Structural and Magnetic Properties of Co?Mn Codoped ZnO Nanoparticles Obtained by Microwave Solvothermal Synthesis
Abstract
:1. Introduction
- -
- reduction of the synthesis duration in comparison with conventional heating methods,
- -
- high homogeneity of NPs and narrow size distribution resulting from a small temperature gradient in the reaction vessel,
- -
- high product purity.
2. Materials and Methods
2.1. Substrates
2.2. Synthesis of Zn(1−x−y)MnxCoyO NPs
- Continuous microwave heating of the feedstock until the preset maximum temperature is reached (Tmax)—195 °C.
- Once the feedstock reaches the maximum temperature of 195 °C, the reactor’s controller switches off the microwave heating and the feedstock temperature drops to the preset minimum temperature (Tmin)—185 °C.
- Once the feedstock reaches Tmin 185 °C, the reactor’s controller switches on the continuous microwave heating and re-heats the sample to Tmax 195 °C.
- Cyclic repetition of stages 2 and 3 until the preset reaction duration of 25 min is reached.
- The reactor is cooled down by activating cold water flow in the metal body, inside which the Teflon reaction vessel containing the obtained post-reaction suspended matter is placed.
2.3. Characterisation Methods
3. Results and Discussion
3.1. Morphology
3.2. Phase Composition and Lattice Parameters
- -
- the amount of the secondary phase was below the detection limit of the XRD method,
- -
- the secondary phase was an amorphous material,
- -
- the secondary phase is lamellar-shaped ZnO.
3.3. Impact of Chemical Composition on the Colour of Codoped ZnO NPs
3.4. Density, Specific Surface Area and Size Distribution of NP
- -
- a different volume and packing of the unit cell of ZnO than in the case of MnO and CoO [6];
- -
- lower atomic mass of the dopants (Co2+—≈58.93 u; Mn2+—≈54.94 u) in comparison to the substituted Zn2+ (≈65.38 u) atoms in the ZnO crystalline structure;
- -
- increasing number of defects in the ZnO crystalline structure resulting from the growth of the content of Co2+ and Mn2+ dopants.
3.5. Magnetic Characterisation of the Zn(1−x−y)MnxCoyO NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fortunato, E.; Gonçalves, A.; Pimentel, A.; Barquinha, P.; Gonçalves, G.; Pereira, L.; Ferreira, I.; Martins, R. Zinc oxide, a multifunctional material: From material to device applications. Appl. Phys. A 2009, 96, 197–205. [Google Scholar] [CrossRef]
- Ali, A.; Phull, A.R.; Zia, M. Elemental Zinc to Zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological and environmental concerns. Nanotechnol. Rev. 2018, 7, 413–441. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jagadish, C. Review of zincblende ZnO: Stability of metastable ZnO phases. J. Appl. Phys. 2007, 102, 071101. [Google Scholar] [CrossRef]
- Ozgur, U.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.J.; Morkoc, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Jagadish, C.; Pearton, S. Zinc Oxide Bulk, Thin Films and Nanostructures, 1st ed.; Elsevier: Oxford, UK, 2006; ISBN 978-0-08-044722-3. [Google Scholar]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology, 1st ed.; WILEY-VCH: Weinheim, Germany, 2009; ISBN 978-3-527-40813-9. [Google Scholar]
- Ozgur, U.; Hofstetter, D.; Morkoc, H. ZnO devices and applications: A review of current status and future prospects. Proc. IEEE 2010, 98, 1255–1268. [Google Scholar] [CrossRef]
- Litton, C.W.; Reynolds, D.C.; Collins, T.C. Zinc Oxide Materials for Electronic and Optoelectronic Device Applications, 1st ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; ISBN 9780470519714. [Google Scholar]
- Willander, M. Zinc Oxide Nanostructures: Advances and Applications, 1st ed.; Pan Stanford: New York, NY, USA, 2014; ISBN 9789814411332. [Google Scholar]
- Djurišić, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO Nanostructures for Optoelectronics: Material Properties and Device Applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Oprea, O.; Andronescu, E.; Ficai, D.; Ficai, A.; Oktar, F.N.; Yetmez, M. ZnO Applications and Challenges. Curr. Org. Chem. 2014, 18, 192–203. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Gun’ko, Y.; Vallet-Regí, M. ZnO Nanostructures for Drug Delivery and Theranostic Applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, S.; Kaus, A.S.N.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical Applications of Zinc Oxide Nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, S.; Mohanan, P.V. Engineered Zinc Oxide Nanoparticles; Biological Interactions at the Organ Level. Curr. Med. Chem. 2016, 23, 4057–4068. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, V.N.; Devi Rajeswari, V. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef] [PubMed]
- Cierech, M.; Kolenda, A.; Grudniak, A.M.; Wojnarowicz, J.; Woźniak, B.; Gołaś, M.; Swoboda-Kopeć, E.; Łojkowski, W.; Mierzwińska-Nastalska, E. Significance of polymethylmethacrylate (PMMA) modification by zinc oxide nanoparticles for fungal biofilm formation. Int. J. Pharm. 2016, 510, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Cierech, M.; Wojnarowicz, J.; Szmigiel, D.; Bączkowski, B.; Grudniak, A.; Wolska, K.; Łojkowski, W.; Mierzwińska-Nastalska, E. Preparation and characterization of ZnO-PMMA resin nanocomposites for denture bases. Acta Bioeng. Biomech. 2016, 18, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Cierech, M.; Osica, I.; Kolenda, A.; Wojnarowicz, J.; Szmigiel, D.; Łojkowski, W.; Kurzydłowski, K.; Ariga, K.; Mierzwienska-Nastalska, E. Mechanical and Physicochemical Properties of Newly Formed ZnO-PMMA Nanocomposites for Denture Bases. Nanomaterials 2018, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Paszek, E.; Czyz, J.; Woźniacka, O.; Jakubiak, D.; Wojnarowicz, J.; Łojkowski, W.; Stępień, E. Zinc oxide nanoparticles impair the integrity of human umbilical vein endothelial cell monolayer in vitro. J. Biomed. Nanotechnol. 2012, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Pokrowiecki, R.; Pałka, K.; Mielczarek, A. Nanomaterials in dentistry: A cornerstone or a black box? Nanomedicine 2018, 13, 639–667. [Google Scholar] [CrossRef] [PubMed]
