Synthesis of Two Novels-Shaped Dibenzo[c,l] Chrysene Derivatives, Crystal Structure, and the Evaluation of their Photophysical Properties
Abstract
:1. Introduction
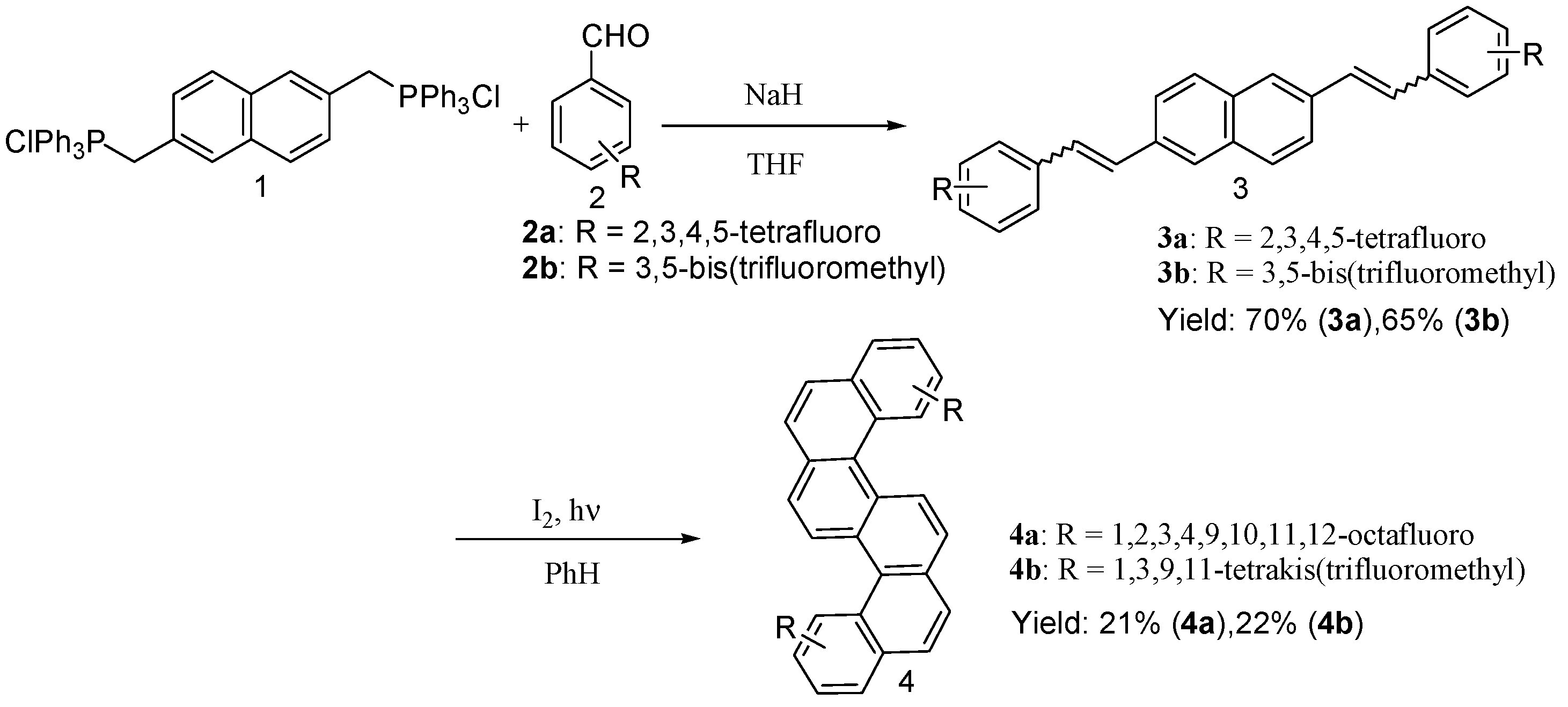
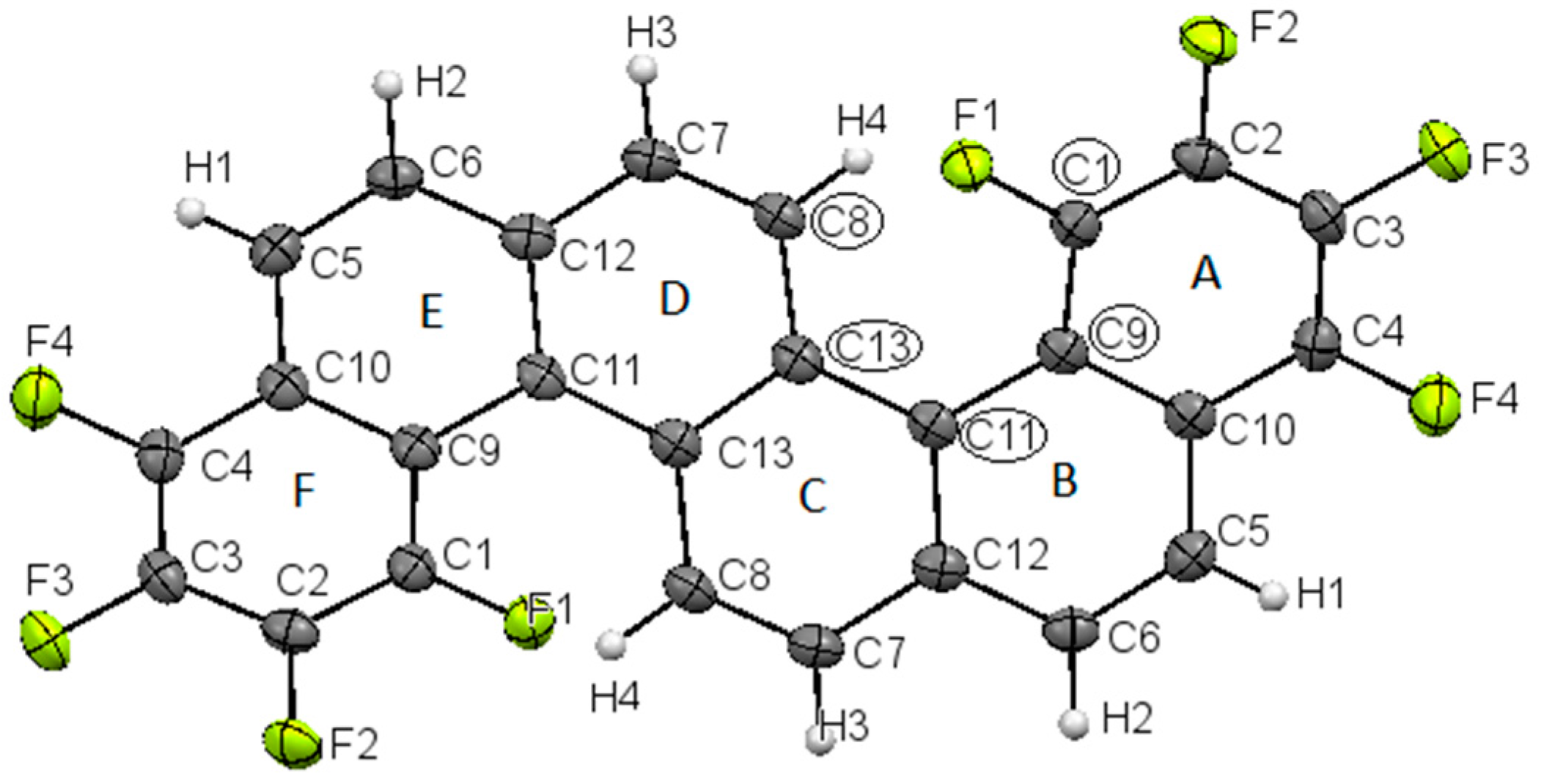
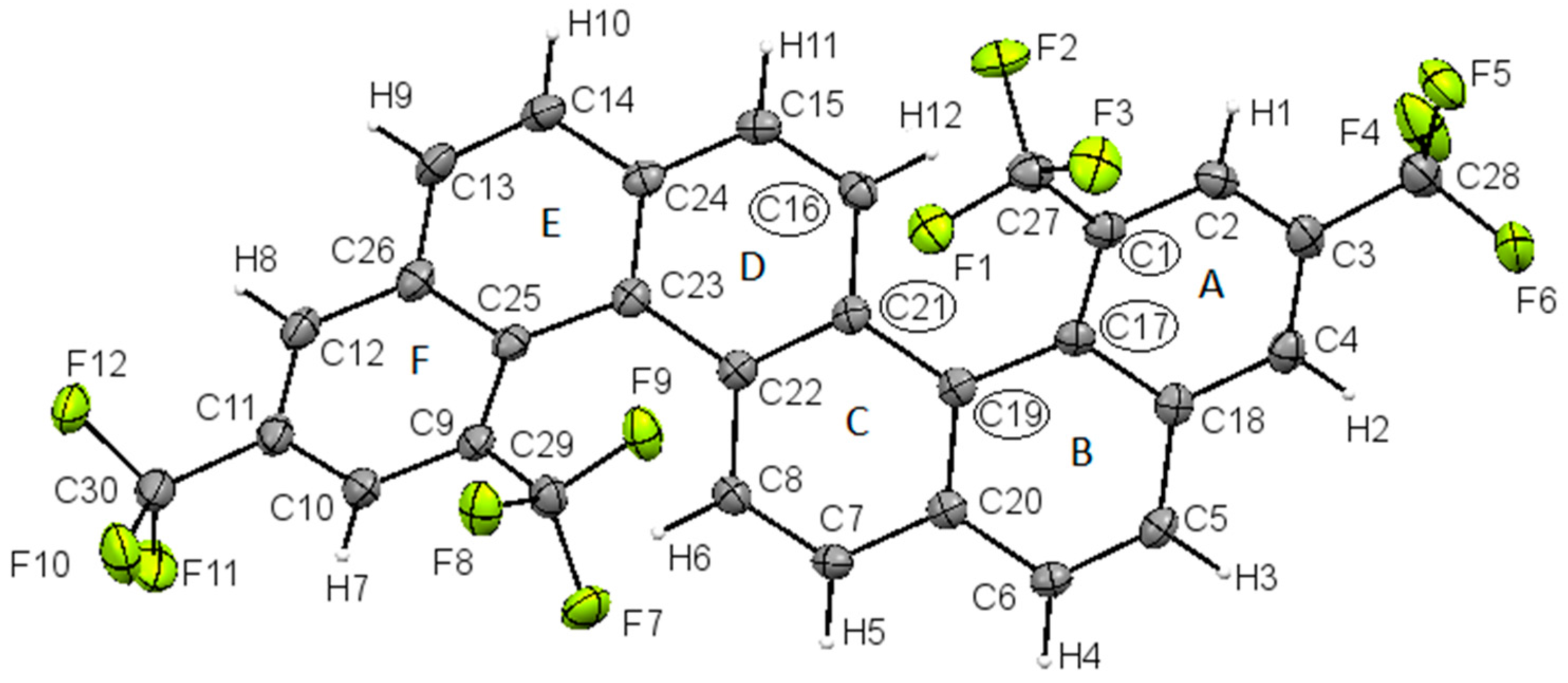
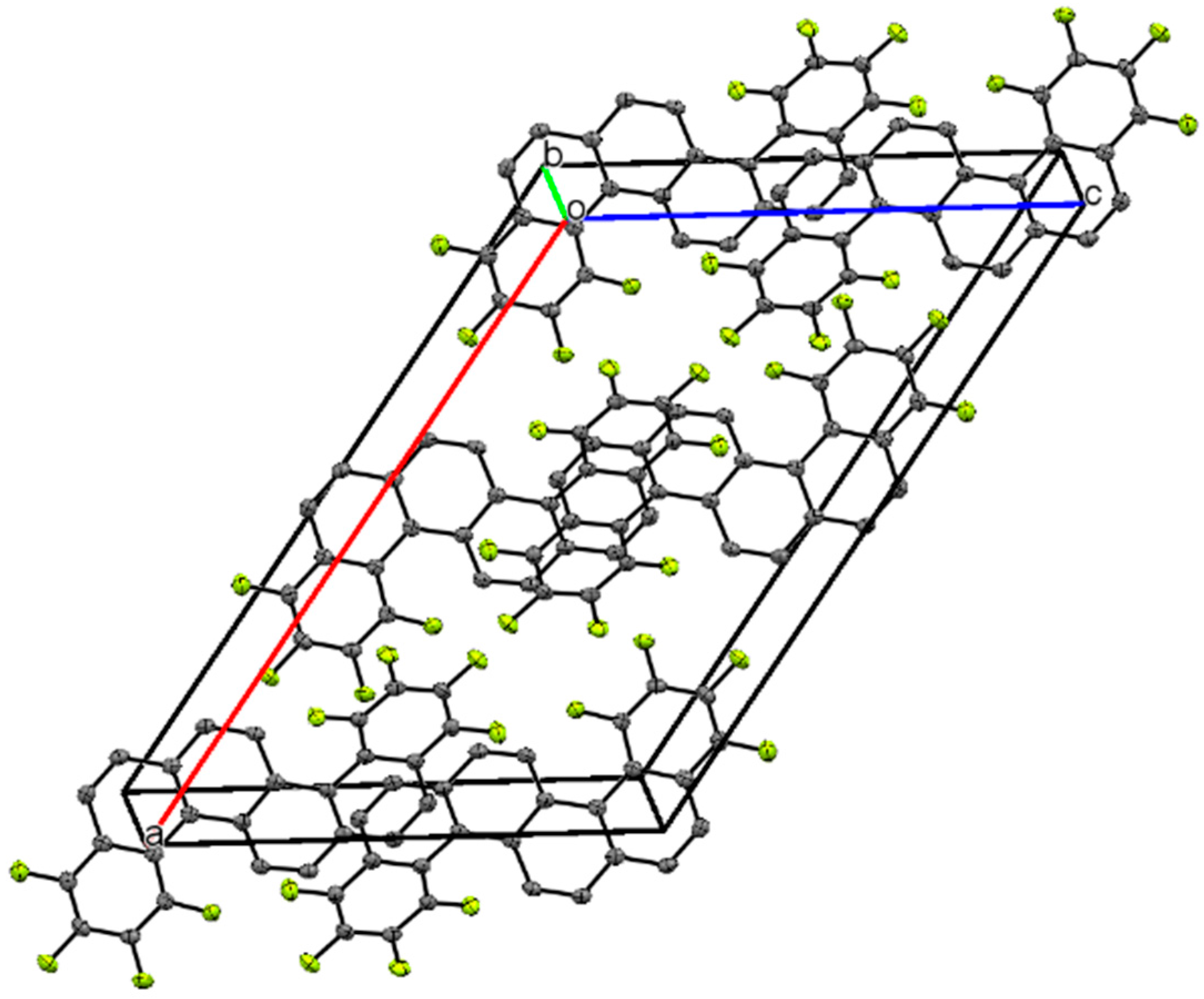
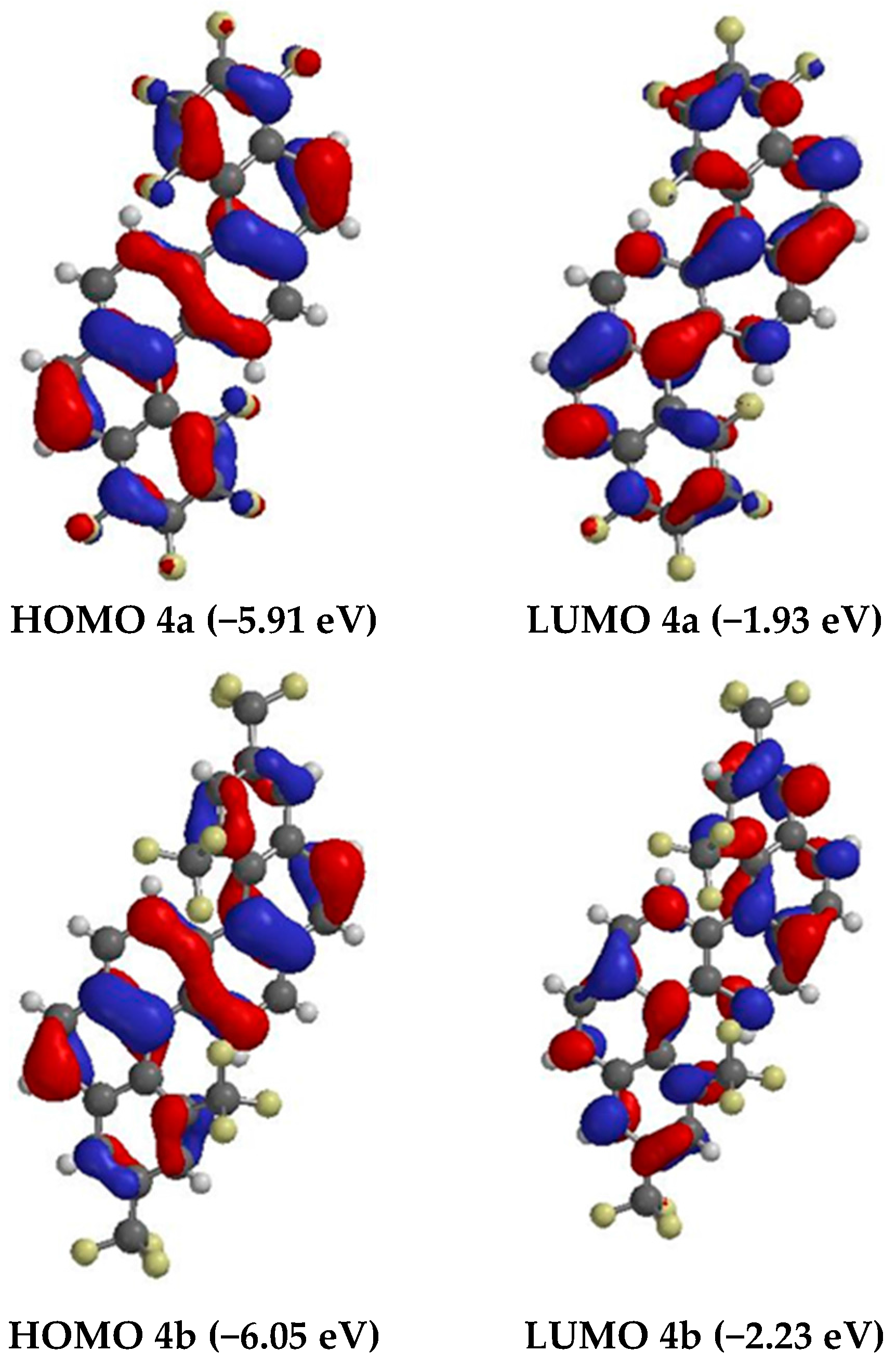
2. Results and Discussion
Synthesis and Spectra Analysis
3. Experiments
3.1. Materials and Instrumentation
3.2. Synthesis
3.2.1. Experimental procedure for the synthesis of molecules 3a and 3b
3.2.2. Typical Procedure for the Photo Cyclization of the Compounds 4a and 4b
3.2.3. Analytical Data for Compounds 4a and 4b
3.3.Data Collection, Refinement, and Structural Determination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-Conjugated Systems in Field-Effect Transistors: A Material Odyssey of Organic Electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028–5048. [Google Scholar] [CrossRef] [PubMed]
