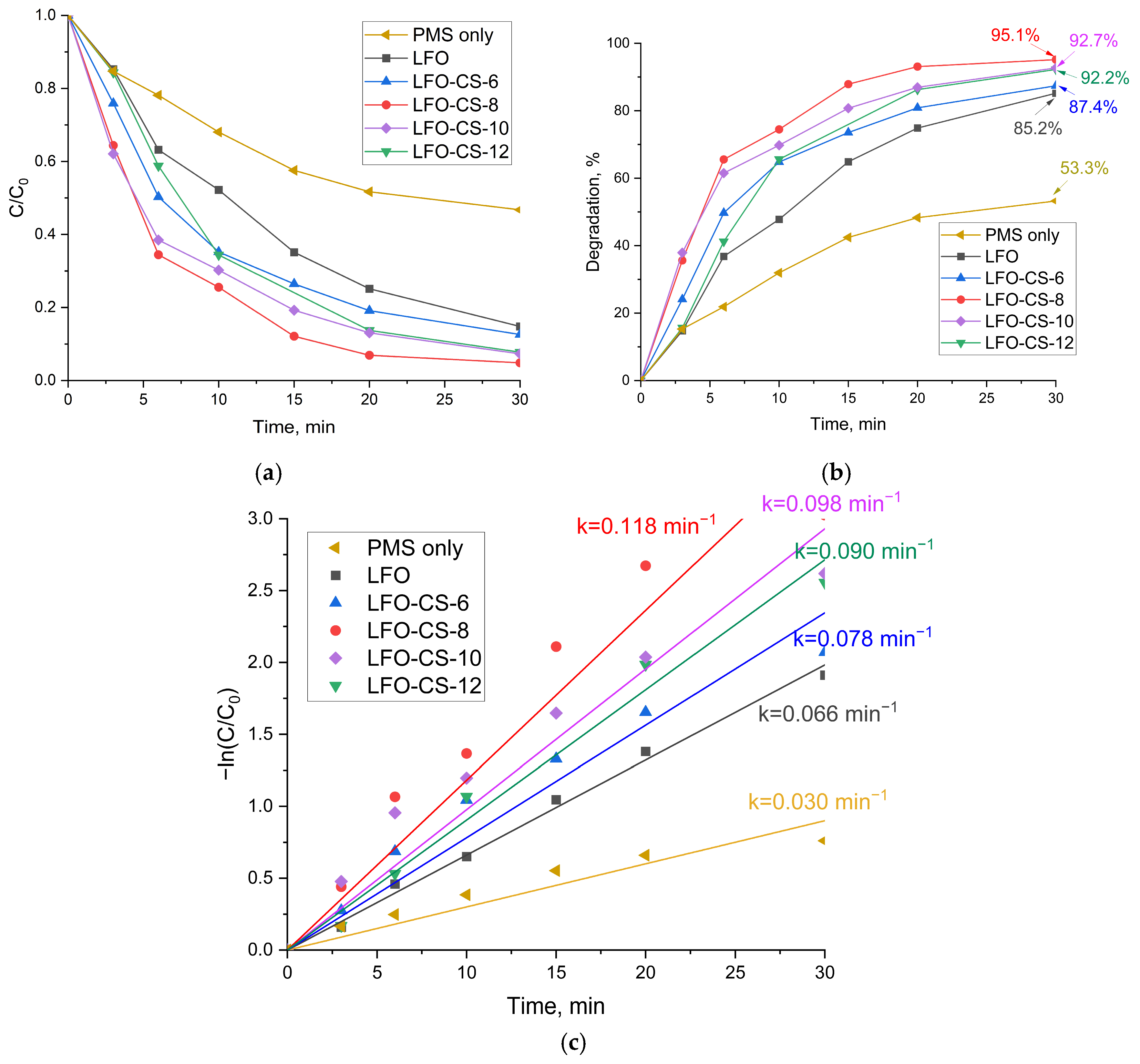
3.2.1. Degradation of the Pollutant TCH Under Visible-Light Irradiation in the Presence of PMS and the Activator LFO-CS-X
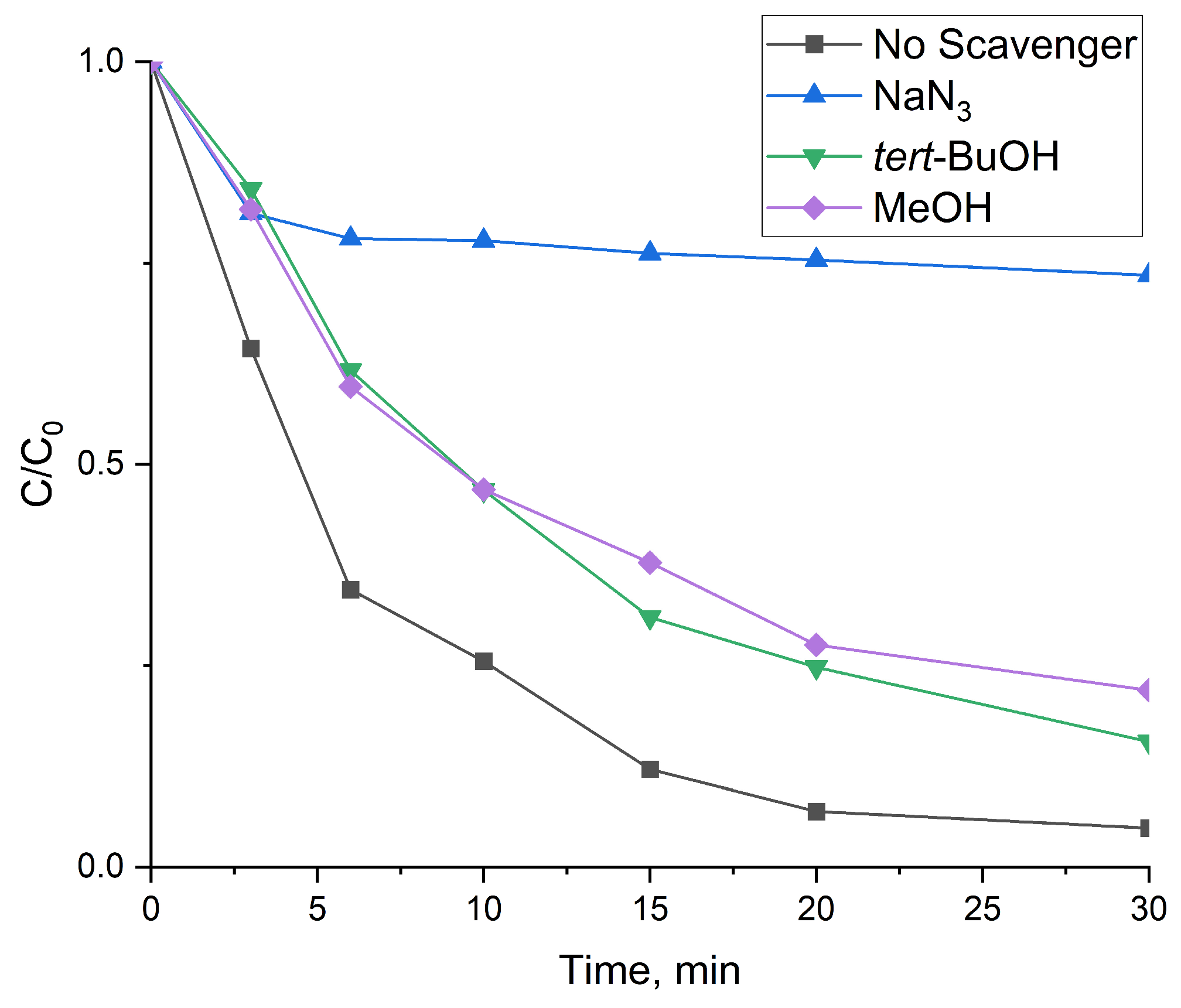
3.2.2. Reaction Mechanism
3.3. Factors Influencing the Process of the Decomposition of TCH
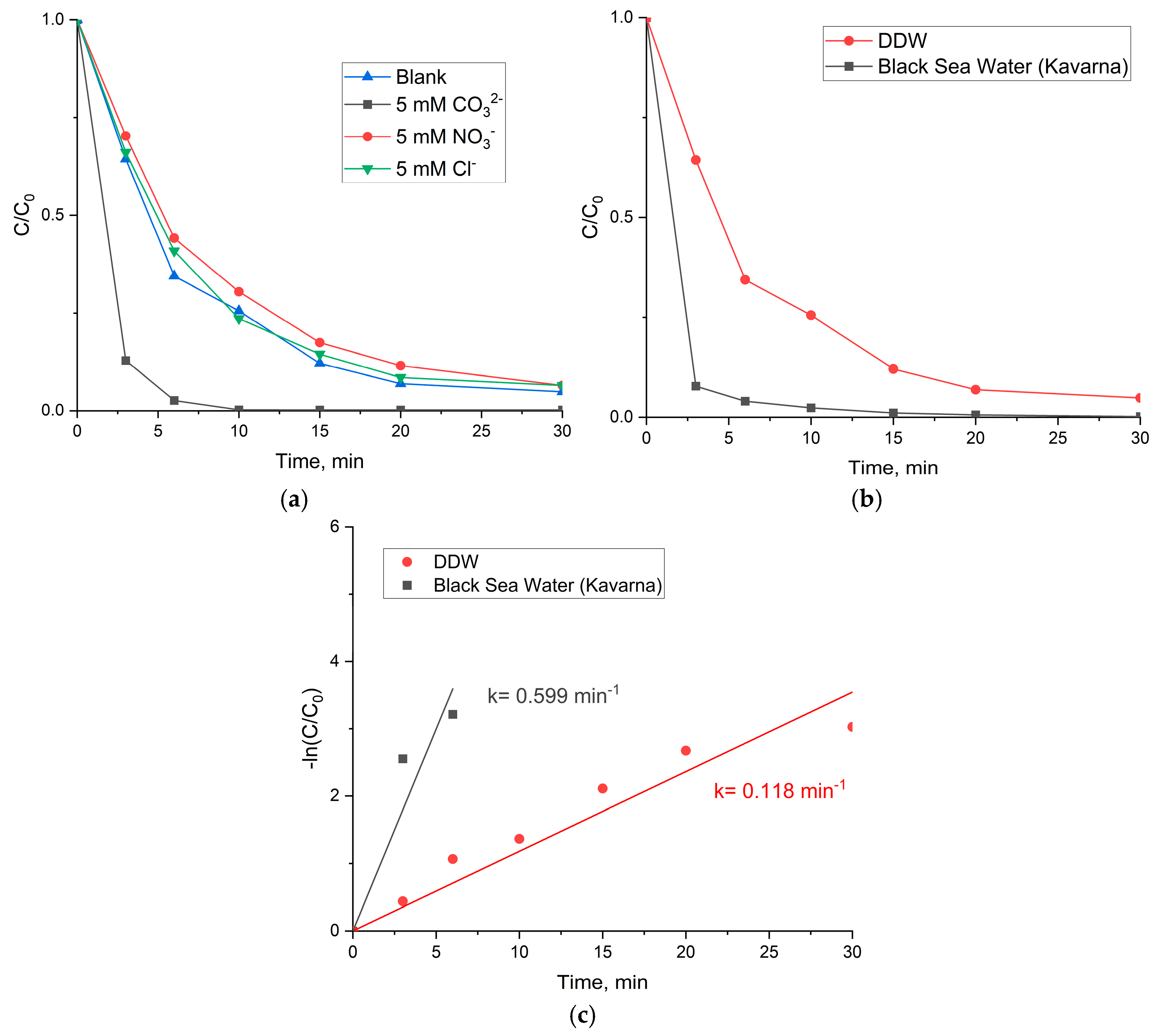
3.3.1. Influence of the Water Matrix
3.3.2. Influence of the Kind of Irradiation and of the Application of Ultrasound
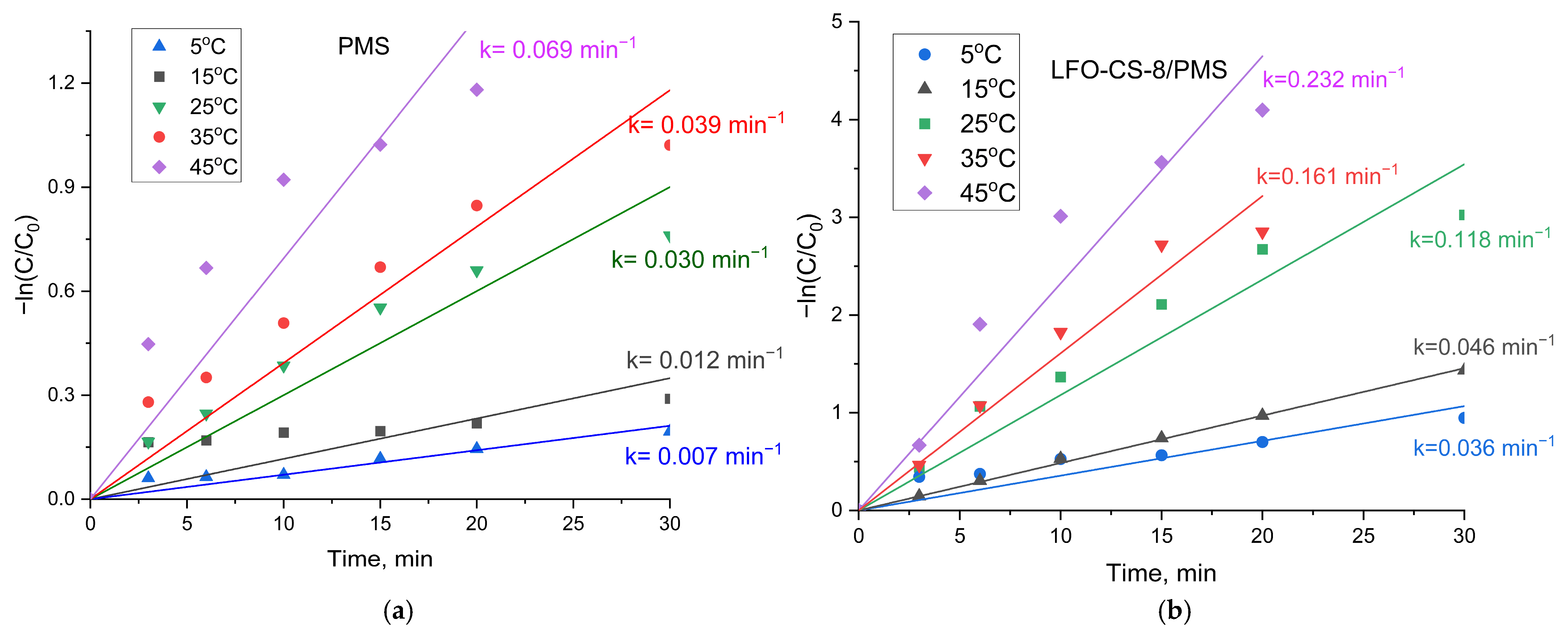
3.3.3. Influence of the Temperature
3.4. Recycling of the Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Liu, Y.; Liu, W.; Li, X.; Mao, L. Synthesis of Honeycomb-like Mesoporous Nitrogen-Doped Carbon Nanospheres as Pt Catalyst Supports for Methanol Oxidation in Alkaline Media. Appl. Surf. Sci. 2017, 407, 64–71. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W.; Kong, F.; Chen, H.; Wang, Z.; Liu, S.; Jin, C. Carbon Spheres Derived from Biomass Residue via Ultrasonic Spray Pyrolysis for Supercapacitors. Mater. Chem. Phys. 2018, 219, 461–467. [Google Scholar] [CrossRef]
- Roberts, A.D.; Li, X.; Zhang, H. Porous Carbon Spheres and Monoliths: Morphology Control, Pore Size Tuning and Their Applications as Li-Ion Battery Anode Materials. Chem. Soc. Rev. 2014, 43, 4341–4356. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh Mehrizi, M.; Abdi, J.; Rezakazemi, M.; Salehi, E. A Review on Recent Advances in Hollow Spheres for Hydrogen Storage. Int. J. Hydrogen Energy 2020, 45, 17583–17604. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Mhlanga, S.D.; Coville, N.J. Carbon Spheres. Mater. Sci. Eng. R Rep. 2010, 70, 1–28. [Google Scholar] [CrossRef]
- Panickar, R.; Sobhan, C.B.; Chakravorti, S. Chemical Vapor Deposition Synthesis of Carbon Spheres: Effects of Temperature and Hydrogen. Vacuum 2020, 172, 109108. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Dubey, P.K.; Yadav, R.M.; Singh, D.P.; Tiwari, R.S.; Srivastava, O.N. Highly Zone-Dependent Synthesis of Different Carbon Nanostructures Using Plasma-Enhanced Arc Discharge Technique. J. Nanopart. Res. 2015, 17, 24. [Google Scholar] [CrossRef]
- Ganash, E.A.; Al-Jabarti, G.A.; Altuwirqi, R.M. The Synthesis of Carbon-Based Nanomaterials by Pulsed Laser Ablation in Water. Mater. Res. Express 2020, 7, 015002. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Braghiroli, F.L.; Izquierdo, M.T.; Parmentier, J.; Celzard, A.; Fierro, V. Synthesis and Properties of Carbon Microspheres Based on Tannin–Sucrose Mixtures Treated in Hydrothermal Conditions. Ind. Crops Prod. 2020, 154, 112564. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and Structural Differences between Glucose, Cellulose and Lignocellulosic Biomass Derived Hydrothermal Carbons. Green Chem. 2011, 13, 3273. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M. Chemistry and Materials Options of Sustainable Carbon Materials Made by Hydrothermal Carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, L.; Shahbazi, A. A Review of Hydrothermal Carbonization of Carbohydrates for Carbon Spheres Preparation. Trends Renew. Energy 2015, 1, 43–56. [Google Scholar] [CrossRef]
- Tooming, T.; Thomberg, T.; Romann, T.; Palm, R.; Jänes, A.; Lust, E. Carbon Materials for Supercapacitor Application by Hydrothermal Carbonization of D-Glucose. IOP Conf. Ser. Mater. Sci. Eng. 2013, 49, 012020. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Y.; Wang, J.; Shao, Z. Preparation and Characterization of Macroporous LiNi1/3Co1/3Mn1/3O2 Using Carbon Sphere as Template. Mater. Chem. Phys. 2011, 129, 296–300. [Google Scholar] [CrossRef]
- Chen, G.; Song, G.; Zhao, W.; Gao, D.; Wei, Y.; Li, C. Carbon Sphere-Assisted Solution Combustion Synthesis of Porous/Hollow Structured CeO2-MnOx Catalysts. Chem. Eng. J. 2018, 352, 64–70. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Wang, R.; Wang, Y.; Zhang, X. Reactivity of Carbon Spheres Templated Ce/LaCo0.5Cu0.5O3 in the Microwave Induced H2O2 Catalytic Degradation of Salicylic Acid: Characterization, Kinetic and Mechanism Studies. J. Colloid Interface Sci. 2020, 574, 74–86. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-Type Lanthanum Ferrite Based Photocatalysts: Preparation, Properties, and Applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Andoulsi, R.; Horchani-Naifer, K.; Férid, M. Preparation of Lanthanum Ferrite Powder at Low Temperature. Cerâmica 2012, 58, 126–130. [Google Scholar] [CrossRef]
- Bhargav, K.K.; Ram, S.; Majumder, S.B. Physics of the Multi-Functionality of Lanthanum Ferrite Ceramics. J. Appl. Phys. 2014, 115, 204109. [Google Scholar] [CrossRef]
- Tsvetkov, M.; Encheva, E.; Petrova, S.; Spassova, I.; Milanova, M. Mechanochemically Synthesized Solid Solutions La1−xCexFeO3+x/2 for Activation of Peroxydisulfate in Catalytical Reaction for Tetracycline Degradation. Crystals 2023, 13, 769. [Google Scholar] [CrossRef]
- Hao, L.; Guo, C.; Hu, Z.; Guo, R.; Liu, X.; Liu, C.; Tian, Y. Single–Atom Catalysts Based on Fenton-like/Peroxymonosulfate System for Water Purification: Design and Synthesis Principle, Performance Regulation and Catalytic Mechanism. Nanoscale 2022, 14, 13861–13889. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, B.; Zhang, H.; Ma, J.; Mu, B.; Zhang, W. A Novel Biochar Modified by Chitosan-Fe/S for Tetracycline Adsorption and Studies on Site Energy Distribution. Bioresour. Technol. 2019, 294, 122152. [Google Scholar] [CrossRef] [PubMed]