- Jamalullail, N.; Mohamad, I.S.; Norizan, M.N.; Mahmed, N.; Taib, B.N. Recent improvements on TiO2 and ZnO nanostructure photoanode for dye sensitized solar cells: A brief review. EPJ Web Conf. 2017, 162, 01045. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Zhang, Q. Progress in perovskite solar cells based on ZnO nanostructures. Sol. Energy 2018, 163, 289–306. [Google Scholar] [CrossRef]
- Melo, A.H.N.; Macêdo, M.A. Permanent Data Storage in ZnO Thin Films by Filamentary Resistive Switching. PLoS ONE 2016, 11, e0168515. [Google Scholar] [CrossRef] [PubMed]
- Omelchenko, M.M.; Wojnarowicz, J.; Salamonczyk, M.; Lojkowski, W. Lyotropic liquid crystal based on zinc oxide nanoparticles obtained by microwave solvothermal synthesis. Mater. Chem. Phys. 2017, 192, 383–391. [Google Scholar] [CrossRef]
- Salzano de Luna, M.; Galizia, M.; Wojnarowicz, J.; Rosa, R.; Lojkowski, W.; Acierno, D.; Filippone, G.; Leonelli, C. Dispersing hydrophilic nanoparticles in hydrophobic polymers: HDPE/ZnO nanocomposites by a novel template-based approach. Express Polym. Lett. 2014, 8, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Huang, G.; An, C.; He, Y.; Yao, Y.; Zhang, P.; Shen, J. Performance of ceramic disk filter coated with nano ZnO for removing Escherichia coli from water in small rural and remote communities of developing regions. Environ. Pollut. 2018, 238, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Teh, S.J.; Lai, C.W. Photocatalytic Water Oxidation on ZnO: A Review. Catalysts 2017, 7, 93. [Google Scholar] [CrossRef]
- Tudose, I.V.; Suchea, M. ZnO for photocatalytic air purification applications. IOP Conf. Ser. Mater. Sci. Eng. 2016, 133, 012040. [Google Scholar] [CrossRef] [Green Version]
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Ng, A.M.C. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2012, 22, 6526–6535. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A. ZnO Nanostructures and Their Sensing Applications: A Review. Nanosci. Nanotechnol. Lett. 2017, 9, 1787–1826. [Google Scholar] [CrossRef]
- Woźniak, B.; Dąbrowska, S.; Wojnarowicz, J.; Chudoba, T.; Łojkowski, W. Content available remote Coating synthetic materials with zinc oxide nanoparticles acting as a UV filter. Glass Ceram. 2017, 3, 15–17. [Google Scholar]
- Fu, Y.; Fu, W.; Liu, Y.; Zhang, G.; Liu, Y.; Yu, H. Comparison of ZnO nanorod array coatings on wood and their UV prevention effects obtained by microwave-assisted hydrothermal and conventional hydrothermal synthesis. Holzforschung 2015, 69, 1009–1014. [Google Scholar] [CrossRef]
- Wallenhorst, L.; Gurău, L.; Gellerich, A.; Militz, H.; Ohms, G.; Viöl, W. UV-blocking properties of Zn/ZnO coatings on wood deposited by cold plasma spraying at atmospheric pressure. Appl. Surf. Sci. 2018, 434, 1183–1192. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, K.C. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Pearton, S.J.; Norton, D.P.; Heo, Y.W.; Tien, L.C.; Ivill, M.P.; Li, Y.; Kang, B.S.; Ren, F.; Kelly, J.; Hebard, A.F. ZnO spintronics and nanowire devices. J. Electron. Mater. 2006, 35, 862–868. [Google Scholar] [CrossRef]
- Lu, J.W.; Chen, E.; Kabir, M.; Stan, M.R.; Wolf, S.A. Spintronics technology: Past, present and future. Int. Mater. Rev. 2016, 61, 456–472. [Google Scholar] [CrossRef]
- Awschalom, D.D.; Flatté, M.E. Challenges for semiconductor spintronics. Nat. Phys. 2007, 3, 153–159. [Google Scholar] [CrossRef]
- Dietl, T.; Ohno, H.; Matsukura, M.; Cibert, J.; Ferrand, D. Zener Model Description of Ferromagnetism in Zinc-Blende Magnetic Semiconductors. Science 2000, 287, 1019–2000. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Katayama-Yosida, H. First principles materials design for semiconductor spintronics. Semicond. Sci. Technol. 2002, 17, 367–376. [Google Scholar] [CrossRef]
- Mustaqima, M.; Liu, C. ZnO-based nanostructures for diluted magnetic semiconductor. Turk. J. Phys. 2014, 38, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Djerdj, I.; Jaglicić, Z.; Arcon, D.; Niederberger, M. Co-Doped ZnO nanoparticles: Minireview. Nanoscale 2010, 2, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. A perspective of recent progress in ZnO diluted magnetic semiconductors. Appl. Phys. A 2013, 112, 241–254. [Google Scholar] [CrossRef]
- Liu, C.; Yun, F.; Morkoç, H. Ferromagnetism of ZnO and GaN: A Review. J. Mater. Sci. Mater. Electron. 2005, 16, 555–597. [Google Scholar] [CrossRef]
- Naeem, M.; Hasanain, S.K.; Kobayashi, M.; Ishida, Y.; Fujimori, A.; Buzby, S.; Shah, S.I. Effect of reducing atmosphere on the magnetism of Zn1−xCoxO (0 ≤ x ≤ 0.10) nanoparticles. Nanotechnology 2006, 17, 2675. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.W.; Wu, X.L.; Qiu, T. Synthesis and magnetic properties of Zn1−xCoxO nanorods. J. Appl. Phys. 2006, 99, 074303. [Google Scholar] [CrossRef]
- Sharrouf, M.; Awad, R.; Marhaba, S.; El-Said Bakeer, D. Structural, Optical and Room Temperature Magnetic Study of Mn-Doped ZnO Nanoparticles. Nano 2016, 11, 1650042. [Google Scholar] [CrossRef]
- Fu, J.; Pen, X.; Yan, S.; Gong, Y.; Tan, Y.; Liang, R.; Du, R.; Xing, X.; Mo, G.; Chen, Z.; et al. Synthesis and structural characterization of ZnO doped with Co. J. Alloys Compd. 2013, 558, 212–221. [Google Scholar] [CrossRef]