- Blouin, N.; Leclerc, M. Poly(2,7-carbazole)s: Structure−Property Relationships. Acc. Chem. Res. 2008, 41, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Pron, A.; Gawrys, P.; Zagorska, M.; Djurado, D.; Demadrille, R. Electroactive materials for organic electronics: Preparation strategies, structural aspects and characterization techniques. Chem. Soc. Rev. 2010, 39, 2577–2632. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, Y.; Zhu, D. π-Conjugated molecules with fused rings for organic field-effect transistors: Design, synthesis and applications. Chem. Soc. Rev. 2010, 39, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, Y.; Zhan, X. Small molecule semiconductors for high-efficiency organic photovoltaics. Chem. Soc. Rev. 2012, 41, 4245–4272. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.N.; Roberts, M.E.; Bao, Z. Graphene speeds up computers: Carbon. Mater. Today 2009, 12, 12. [Google Scholar] [CrossRef]
- Aleshin, A.N.; Lee, J.Y.; Chu, S.W.; Kim, J.S.; Park, Y.W. Mobility studies of field-effect transistor structures based on anthracene single crystals. Appl. Phys. Lett. 2004, 84, 5383. [Google Scholar] [CrossRef]
- Kelley, T.W.; Boardman, L.D.; Dunbar, T.D.; Muyres, D.V.; Pellerite, M.J.; Smith, T.Y.P. High-Performance OTFTs Using Surface-Modified Alumina Dielectrics. J. Phys. Chem. B 2003, 107, 5877–5881. [Google Scholar] [CrossRef]
- Garnier, F.; Yassar, A.; Hajlaoui, R.; Horowitz, G.; Deloffre, F.; Servet, B.; Ries, S.; Alnot, P. Molecular engineering of organic semiconductors: Design of self-assembly properties in conjugated thiophene oligomers. J. Am. Chem. Soc. 1993, 115, 8716–8721. [Google Scholar] [CrossRef]
- Sharma, A.K.; Lin, J.-M.; Desai, D.; Amin, S. Convenient Syntheses of Dibenzo[c,p]chrysene and Its Possible Proximate and Ultimate Carcinogens: In Vitro Metabolism and DNA Adduction Studies. J. Org. Chem. 2004, 70, 4962–4970. [Google Scholar] [CrossRef] [PubMed]
- Brison, J.; De Bakker, C.; Defay, N.; Geerts-Evrard, F.; Marchant, M.-J.; Martin, R.H. Synthèse Photochimique D'Hydrocarbures Polycycliques Aromatiques et Étude en RMN-1H Des Protons de Baie. Effets de Solvant Specifiques et Effets Nucleaires Overhauser. Bulletin des Sociétés Chimiques Belges 1983, 92, 901–912. [Google Scholar] [CrossRef]