- Danner, M.-C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic Pollution in Surface Fresh Waters: Occurrence and Effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Dong, S.; Zhang, J.; Zhou, D.; Rittmann, B.E. Roles of an Easily Biodegradable Co-Substrate in Enhancing Tetracycline Treatment in an Intimately Coupled Photocatalytic-Biological Reactor. Water Res. 2018, 136, 75–83. [Google Scholar] [CrossRef]
- Xiong, H.; Zou, D.; Zhou, D.; Dong, S.; Wang, J.; Rittmann, B.E. Enhancing Degradation and Mineralization of Tetracycline Using Intimately Coupled Photocatalysis and Biodegradation (ICPB). Chem. Eng. J. 2017, 316, 7–14. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B Condens. Matter. 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Datt, G.; Raja, M.M.; Abhyankar, A.C. Steering of Magnetic Interactions in Ni0.5Zn0.5Fe2–x(Mn)xO4 Nanoferrites via Substitution-Induced Cationic Redistribution. J. Phys. Chem. C 2021, 125, 10693–10707. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi (B) 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Hernández-Laverde, M.; Morante, N.; Gutiérrez, B.L.; Murcia, J.J.; Monzillo, K.; Sannino, D.; Vaiano, V. Solar Light Elimination of Bacteria, Yeast and Organic Pollutants by Effective Photocatalysts Based on Ag/Cr-TiO2 and Pd/Cr-TiO2. Nanomaterials 2024, 14, 1730. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Prediction of Flatband Potentials at Semiconductor-Electrolyte Interfaces from Atomic Electronegativities. J. Electrochem. Soc. 1978, 125, 228–232. [Google Scholar] [CrossRef]