- Omri, K.; El Ghoul, J.; Lemine, O.M.; Bououdina, M.; Zhang, B.; El Mir, L. Magnetic and optical properties of manganese doped ZnO nanoparticles synthesized by sol–gel technique. Superlattices Microstruct. 2013, 60, 139–147. [Google Scholar] [CrossRef]
- Sharma, V.K.; Najim, M.; Srivastava, A.K.; Varma, G.D. Structural and magnetic studies on transition metal (Mn, Co) doped ZnO nanoparticles. J. Magn. Magn. Mater. 2012, 324, 683–689. [Google Scholar] [CrossRef]
- Blasco, J.; Bartolomé, F.; García, L.M.; García, J.J. Extrinsic origin of ferromagnetism in doped ZnO. J. Mater. Chem. 2006, 16, 2282–2288. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Cao, C. Hydrothermal synthesis of Co-doped ZnO flakes with room temperature ferromagnetism. J. Alloys Compd. 2010, 501, 265–268. [Google Scholar] [CrossRef]
- Mandal, S.K.; Das, A.K.; Natha, T.K. Microstructural and magnetic properties of ZnO:TM (TM = Co, Mn) diluted magnetic semiconducting nanoparticles. J. Appl. Phys. 2006, 100, 104315. [Google Scholar] [CrossRef]
- Kuryliszyn-Kudelska, I.; Dobrowolski, W.D.; Kilański, Ł.; Hadžić, B.; Romčević, N.; Sibera, D.; Narkiewicz, U.; Dziawa, P. Magnetic properties of nanocrystalline ZnO doped with MnO and CoO. J. Phys. Conf. Ser. 2010, 200, 072058. [Google Scholar] [CrossRef] [Green Version]
- Kuryliszyn-Kudelska, I.; Dobrowolski, W.; Arciszewska, M.; Romčević, N.; Romčević, M.; Hadžić, B.; Sibera, D.; Narkiewicz, U.; Lojkowski, W. Transition metals in ZnO nanocrystals: Magnetic and structural properties. Sci. Sinter. 2013, 45, 31–48. [Google Scholar] [CrossRef]
- Kuryliszyn-Kudelska, I.; Dobrowolski, W.; Arciszewska, M.; Romčević, N.; Romčević, M.; Hadžić, B.; Sibera, D.; Narkiewicz, U. Superparamagnetic and ferrimagnetic behavior of nanocrystalline ZnO(MnO). Phys. E Low-Dimens. Syst. Nanostruct. 2018, 98, 10–16. [Google Scholar] [CrossRef]
- Żołnierkiewicz, G.; Typek, J.; Guskos, N.; Narkiewicz, U.; Sibera, D. Magnetic resonance study of nanocrystalline 0.10MnO/0.90ZnO. Cent. Eur. J. Phys. 2013, 11, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Shen, D.Z.; Zhang, J.Y.; Zhao, D.X.; Li, B.S.; Lu, Y.M.; Liu, Y.C.; Fan, X.W. Magnetism origin of Mn-doped ZnO nanoclusters. J. Magn. Magn. Mater. 2006, 302, 118–121. [Google Scholar] [CrossRef]
- Samanta, K.; Bhattacharya, P.; Katiyar, R.S.; Iwamoto, W.; Pagliuso, P.G.; Rettori, C. Raman scattering studies in dilute magnetic semiconductor Zn1−xCoxO. Phys. Rev. B 2006, 73, 245213. [Google Scholar] [CrossRef]
- Kuryliszyn-Kudelska, I.; Hadžić, B.; Sibera, D.; Romčević, M.; Romčević, N.; Narkiewicz, U.; Łojkowski, W.; Arciszewska, M.; Dobrowolski, W. Magnetic properties of ZnO(Co) nanocrystals. J. Alloys Compd. 2013, 561, 247–251. [Google Scholar] [CrossRef]
- Typek, J.; Guskos, N.; Zolnierkiewicz, G.; Sibera, D.; Narkiewicz, U. Magnetic resonance study of Co-doped ZnO nanomaterials: A case of high doping. Rev. Adv. Mater. Sci. 2017, 50, 76–87. [Google Scholar]
- Birajdar, S.D.; Alange, R.C.; More, S.D.; Murumkar, V.D.; Jadhav, K.M. Sol-gel Auto Combustion Synthesis, Structural and Magnetic Properties of Mn doped ZnO Nanoparticles. Procedia Manuf. 2018, 20, 174–180. [Google Scholar] [CrossRef]
- Luo, X.; Lee, W.T.; Xing, G.; Bao, N.; Yonis, A.; Chu, D.; Lee, J.; Ding, J.; Li, S.; Yi, J. Ferromagnetic ordering in Mn-doped ZnO nanoparticles. Nanoscale Res. Lett. 2014, 9, 625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.S.; Huang, J.C.A.; Huang, Y.H.; Liao, Y.F.; Lin, M.Z.; Lee, C.H.; Lee, J.F.; Chen, S.F.; Lai, L.Y.; Liu, C.P. Evidence of oxygen vacancy enhanced room-temperature ferromagnetism in Co-doped ZnO. Appl. Phys. Lett. 2006, 88, 242507. [Google Scholar] [CrossRef]
- da Silva, R.T.; Mesquita, A.; de Zevallos, A.O.; Chiaramonte, T.; Gratens, X.; Chitta, V.A.; Morbec, J.M.; Rahman, G.; García-Suárez, V.M.; Doriguetto, A.C.; et al. Multifunctional nanostructured Co-doped ZnO: Co spatial distribution and correlated magnetic properties. Phys. Chem. Chem. Phys. 2018, 20, 20257–20269. [Google Scholar] [CrossRef] [PubMed]
- Shatnawi, M.; Alsmadi, A.M.; Bsoul, I.; Salameh, B.; Mathai, M.; Alnawashi, G.; Alzoubi, G.M.; Al-Dweri, F.; Bawa’aneh, M.S. Influence of Mn doping on the magnetic and optical properties of ZnO nanocrystalline particles. Results Phys. 2016, 6, 1064–1071. [Google Scholar] [CrossRef]
- Mamani, N.C.; da Silva, R.T.; de Zevallos, A.O.; Cotta, A.A.C.; Macedo, W.A.D.; Li, M.S.; Bernardi, M.I.B.; Doriguetto, A.C.; de Carvalho, H.B. On the nature of the room temperature ferromagnetism in nanoparticulate co-doped ZnO thin films prepared by EB-PVD. J. Alloys Compd. 2017, 695, 2682–2688. [Google Scholar] [CrossRef]
- Othman, A.A.; Osman, M.A.; Ibrahim, E.M.M.; Ali, M.A.; Abd-Elrahim, A.G. Mn-doped ZnO nanocrystals synthesized by sonochemical method: Structural, photoluminescence, and magnetic properties. Mater. Sci. Eng. B 2017, 219, 1–9. [Google Scholar] [CrossRef]
- Martínez, B.; Sandiumenge, F.; Balcells, L.; Arbiol, J.; Sibieude, F.; Monty, C. Structure and magnetic properties of Co-doped ZnO nanoparticles. Phys. Rev. B 2005, 72, 165202. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Kusnieruk, S.; Chudoba, T.; Gierlotka, S.; Lojkowski, W.; Knoff, W.; Lukasiewicz, M.I.; Witkowski, B.S.; Wolska, A.; Klepka, M.T.; et al. Paramagnetism of cobalt-doped ZnO nanoparticles obtained by microwave solvothermal synthesis. Beilstein J. Nanotechnol. 2015, 6, 1957–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Tse, K.; Wong, M.; Zhang, Y.; Zhu, J. A brief review of co-doping. Front. Phys. 2016, 11, 117405. [Google Scholar] [CrossRef]