- Swartz, C.R.; Parkin, S.R.; Bullock, J.E.; Anthony, J.E.; Mayer, A.C.; Malliaras, G.G. Synthesis and Characterization of Electron-Deficient Pentacenes. Org. Lett. 2005, 7, 3163–3166. [Google Scholar] [CrossRef] [PubMed]
- Kikuzawa, Y.; Mori, T.; Takeuchi, H. Synthesis of 2,5,8,11,14,17-Hexafluoro-hexa-peri-hexabenzocoronene for n-Type Organic Field-Effect Transistors. Org. Lett. 2007, 9, 4817–4820. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Wang, H.B.; Zhu, F.; Yang, J.L.; Tian, H.K.; Geng, Y.H.; Yan, D.H. Phthalocyanato Tin(IV) Dichloride: An Air-Stable, High-Performance, n-Type Organic Semiconductor with a High Field-Effect Electron Mobility. Adv. Mater. 2008, 20, 2142–2144. [Google Scholar] [CrossRef]
- Hakan, U.; Facchetti, A.; Tobin, J.M. n-Channel Semiconductor Materials Design for Organic Complementary Circuits. Acc. Chem. Res. 2011, 44, 501–510. [Google Scholar]
- Liu, J.; Ma, J.; Zhang, K.; Ravat, P.; Machata, P.; Avdoshenko, S.; Hennersdorf, F.; Komber, H.; Pisula, W.; Weigand, J.J.; et al. π-Extended and Curved Antiaromatic Polycyclic Hydrocarbons. J. Am. Chem. Soc. 2017, 139, 7513–7521. [Google Scholar] [CrossRef] [PubMed]
- Olena, P.; Vladimir, A.A.; Alexey, A.G.; Frank, H.; Frank, W.H.; Konstantin, Y.A. Synthesis of Rationally Halogenated Buckybowls by Chemoselective Aromatic C−F Bond Activation. Angew. Chem. Int. Ed. 2017, 56, 4834–4838. [Google Scholar]
- Nagamatsu, S.; Moriguchi, T.; Nagase, T.; Oku, S.; Kuramoto, K.; Takashima, W.; Okauchi, T.; Mizoguchi, K.; Hayase, S.; Kaneto, K. A steady operation of n-type organic thin-film transistors with cyano-substituted distyrylbenzene derivative. Appl. Phys. Express 2009, 2, 1–3. [Google Scholar] [CrossRef]
- Nagamatsu, S.; Oku, S.; Kuramoto, K.; Moriguchi, T.; Takashima, W.; Okauchi, T.; Hayase, S. Long-Term Air-Stable n-Channel Organic Thin-Film Transistors Using 2,5-Difluoro-1,4-phenylene-bis{2-[4-(trifluoromethyl)phenyl]acrylonitrile}. Appl. Mater. Interfaces 2014, 6, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Kitou, N.; Prasad, J.V.; Yoza, K.; Nagamatsu, S.; Okauchi, T.; Tsuge, A.; Takashima, W. Molecular structures of n-type semiconducting material 2,5-difluoro-1,4-phenylene-3,3-bis{2-[(4-trifluoromethyl)phenyl]acrylonitrile} and its photo dimerization product. J. Mol. Struct. 2016, 1118, 372–377. [Google Scholar] [CrossRef]
- Moriguchi, T.; Higashi, M.; Yakeya, D.; Prasad, J.V.; Tsuge, A.; Okauchi, T.; Nagamatsu, S.; Takashima, W. Synthesis, characterization and air stable semiconductor properties of thiophene-condensed pyrene derivatives. J. Mol. Struct. 2017, 1127, 413–418. [Google Scholar] [CrossRef]
- Yamamoto, T.; Komarudin, D.; Arai, M.; Lee, B.-L.; Suganuma, H.; Asakawa, N.; Inoue, Y.; Kubota, K.; Sasaki, S.; Fukuda, T.; et al. Extensive Studies on π-Stacking of Poly(3-alkylthiophene-2,5-diyl)s and Poly(4-alkylthiazole-2,5-diyl)s by Optical Spectroscopy, NMR Analysis, Light Scattering Analysis, and X-ray Crystallography. J. Am. Chem. Soc. 1998, 120, 2047–2058. [Google Scholar] [CrossRef]
- APEX2 Version 2009.9; Bruker AXS Inc.: Tokyo, Japan, 2009.