- Peng, J.; Xu, J.; Wang, Z.; Ding, Z.; Wang, S. Developing an Efficient NiCo2S4 Cocatalyst for Improving the Visible Light H2 Evolution Performance of CdS Nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 25919–25926. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Zhang, Y.; Gao, Y.; Xiao, X.; Zhang, Z.; Chen, T.; Zhao, Y. Tetracycline Degradation Mechanism of Peroxymonosulfate Activated by Oxygen-Doped Carbon Nitride. RSC Adv. 2023, 13, 6368–6377. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, M.; Liu, H.; Tang, J.; Yu, X.; Wu, H.; Jin, L.; Sun, J. Efficient Degradation of Tetracycline by Peroxymonosulfate Activated with Ni-Co Bimetallic Oxide Derived from Bimetallic Oxalate. Toxics 2024, 12, 816. [Google Scholar] [CrossRef]
- Schneider, J.T.; Firak, D.S.; Ribeiro, R.R.; Peralta-Zamora, P. Use of Scavenger Agents in Heterogeneous Photocatalysis: Truths, Half-Truths, and Misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723–15733. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.M.; Márquez, G.; Tena, M.; Álvarez, P.M.; Beltrán, F.J. Determination of Main Species Involved in the First Steps of TiO2 Photocatalytic Degradation of Organics with the Use of Scavengers: The Case of Ofloxacin. Appl. Catal. B 2015, 178, 44–53. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Zhang, G.; Liu, G.; Li, Y.; Shen, T.; Crittenden, J.C. Enhanced Persulfate Oxidation of Organic Pollutants and Removal of Total Organic Carbons Using Natural Magnetite and Microwave Irradiation. Chem. Eng. J. 2020, 383, 123140. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A Review on Fenton-like Processes for Organic Wastewater Treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Zazo, J.A.; Pliego, G.; Blasco, S.; Casas, J.A.; Rodriguez, J.J. Intensification of the Fenton Process by Increasing the Temperature. Ind. Eng. Chem. Res. 2011, 50, 866–870. [Google Scholar] [CrossRef]

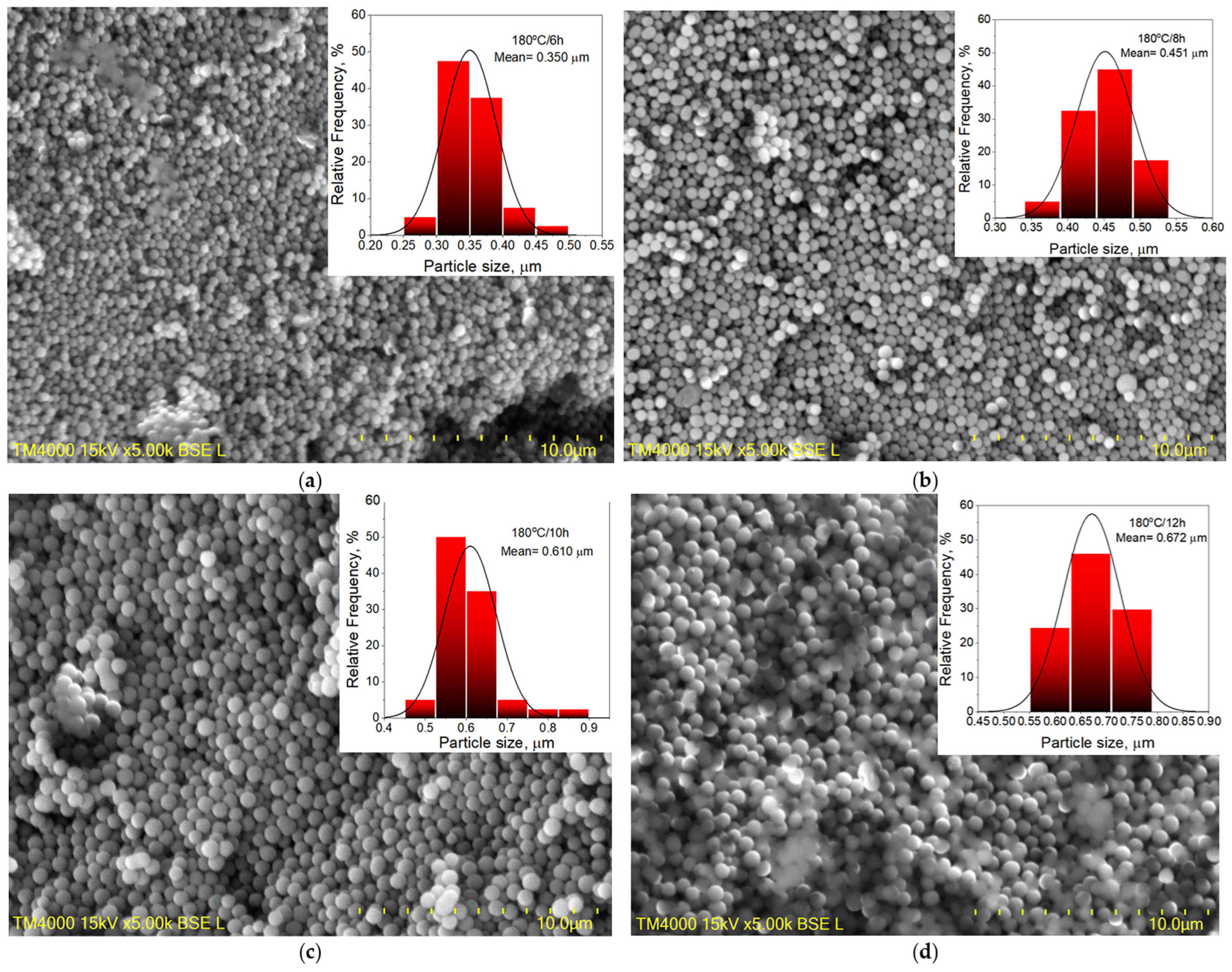
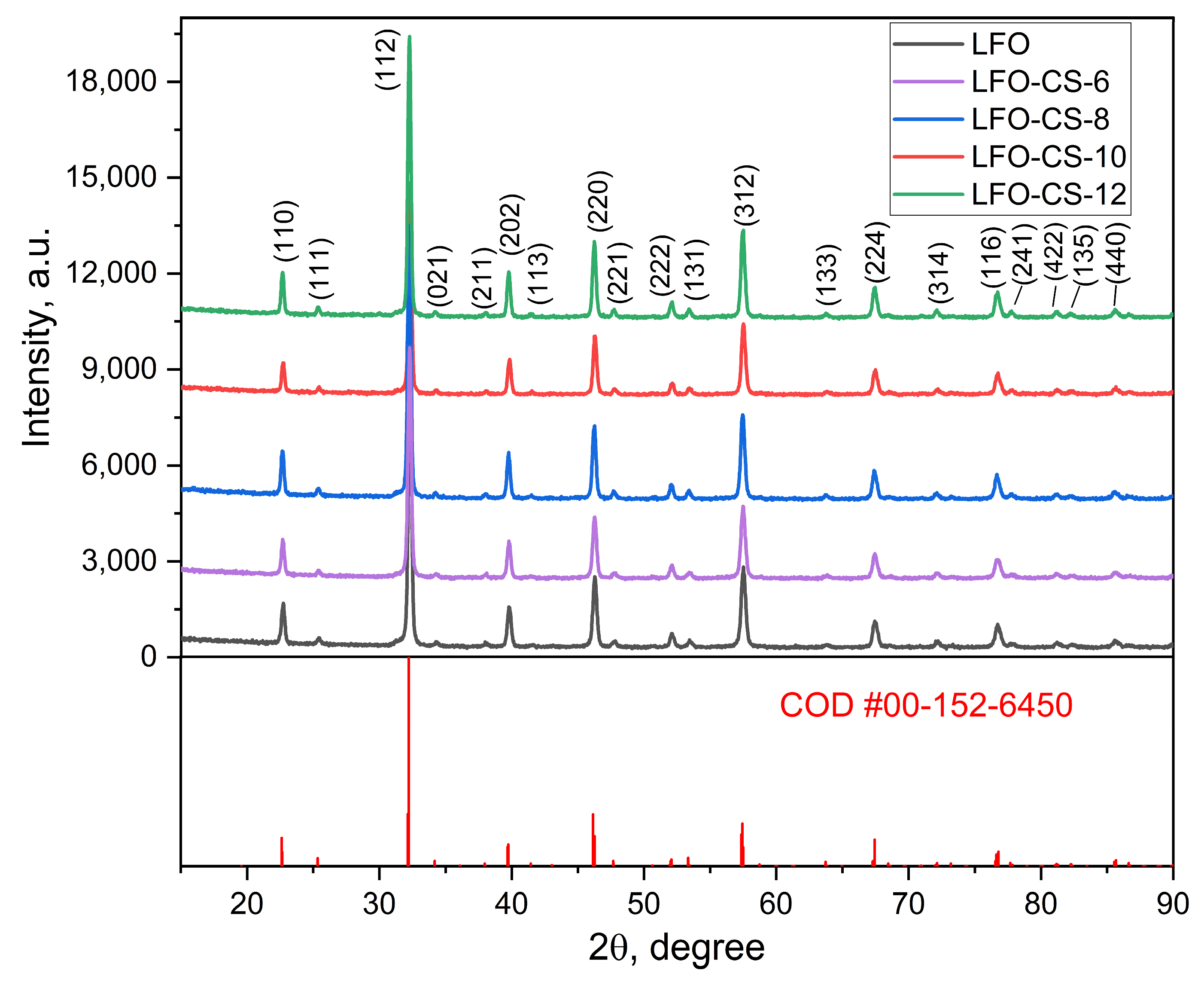
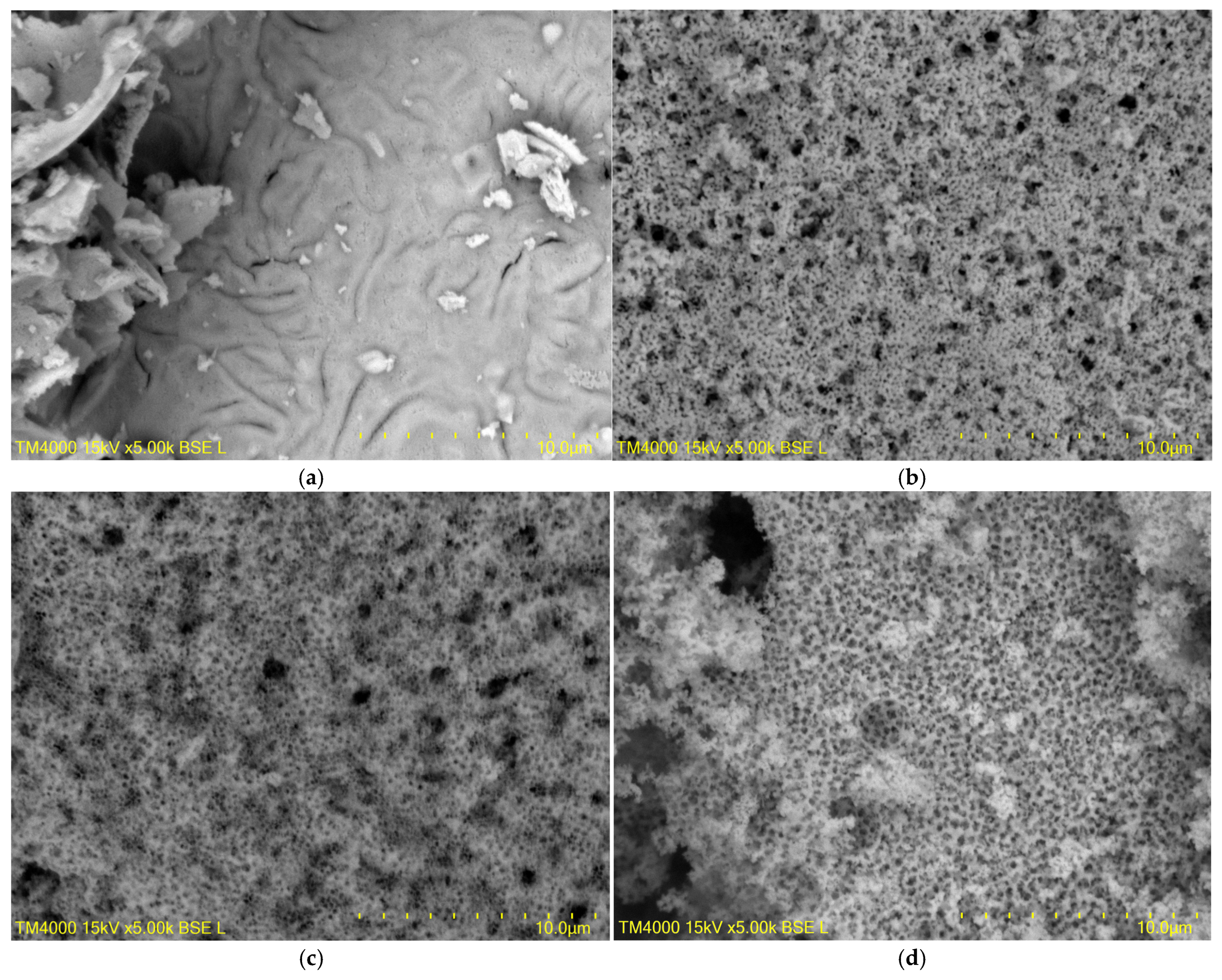




| Sample | Unit Cell Parameters, Å | Crystallites Size, nm | Microstrains, × 10−3 a.u. | Rwp, % | χ2 |
|---|---|---|---|---|---|
| LaFeO3 | a = 5.5608(1) b = 5.5653(1) c = 7.8614(1) | 88.5 ± 1.5 | 0.0016 | 6.82 | 2.13 |
| LFO-CS-6 | a = 5.5608(1) b = 5.5639(1) c = 7.8609(1) | 98.4 ± 1.4 | 0.0016 | 6.14 | 1.51 |
| LFO-CS-8 | a = 5.5582(1) b = 5.5680(1) c = 7.8617(1) | 106.8 ± 1.5 | 0.0013 | 7.28 | 2.37 |
| LFO-CS-10 | a = 5.5587(1) b = 5.5686(1) c = 7.8604(1) | 108.6 ± 1.7 | 0.0013 | 8.00 | 2.31 |
| LFO-CS-12 | a = 5.5581(1) b = 5.5680(1) c = 7.8594(1) | 128.4 ± 2.2 | 0.0013 | 6.10 | 1.67 |
| Sample | Indirect Band Gap | Direct Band Gap | ||||
|---|---|---|---|---|---|---|
| Eg, eV/λ, nm | ECB, eV | EVB, eV | Eg, eV/λ, nm | ECB, eV | EVB, eV | |