- Katayama-Yoshida, H.; Nishimatsu, T.; Yamamoto, T.; Orita, N. Codoping method for the fabrication of low-resistivity wide band-gap semiconductors in p-type GaN, p-type AlN and n-type diamond: Prediction versus experiment. J. Phys. Condens. Matter 2001, 13, 8901–8914. [Google Scholar] [CrossRef]
- Eringen, A.C.; Maugin, G.A. Electrodynamics of Continua I: Foundations and Solid Media; Springer: New York, NY, USA, 1990; ISBN 978-1-4612-3226-1. [Google Scholar]
- Jung, S.W.; An, S.J.; Yi, G.C.; Jung, C.U.; Lee, S.; Cho, S. Ferromagnetic properties of Zn1−xMnxO epitaxial thin films. Appl. Phys. Lett. 2002, 80, 4561–4563. [Google Scholar] [CrossRef]
- Jaćimović, J.; Micković, Z.; Gaál, R.; Smajda, R.; Vâju, C.; Sienkiewicz, A.; Forró, L.; Magrez, A. Synthesis, electrical resistivity, thermo-electric power and magnetization of cubic ZnMnO3. Solid State Commun. 2011, 151, 487–490. [Google Scholar] [CrossRef]
- Blasco, J.; García, J. Stable cubic spinels in the Zn–Mn–O system in air. J. Solid State Chem. 2006, 179, 2199–2205. [Google Scholar] [CrossRef]
- Han, S.J.; Jang, T.H.; Kim, Y.B.; Park, B.G.; Park, J.H.; Jeong, Y.H. Magnetism in Mn-doped ZnO bulk samples prepared by solid state reaction. Appl. Phys. Lett. 2003, 83, 920–922. [Google Scholar] [CrossRef] [Green Version]
- Christensen, A.N.; Ollivier, G. Hydrothermal preparation and low temperature magnetic properties of Mn(OH)2. Solid State Commun. 1972, 10, 609–614. [Google Scholar] [CrossRef]
- Norton, D.P.; Overberg, M.E.; Pearton, S.J.; Pruessner, K.; Budai, J.D.; Boatner, L.A.; Chisholm, M.F.; Lee, J.S.; Khim, Z.G.; Park, Y.D.; et al. Ferromagnetism in cobalt-implanted ZnO. Appl. Phys. Lett. 2003, 83, 5488–5490. [Google Scholar] [CrossRef]
- Brumage, W.H.; Dorman, C.F.; Quade, C.R. Temperature-dependent paramagnetic susceptibilities of Cu2+ and Co2+ as dilute impurities in ZnO. Phys. Rev. B Condens. Matter 2001, 63, 104411. [Google Scholar] [CrossRef]
- Cho, Y.M.; Choo, W.K.; Kim, H.; Kim, D.; Ihm, Y.E. Effects of rapid thermal annealing on the ferromagnetic properties of sputtered Zn1−x(Co0.5Fe0.5)xO thin films. Appl. Phys. Lett. 2002, 80, 3358. [Google Scholar] [CrossRef]
- Seidov, Z.; Açıkgöz, M.; Kazan, S.; Mikailzade, F. Magnetic properties of Co3O4 polycrystal powder. Ceram. Int. 2016, 42, 12928–12931. [Google Scholar] [CrossRef]
- Singh, V.; Major, D.T. Electronic Structure and Bonding in Co-Based Single and Mixed Valence Oxides: A Quantum Chemical Perspective. Inorg. Chem. 2016, 55, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, C.R.; Kumara, R.; Vijaya Prakash, G. Functional properties of ZnCo2O4 nano-particles obtained by thermal decomposition of a solution of binary metal nitrates. RSC Adv. 2015, 5, 26843–26849. [Google Scholar] [CrossRef]
- Gupta, A.; Tiwari, S.D. Magnetic Properties of Undoped and Al doped Layered α-Co(OH)2. Phys. B Condens. Matter 2017, 525, 21–25. [Google Scholar] [CrossRef]
- Yan, L.; Ong, C.K.; Rao, X.S. Magnetic order in Co-doped and (Mn, Co) codoped ZnO thin films by pulsed laser deposition. J. Appl. Phys. 2004, 96, 508–511. [Google Scholar] [CrossRef]
- Gu, Z.B.; Yuan, C.S.; Lu, M.H.; Wang, J.; Wu, D.; Zhang, S.T.; Zhu, S.N.; Zhu, Y.Y.; Chena, Y.F. Magnetic and transport properties of (Mn, Co)-codoped ZnO films prepared by radio-frequency magnetron cosputtering. J. Appl. Phys. 2005, 98, 053908. [Google Scholar] [CrossRef]
- Gu, Z.-B.; Lu, M.-H.; Wang, J.; Du, C.-L.; Yuan, C.-S.; Wu, D.; Zhang, S.-T.; Zhu, Y.-Y.; Zhu, S.-N.; Chen, Y.-F. Optical properties of (Mn, Co) co-doped ZnO films prepared by dual-radio frequency magnetron sputtering. Thin Solid Films 2006, 515, 2361–2365. [Google Scholar] [CrossRef]
- Du, C.L.; Gu, Z.B.; You, Y.M.; Kasim, J.; Yu, T.; Shen, Z.X.; Ni, Z.H.; Ma, Y.; Cheng, G.X.; Chen, Y.F. Resonant Raman spectroscopy of (Mn,Co)-codoped ZnO films. J. Appl. Phys. 2008, 103, 023521. [Google Scholar] [CrossRef]
- Duan, L.B.; Rao, G.H.; Wang, Y.C.; Yu, J.; Wang, T. Magnetization and Raman scattering studies of (Co,Mn) codoped ZnO nanoparticles. J. Appl. Phys. 2008, 104, 013909. [Google Scholar] [CrossRef]
- Nirmala, M.; Smitha, P.; Anukaliani, A. Optical and electrical properties of undoped and (Mn, Co) co-doped ZnO nanoparticles synthesized by DC thermal plasma method. Superlattices Microstruct. 2011, 50, 563–571. [Google Scholar] [CrossRef]
- Nirmala, M.; Anukaliani, A. Synthesis and characterization of undoped and TM (Co, Mn) doped ZnO nanoparticles. Mater. Lett. 2011, 65, 2645–2648. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Zhang, Q.; Qiao, Y.; Gu, Y.; Liu, J.; Zhang, Y. Facile synthesis of highly uniform Mn/Co-codoped ZnO nanowires: Optical, electrical, and magnetic properties. Nanoscale 2011, 3, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, D.Y.; Lad, A.D.; Pathak, A.K.; Dubenko, I.; Ali, N.; Mahamuni, S. Ferromagnetism in ZnO Nanocrystals: Doping and Surface Chemistry. J. Phys. Chem. C 2010, 114, 1451–1459. [Google Scholar] [CrossRef]
- Phan, L.; Vincent, R.; Cherns, D.; Dan, N.H.; Yu, S.C. Characterization of (Mn, Co)-Codoped ZnO Nanorods Prepared by Thermal Diffusion. IEEE Trans. Magn. 2009, 45, 2435–2438. [Google Scholar] [CrossRef]
- Naeem, M.; Hasanain, S.K. Role of donor defects in stabilizing room temperature ferromagnetism in (Mn, Co) co-doped ZnO nanoparticles. J. Phys. Condens. Matter 2012, 24, 245305. [Google Scholar] [CrossRef] [PubMed]
- Yin-Hua, Y.; Xi, C.Q. Infrared emissivities of Mn, Co co-doped ZnO powders. Chin. Phys. B 2012, 21, 124205. [Google Scholar] [CrossRef]
- Sato, W.; Kano, Y.; Suzuki, T.; Nakagawa, M.; Kobayashi, Y. Local fields in Co and Mn Co-doped ZnO. Hyperfine Interact. 2016, 237, 28. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Zheng, Z.J. Magnetic behavior of Co–Mn co-doped ZnO nanoparticles. Magn. Magn. Mater. 2014, 372, 37–40. [Google Scholar] [CrossRef]