- SAINT Version 7; Bruker AXS Inc.: Tokyo, Japan, 2009.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
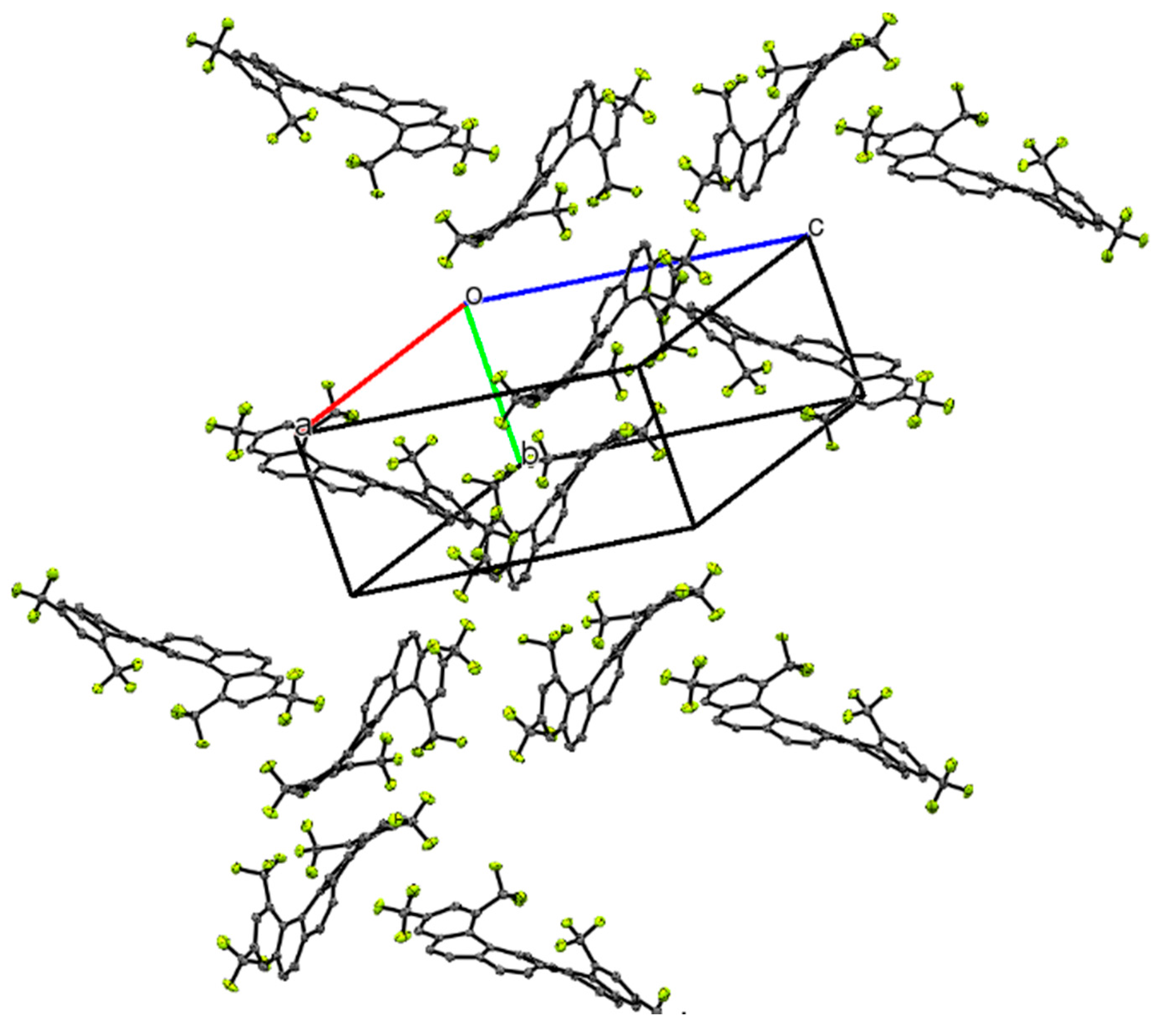


| Short Contacts (between the Side Columns) | Short Contacts (between Molecules in the Column) | ||
|---|---|---|---|
| F1−F4 | 2.862 | C2−C11 | 3.327 |
| F2−F2 | 2.918 | C4−C8 | 3.252 |
| H7−F1 | 2.525 | C7−C9 | 3.324 |
| F3−H8 | 2.565 | F3−C4 | 3.147 |
| Short Contacts (between the Side Columns) | Short Contacts (between Molecules in the Column) | ||
|---|---|---|---|
| F4−F12 | 2.905 | F9−C3 | 3.130 |
| H21−F5 | 2.6 | ||
| F2−F4 | 2.925 | ||
| F4−F4 | 2.704 | ||
| F3−F3 | 2.882 | ||
| F1−F6 | 2.815 | ||
| Parameters Measured | 4a | 4b |
|---|---|---|
| Empirical Formula | C26H8F8 | C30H12F12 |
| Formula Weight (g·mol−1) | 472.32 | 600.40 |
| Crystal shape, color | Prism, yellow | Prism, yellow |
| Temperature | 120 K | 120 K |
| Radiation type | Mo Kα | Mo Kα |
| Wavelength (Å) | 0.7107 | 0.7107 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | P21/n |
| Unit cell dimensions | a = 20.615(4) Å | a = 15.779(3) Å |
| b = 7.3711(13) Å | b = 8.0502(15) Å | |
| c = 14.044(3) Å | c = 18.327(3) Å | |
| α = 90° | α = 90° | |
| β = 124.2960(10)° | β = 96.565(2)° | |
| γ = 90° | γ = 90° | |
| Volume | 1763(5) Å3 | 2312.8(7) Å3 |
| Z | 4 | 4 |
| Calculated density (Mg·m−3) | 1.779 | 1.724 |
| Absorption coefficient mm−1 | 0.162 | 0.168 |
| F(000) | 944 | 1200 |
| Crystal Size (mm) | 0.25 × 0.15 × 0.10 | 0.40 × 0.25 × 0.15 |
| Theta range for data collection | 2.39 to 24.99° | 1.61 to 25.03° |
| Limiting indices | −24 < h < 24 | −18 < h < 18 |
| −8 < k < 8 | −9 < k < 9 | |
| −16 < l < 16 | −21 < l < 21 | |
| Reflections collected/unique | 8044/1548 | 21481/4070 |