| LaFeO3 | 2.12/584 | −0.0132 | 2.1014 | 2.39/519 | −0.1481 | 2.2418 |
| LFO-CS-6 | 2.12/584 | −0.0132 | 2.1014 | 2.37/523 | −0.1382 | 2.2318 |
| LFO-CS-8 | 2.15/575 | −0.0282 | 2.1218 | 2.55/486 | −0.2282 | 2.3218 |
| LFO-CS-10 | 2.16/573 | −0.0225 | 2.1375 | 2.57/482 | −0.2382 | 2.3318 |
| LFO-CS-12 | 2.15/575 | −0.0282 | 2.1218 | 2.54/488 | −0.2231 | 2.3168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encheva, E.; Koleva, S.; Tsvetkov, M.; Milanova, M. Enhanced Fenton-like Catalytic Activation of Peroxymonosulfate over Macroporous LaFeO3 for Water Remediation. Crystals 2025, 15, 394. https://doi.org/10.3390/cryst15050394
Encheva E, Koleva S, Tsvetkov M, Milanova M. Enhanced Fenton-like Catalytic Activation of Peroxymonosulfate over Macroporous LaFeO3 for Water Remediation. Crystals. 2025; 15(5):394. https://doi.org/10.3390/cryst15050394
Chicago/Turabian StyleEncheva, Elzhana, Savina Koleva, Martin Tsvetkov, and Maria Milanova. 2025. "Enhanced Fenton-like Catalytic Activation of Peroxymonosulfate over Macroporous LaFeO3 for Water Remediation" Crystals 15, no. 5: 394. https://doi.org/10.3390/cryst15050394
APA StyleEncheva, E., Koleva, S., Tsvetkov, M., & Milanova, M. (2025). Enhanced Fenton-like Catalytic Activation of Peroxymonosulfate over Macroporous LaFeO3 for Water Remediation. Crystals, 15(5), 394. https://doi.org/10.3390/cryst15050394