- Neena, D.; Shah, A.H.; Deshmukh, K.; Ahmad, H.; Fu, D.J.; Kondamareddy, K.K.; Kumar, P.; Dwivedi, R.K.; Sing, V. Influence of (Co-Mn) co-doping on the microstructures, optical properties of sol-gel derived ZnO nanoparticles. Eur. Phys. J. D 2016, 70, 53. [Google Scholar] [CrossRef]
- Sivaselvan, S.; Muthukumaran, S.; Ashokkumar, M. Influence of Co-doping on the structural, optical and morphological properties of Zn0.96Mn0.04O nanoparticles by sol–gel method. Opt. Mater. 2014, 36, 797–803. [Google Scholar] [CrossRef]
- Abdullahi, S.S.; Köseog, Y.; Güner, S.; Kazan, S.; Kocaman, B.; Ndikilar, C.E. Synthesis and characterization of Mn and Co codoped ZnO nanoparticles. Superlattices Microstruct. 2015, 83, 342–352. [Google Scholar] [CrossRef]
- Köseoğlu, Y. Fuel aided rapid synthesis and room temperature ferromagnetism of M0.1Co0.1Zn0.8O (M = Mn, Ni, Fe and Cu) DMS nanoparticles. Ceram. Int. 2016, 42, 9190–9195. [Google Scholar] [CrossRef]
- Pazhanivelu, V.; Blessington Selvadurai, A.P.; Zhao, Y.; Thiyagarajan, R.; Murugaraj, R. Room temperature ferromagnetism in Mn doped ZnO: Co nanoparticles by co-precipitation method. Phys. B Condens. Matter 2016, 481, 91–96. [Google Scholar] [CrossRef]
- Pazhanivelu, V.; Blessington Selvadurai, A.P.; Murugaraj, R. Sintering Effect on Structural, Optical and Unusual Magnetic Behaviour in Zn0.95Co0.05O-Based DMS Materials. J. Supercond. Nov. Magn. 2015, 28, 2575–2581. [Google Scholar] [CrossRef]
- Pazhanivelu, V.; Blessington Selvadurai, A.P.; Murugaraj, R. Room temperature magnetic behaviour of Mn codoping in ZnO: Co nanoparticles synthesized by co-precipitation method. J. Mater. Sci. Mater. Electron. 2018, 29, 3087–3094. [Google Scholar] [CrossRef]
- Yakout, S.M.; El-Sayed, A.M. Synthesis, Structure, and Room Temperature Ferromagnetism of Mn and/or Co Doped ZnO Nanocrystalline. J. Supercond. Nov. Magn. 2016, 29, 1593–1599. [Google Scholar] [CrossRef] [Green Version]
- Karthika, K.; Ravichandran, K. Enhancing the magnetic and antibacterial properties of ZnO nanopowders through Mn+Co doping. Ceram. Int. 2015, 41, 7944–7951. [Google Scholar] [CrossRef]
- Sharma, D.; Jha, R. Transition metal (Co, Mn) co-doped ZnO nanoparticles: Effect on structural and optical properties. J. Alloys Compd. 2017, 698, 532–538. [Google Scholar] [CrossRef]
- Khan, R.; de Araujo, C.I.L.; Khan, T.; Khan, A.; Ullah, B.; Fashu, S. Influence of oxygen vacancies on the structural, dielectric, and magnetic properties of (Mn, Co) co-doped ZnO nanostructures. J. Mater. Sci. Mater. Electron. 2018, 29, 9785–9795. [Google Scholar] [CrossRef]
- Khan, R.; Fashu, Z.S.; Rehman, Y.U.; Khan, A.; Rahman, M.U. Structure and magnetic properties of (Co, Mn) co-doped ZnO diluted magnetic semiconductor nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 32–37. [Google Scholar] [CrossRef]
- Li, G.R.; Qu, D.L.; Zhao, W.X.; Tong, Y.X. Electrochemical deposition of (Mn, Co)-codoped ZnO nanorod arrays without any template. Electrochem. Commun. 2007, 9, 1661–1666. [Google Scholar] [CrossRef]
- Dai, J.; Meng, C.; Li, Q. First-principles study on the magnetism of Mn and Co codoped ZnO. Phys. B Condens. Matter 2013, 409, 5–9. [Google Scholar] [CrossRef]
- Stashans, A.; Rivera, K. Electronic and Magnetic Properties of Co- and Mn-codoped ZnO by Density Functional Theory. Chin. Phys. Lett. 2016, 33, 097102. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, X.; Yang, P. Investigation on electronic and magnetic properties of Co and Mn in ZnO with different doping types. J. Magn. Magn. Mater. 2018, 461, 1–5. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Microwaves in Nanoparticle Synthesis: Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 9783527331970. [Google Scholar]
- Chikan, V.; McLaurin, E.J. Rapid Nanoparticle Synthesis by Magnetic and Microwave Heating. Nanomaterials 2016, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Majeed, S.; Nayak, A.; Bhushan, B. Principles and Advantages of Microwave—Assisted Methods for the Synthesis of Nanomaterials for Water Purification. In Advanced Nanomaterials for Water Engineering, Treatment, and Hydraulics, 2nd ed.; Saleh, T.A., Ed.; IGI Global: Hershey, PA, USA, 2017; pp. 40–57. ISBN -10 1522521364. [Google Scholar]
- Motshekga, S.C.; Pillai, S.K.; Ray, S.S.; Jalama, K.; Krause, R.W.M. Recent Trends in the Microwave-Assisted Synthesis of Metal Oxide Nanoparticles Supported on Carbon Nanotubes and Their Applications. J. Nanomater. 2012, 2012, 691503. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Koltsov, I.; Prześniak-Welenc, M.; Wojnarowicz, J.; Rogowska, A.; Mizeracki, J.; Malysa, M.; Kimmel, G. Thermal and physical properties of ZrO2-AlO(OH) nanopowders synthesised by microwave hydrothermal method. J. Therm. Anal. Calorim. 2017, 13, 2273–2284. [Google Scholar] [CrossRef]
- Guenin, E. Microwave Engineering of Nanomaterials: From Mesoscale to Nanoscale, 1st ed.; Pan Stanford Publishing Pte. Ltd.: Boca Raton, FL, USA, 2016; ISBN 9789814669429. [Google Scholar]
- Polshettiwar, V.; Nadagouda, M.N.; Varma, R.S. Microwaveassisted chemistry: A rapid and sustainable route to synthesis of organics and nanomaterials. Aust. J. Chem. 2009, 62, 16–26. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Majcher, A.; Łojkowski, W. Microwaves Applied to Hydrothermal Synthesis of Nanoparticles. In Microwave Chemistry, 1st ed.; Cravotto, G., Carnaroglio, D., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2017; pp. 205–224. ISBN 9783110479935. [Google Scholar]