| [Rint = 0.233] | [Rint = 0.304] | |
| Completeness to theta (%) | 99.9 | 99.6 |
| Max. and min. transmission | 0.984 and 0.864 | 0.975 and 0.717 |
| Refinement method | Full-matrix LS on F2 | Full-matrix LS on F2 |
| Data/restraints/parameters | 1548/108/154 | 4070/0/379 |
| Goodness-of-fit on F2 | 1.073 | 1.128 |
| Final R indices [I > 2 sigma (I)] | R1 = 0.0346 | R1 = 0.0299 |
| wR2 = 0.0983 | wR2 = 0.0800 | |
| R indices (all data) | R1 = 0.0410 | R1 = 0.0345 |
| wR2 = 0.1034 | wR2 = 0.0943 | |
| Largest diff. peak and hole | 0.276 and −0.188 | 0.239 and −0.290 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriguchi, T.; Tabuchi, D.; Yakeya, D.; Tsuge, A.; Jalli, V. Synthesis of Two Novels-Shaped Dibenzo[c,l] Chrysene Derivatives, Crystal Structure, and the Evaluation of their Photophysical Properties. Crystals 2017, 7, 251. https://doi.org/10.3390/cryst7080251
Moriguchi T, Tabuchi D, Yakeya D, Tsuge A, Jalli V. Synthesis of Two Novels-Shaped Dibenzo[c,l] Chrysene Derivatives, Crystal Structure, and the Evaluation of their Photophysical Properties. Crystals. 2017; 7(8):251. https://doi.org/10.3390/cryst7080251
Chicago/Turabian StyleMoriguchi, Tetsuji, Daichi Tabuchi, Daisuke Yakeya, Akihiko Tsuge, and Venkataprasad Jalli. 2017. "Synthesis of Two Novels-Shaped Dibenzo[c,l] Chrysene Derivatives, Crystal Structure, and the Evaluation of their Photophysical Properties" Crystals 7, no. 8: 251. https://doi.org/10.3390/cryst7080251
APA StyleMoriguchi, T., Tabuchi, D., Yakeya, D., Tsuge, A., & Jalli, V. (2017). Synthesis of Two Novels-Shaped Dibenzo[c,l] Chrysene Derivatives, Crystal Structure, and the Evaluation of their Photophysical Properties. Crystals, 7(8), 251. https://doi.org/10.3390/cryst7080251