- Lojkowski, W.; Leonelli, C.; Chudoba, T.; Wojnarowicz, J.; Majcher, A.; Mazurkiewicz, A. High-Energy-low-temperature technologies for the synthesis of nanoparticles: Microwaves and high pressure. Inorganics 2014, 2, 606–619. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide-From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Hasanpoor, M.; Aliofkhazraei, M.; Delavari, H. Microwave-assisted Synthesis of Zinc Oxide Nanoparticles. Proc. Mater. Sci. 2015, 11, 320–325. [Google Scholar] [CrossRef]
- Shao, D.; Wei, Q. Microwave-Assisted Rapid Preparation of Nano-ZnO/Ag Composite Functionalized Polyester Nonwoven Membrane for Improving Its UV Shielding and Antibacterial Properties. Materials 2018, 11, 1412. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.U.; Kang, M.; Kim, H.S. Microwave-assisted Facile and Ultrafast Growth of ZnO Nanostructures and Proposition of Alternative Microwave-assisted Methods to Address Growth Stoppage. Sci. Rep. 2016, 6, 24870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, A.H.S.; Chang, S.B.; Kim, H.S. NH4OH-Oriented and pH-Dependent Growth of ZnO Nanostructures via Microwave-Assisted Growth Method. J. Nanosci. Nanotechnol. 2018, 18, 2125–2127. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Ferreira, S.H.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave Synthesized ZnO Nanorod Arrays for UV Sensors: A Seed Layer Annealing Temperature Study. Materials 2016, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Nunes, D.; Duarte, P.; Rodrigues, J.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Synthesis of Long ZnO Nanorods under Microwave Irradiation or Conventional Heating. J. Phys. Chem. C 2014, 118, 14629–14639. [Google Scholar] [CrossRef]
- Barreto, G.P.; Morales, G.; Quintanilla Ma, L.L. Microwave Assisted Synthesis of ZnO Nanoparticles: Effect of Precursor Reagents, Temperature, Irradiation Time, and Additives on Nano-ZnO Morphology Development. J. Mater. 2013, 2013, 478681. [Google Scholar] [CrossRef]
- Barreto, G.; Morales, G.; Cañizo, A.; Eyler, N. Microwave Assisted Synthesis of ZnO Tridimensional Nanostructures. Proc. Mater. Sci. 2015, 8, 535–540. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakraborty, J. Rapid synthesis of zinc oxide nanoforest: Use of microwave and forced seeding. Mater. Res. Express 2016, 3, 125004. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Opalinska, A.; Chudoba, T.; Gierlotka, S.; Mukhovskyi, R.; Pietrzykowska, E.; Sobczak, K.; Lojkowski, W. Effect of water content in ethylene glycol solvent on the size of ZnO nanoparticles prepared using microwave solvothermal synthesis. J. Nanomater. 2016, 2016, 2789871. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Koltsov, I.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis. Nanotechnology 2018, 29, 065601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, E.; Aliofkhazraei, M.; Hasanpoor, M.; Chipara, M. Hierarchical and Complex ZnO Nanostructures by Microwave-Assisted Synthesis: Morphologies, Growth Mechanism and Classification. Crit. Rev. Solid State Mater. Sci. 2018, 43, 475–541. [Google Scholar] [CrossRef]
- Majcher, A.; Wiejak, J.; Przybylski, J.; Chudoba, T.; Wojnarowicz, J. A novel reactor for microwave hydrothermal scale-up nanopowder synthesis. Int. J. Chem. React. Eng. 2013, 11, 361–368. [Google Scholar] [CrossRef]
- Cravotto, G.; Carnaroglio, D. Microwave Chemistry, 1st ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2017; ISBN 9783110479935. [Google Scholar]
- Rinaldi, L.; Carnaroglio, D.; Rotolo, L.; Cravotto, G. A Microwave-Based Chemical Factory in the Lab: From Milligram to Multigram Preparations. J. Chem. 2015, 2015, 879531. [Google Scholar] [CrossRef]
- Dąbrowska, S.; Chudoba, T.; Wojnarowicz, J.; Łojkowski, W. Current Trends in the Development of Microwave Reactors for the Synthesis of Nanomaterials in Laboratories and Industries: A Review. Crystals 2018, 8, 379. [Google Scholar] [CrossRef]
- Dąbrowska, S.; Chudoba, T.; Wojnarowicz, J.; Łojkowski, W. Problems of exploitations of microwave reactors for nanoparticles synthesis. J. Mach. Construct. Maint. 2018, 110, 41–47. [Google Scholar]
- Wojnarowicz, J.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Effect of Microwave Radiation Power on the Size of Aggregates of ZnO NPs Prepared Using Microwave Solvothermal Synthesis. Nanomaterials 2018, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowicz, J.; Mukhovskyi, R.; Pietrzykowska, E.; Kusnieruk, S.; Mizeracki, J.; Lojkowski, W. Microwave solvothermal synthesis and characterization of manganese-doped ZnO nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowicz, J.; Kuśnieruk, S.; Chudoba, T.; Mizeracki, J.; Łojkowski, W. Microwave solvothermal synthesis of Co-doped ZnO nanoparticles. Glass Ceram. 2015, 3, 8–13. [Google Scholar]
- Wojnarowicz, J.; Chudoba, T.; Gierlotka, S.; Sobczak, K.; Lojkowski, W. Size Control of Cobalt-Doped ZnO Nanoparticles Obtained in Microwave Solvothermal Synthesis. Crystals 2018, 8, 179. [Google Scholar] [CrossRef]
- Lojkowski, W.; Gedanken, A.; Grzanka, E.; Opalinska, A.; Strachowski, T.; Pielaszek, R.; Tomaszewska-Grzeda, A.; Yatsunenko, S.; Godlewski, M.; Matysiak, H.; et al. Solvothermal synthesis of nanocrystalline zinc oxide doped with Mn2+, Ni2+, Co2+ and Cr3+ ions. J. Nanopart. Res. 2009, 11, 1991–2002. [Google Scholar] [CrossRef]
- Pielaszek, R. FW15/45M method for determination of the grain size distribution from powder diffraction line profile. J. Alloys Compd. 2004, 37, 128–132. [Google Scholar] [CrossRef]
- Nanopowder XRD Processor Demo, pre⋅α⋅ver.0.0.8, © Pielaszek Research. Available online: http://science24.com/xrd/ (accessed on 10 January 2018).
- FW1/5 4/5M Method of Evaluation of Grain Size Distribution by Powder Diffraction. Available online: http://science24.com/fw145m/ (accessed on 10 January 2018).
- Zhang, Q.; Park, K.; Cao, G. Synthesis of ZnO Aggregates and Their Application in Dye-sensitized Solar Cells. Mater. Matters 2014, 5, 32–39. [Google Scholar]
- Poul, L.; Jouini, N.; Fiévet, F. Layered Hydroxide Metal Acetates (Metal = Zinc, Cobalt, and Nickel): Elaboration via Hydrolysis in Polyol Medium and Comparative Study. Chem. Mater. 2000, 12, 3123–3132. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Hyde, B.G. Crystal Structures: I. Patterns and Symmetry, 1st ed.; Mineralogical Society of America: Washington, DC, USA, 1996; ISBN 0939950405. [Google Scholar]
- Rao, M.S.R.; Okada, T. ZnO Nanocrystals and Allied Materials; Springer: New Delhi, India, 2014; ISBN 978-81-322-1159-4. [Google Scholar]
- Kück, S.; Werheit, H. Non-Tetrahedrally Bonded Binary Compounds II; Springer: Berlin/Heidelberg, Germany, 2000; ISBN 978-3-540-64966-3. [Google Scholar]
- Sarbas, B.; Töpper, W. Mn Manganese; Springer: Berlin/Heidelberg, Germany, 1993; ISBN 978-3-662-08907-1. [Google Scholar]
- Saravanan, R. Solid Oxide Fuel Cell (SOFC) Materials; Materials Research Forum LLC: Millersville, PA, USA, 2018; ISBN 978-1-945291-50-0. [Google Scholar]
- Olesik, J.W. Elemental analysis using ICP-OES and ICP/MS. Anal. Chem. 1991, 63, 12A–21A. [Google Scholar] [CrossRef]
- Barsoum, M.W. Fundamentals of Ceramics, 1st ed.; Taylor & Francis Group: New York, NY, USA; Northampton, UK, 2002; ISBN 9780750309028. [Google Scholar]
- Opalinska, A.; Malka, I.; Dzwolak, W.; Chudoba, T.; Presz, A.; Lojkowski, W. Size-dependent density of zirconia nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusnieruk, S.; Wojnarowicz, S.; Chodara, A.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Influence of hydrothermal synthesis parameters on the properties of hydroxyapatite nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1586–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wejrzanowski, T.; Pielaszek, R.; Opalińska, A.; Matysiak, H.; Łojkowski, W.; Kurzydłowski, K.J. Quantitative methods for nanopowders characterization. Appl. Surf. Sci. 2006, 253, 204–208. [Google Scholar] [CrossRef]
- Zając, M.; Gosk, J.; Grzanka, E.; Kamińska, M.; Twardowski, A.; Strojek, B.; Szyszko, T.; Podsiadło, S. Possible origin of ferromagnetism in (Ga,Mn)N. J. Appl. Phys. 2003, 93, 4715–4717. [Google Scholar] [CrossRef]
- Zając, M.; Gosk, J.; Grzanka, E.; Stelmakh, S.; Palczewska, M.; Wysmołek, A.; Korona, K.; Kamińska, M.; Twardowski, A. Ammonothermal synthesis of GaN doped with transition metal ions (Mn, Fe, Cr). J. Alloys Compd. 2008, 456, 324–338. [Google Scholar] [CrossRef]
- Lawniczak-Jablonska, K.; Wolska, A.; Klepka, M.T.; Kret, S.; Gosk, J.; Twardowski, A.; Wasik, D.; Kwiatkowski, A.; Kurowska, B.; Kowalski, B.J.; et al. Magnetic properties of MnSb inclusions formed in GaSb matrix directly during molecular beam epitaxial growth. J. Appl. Phys. 2011, 109, 074308. [Google Scholar] [CrossRef]
- Gaj, J.A.; Planel, R.; Fishman, G. Relation of magneto-optical properties of free excitons to spin alignment of Mn2+ ions in Cd1−xMnxTe. Solid State Commun. 1979, 29, 435–438. [Google Scholar] [CrossRef]
- Gaj, J.A. On the physical meaning of the modified Brillouin function. Acta Phys. Pol. 1988, 73, 463–466. [Google Scholar]











| Phase | Magnetic Properties | Reference |
|---|---|---|
| Mn | Antiferromagnetic | [76] |
| MnO | Antiferromagnetic | [77] |
| MnO2 | Antiferromagnetic | [77] |
| Mn2O3 | Antiferromagnetic | [77] |
| Mn3O4 | Ferromagnetic | [77] |
| ZnMnO3 | Paramagnetic | [78,79] |
| ZnMn2O4 | Ferromagnetic | [80] |
| Mn(OH)2 | Antiferromagnetic | [81] |
| Co | Ferromagnetic | [82] |
| Co2+cluster | Ferromagnetic | [83] |
| CoO | Antiferromagnetic | [84] |
| Co3O4 | Antiferromagnetic | [85] |
| Co2O3 | Antiferromagnetic | [86] |
| ZnCo2O4 | Ferromagnetic | [87] |
| Co(OH)2 | Paramagnetic | [88] |
| Method | Substrates | Conditions during Preparation | Morphology; Magnetic Properties; Maximum Content of Dopants | Reference |
|---|---|---|---|---|
| pulsed laser deposition | ZnO, Co3O4 with MnO2 | from 400 °C to 600 °C and a low oxygen pressure (5 × 10−5 Pa). | films; room-temperature ferromagnetism; Zn0.7Mn0.15Co0.15O | [89] |
| radio-frequency magnetron co-sputtering and annealing | Zn0.95Mn0.05O, Zn0.80Co0.20O | 550 °C in argon ambient; 400 °C, 600 °C, 800 °C and 900 °C for 1 h in air | films; ferromagnetic and paramagnetic behaviour; Zn0.90Mn0.03Co0.07O | [90,91,92] |
| autocombustion | Zn(NO3)2·6H2O, Co(NO3)2·6H2O, Mn(NO3)2, NH2CH2COOH and H2O | - | nanoparticles; ferromagnetic; Zn0.96Mn0.02Co0.02O | [93] |
| thermal plasma and annealing | zinc, cobalt and manganese metal powders | 450 °C, 550 °C and 650 °C for 1 h, atmospheric air | Particles; | [94,95] |
| thermal evaporation | Zn powders, MnCl2·4H2O, CoCl2·6H2O | 500 °C with a constant argon flow of 50 sccm | nanowires (50–200 nm and lengths up to several tens of microns) | [96] |
| thermal decomposition | Zn(Ac)2·2H2O, Mn(Ac)2·4H2O, Co(Ac)2·4H2O and C16H33NH2 and C4H9PO(OH)2 | at 300 °C under a constant nitrogen flow | nanocrystals (7 nm); room temperature ferromagnetism | [97] |
| thermal diffusion | Zn(NO3)2·6H2O, C6H12N4, Mn and Co (metal powders) | at 850 °C in a vacuum at about 1 × 10−3 Torr. | nanorods (1–2 µm length and 80–200 nm diameter) | [98] |
| chemical route and annealing | Zn(Ac)2·2H2O, Mn(Ac)2·4H2O, Co(Ac)2·4H2O and ethylene glycol | 200 °C for 3 h; 600 °C in (Ar95% H5%) and in air. | nanoparticles (25 nm); paramagnetic and ferromagnetic behaviour; Zn0.94Mn0.04Co0.02O, Zn0.94Mn0.02Co0.04O | [99] |
| solid state reaction and calcination | ZnO was mixed with MnCO3 and Co2O (the mixtures were ball milled) | 2 h in 1000 °C, 1050 °C, 1100 °C, 1150 °C, and 1200 °C in atmospheric air | particles; Zn0.94Mn0.01Co0.05O | [100] |
| solid state reaction and calcination | ZnO was mixed with Mn and Co (metal powders) with the assistance citric acid | 1100 °C for 7 day in air | room temperature ferromagnetism; Zn0.90Mn0.05Co0.05O | [101] |
| sol–gel | Zn(Ac)2·2H2O, Mn(Ac)2·4H2O, Co(Ac)2·4H2O ethanol solution as a solvent, and DEA as a stabilizing agent | 500 °C for 1 h in O2 atmosphere, | particles; exhibited ferromagnetic character; Zn0.94Mn0.04Co0.02O | [102] |
| Zn(Ac)2·2H2O, Mn(Ac)2·4H2O, Co(Ac)2·4H2O, NaOH, water and N,N-dimethylformamide, | 500 °C for 1 h in atmospheric air | nanoparticles; ferromagnetism behaviour; Zn0.7Co0.15Mn0.15O | [103] | |
| Zn(Ac)2·2H2O, Mn(Ac)2·4H2O, Co(Ac)2·4H2O, N,N dimethyl-formamide (DMF) | 500 °C for 4 h in atmospheric air | nanoparticles (20–30 nm); Zn0.88Mn0.04Co0.08O | [104] | |
| microwave assisted combustion synthesis | Mn(NO3)2·4H2O, Co(NO3)6·H2O, Zn(NO3)2·6H2O, water, using urea as a fuel | 800 W for 15 min (kitchen type microwave) | nanoparticles (24 nm); paramagnetic and ferromagnetic behaviour; Zn0.70Mn0.20Co0.10O | [105] |
| Mn(NO3)2·4H2O, Co(NO3)6·H2O, Zn(NO3)2·6H2O, water, using urea as a fuel | 1000 W for 20 min (kitchen type microwave) | nanoparticles (31 nm); room temperature ferromagnetism; Zn0.80Mn0.10Co0.10O | [106] | |
| co-precipitation and calcination | Zn(Ac)2·2H2O, Mn(Ac)2·4H2O, Co(Ac)2·4H2O, (COOH)2·2H2O | 350 °C, 500 °C and 650 °C in atmospheric air | nanoparticles; ferromagnetism behaviour Zn0.90Mn0.05Co0.05O | [107,108,109] |
| Zn(Ac)2·2H2O, MnCl4·4H2O, CoCl2·6H2O, NH4OH | 400 °C for 3 h in atmospheric air | nanoparticles (28 nm); ferromagnetic behaviour; Zn0.98Mn0.01Co0.01O | [110] | |
| Zn(Ac)2·2H2O, Mn(Ac)2·4H2O, Co(Ac)2·4H2O, Na(OH), water | 550 °C for 3 h in atmospheric air | nanoparticles (20 nm); ferromagnetic behaviour; Zn0.85Mn0.10Co0.05O | [111] | |
| Mn(NO3)2·4H2O, Co(NO3)6·H2O, Zn(NO3)2·6H2O, LiOH·H2O, H2O, C2H5OH | 300 °C for 3 h in atmospheric air | nanoparticles; Zn0.92Co0.02Mn0.06O | [112] | |
| Zn(Ac)2·2H2O, Mn(Ac)2·4H2O, Co(Ac)2·4H2O, NH4OH, H2O | 750 °C for 2 h in atmospheric air | nanoparticles (15–17 nm); Zn0.92Mn0.04Co0.04O | [113,114] | |
| electrochemical | ZnCl2, MnCl2, CoCl2, KCl, tartaric acid | densities of electrodepositioning in the range of 0.5–2.0 mA/cm2 | nanorod (diameters: 50–100 nm, no longer than 500 nm) | [115] |
| Name of Precursor | CmZn(Ac)2∙2H2O (mol/dm3) | CmMn(Ac)2∙4H2O (mol/dm3) | CmCo(Ac)2∙4H2O (mol/dm3) |
|---|---|---|---|
| ZnO | 0.3037 | 0 | 0 |
| Zn0.98Mn0.01Co0.01O | 0.3037 | 0.0031 | 0.0031 |
| Zn0.90Mn0.05Co0.05O | 0.3037 | 0.0169 | 0.0169 |
| Zn0.80Mn0.10Co0.10O | 0.3037 | 0.0380 | 0.0380 |
| Zn0.70Mn0.15Co0.15O | 0.3037 | 0.0651 | 0.0651 |
| Sample | Lattice Parameters | Ratio of Lattice Parameters c/a | In hcp Structure, ZnO, Ratio of Lattice Parameters c/a | |
|---|---|---|---|---|
| a ± σ, [Å] | c ± σ, [Å] | |||
| ZnO (JCPDS No. 36-1451) | 3.2498 | 5.2066 | 1.6021 | 1.6330 |
| ZnO | 3.2508 | 5.2074 | 1.6017 | |
| Zn0.98Mn0.01Co0.01O | 3.2520 | 5.2083 | 1.6010 | |
| Zn0.90Mn0.05Co0.05O | 3.2547 | 5.2107 | 1.6007 | |
| Zn0.80Mn0.10Co0.10O | 3.2563 | 5.2122 | 1.6007 | |
| Zn0.70Mn0.15Co0.15O | 3.2569 | 5.2134 | 1.6007 | |
| Zn0.85Mn0.15O [149] | 3.2564 | 5.2138 | 1.6011 | |
| Zn0.85Co0.15O [73] | 3.2520 | 5.2040 | 1.6003 | |
| Sample | Actual Dopant Content, mol % | |||||
|---|---|---|---|---|---|---|
| EDS | ICP-OES | |||||
| Zinc | Manganese | Cobalt | Zinc | Manganese | Cobalt | |
| Zn0.98Mn0.01Co0.01O | 98.86 | 0.26 | 0.88 | 99.06 | 0.25 | 0.69 |
| Zn0.90Mn0.05Co0.05O | 94.36 | 1.42 | 4.21 | 95.29 | 1.18 | 3.53 |
| Zn0.80Mn0.10Co0.10O | 88.08 | 2.57 | 9.35 | 89.94 | 2.11 | 7.96 |
| Zn0.70Mn0.15Co0.15O | 83.48 | 2.86 | 13.65 | 85.58 | 2.35 | 12.07 |
| Sample | Efficiency of Codoping Calculated Based on the Results of ICP-OES (%) | |
|---|---|---|
| Manganese | Cobalt | |
| Zn0.98Mn0.01Co0.01O | 25.00 | 69.00 |
| Zn0.90Mn0.05Co0.05O | 23.60 | 70.60 |
| Zn0.80Mn0.10Co0.10O | 21.10 | 79.60 |
| Zn0.70Mn0.15Co0.15O | 15.67 | 80.47 |
| Sample | R (Red) | G (Green) | B (Blue) | H (Hue) | S (Saturation) | L (Luminance) |
|---|---|---|---|---|---|---|
| ZnO | 1023 | 1023 | 1023 | 000 | 000 | 1000 |
| Zn0.85Co0.15O | 246 | 376 | 292 | 392 | 209 | 304 |
| Zn0.85Mn0.15O | 614 | 438 | 211 | 093 | 488 | 403 |
| Zn0.98Mn0.01Co0.01O | 660 | 702 | 547 | 211 | 194 | 610 |
| Zn0.90Mn0.05Co0.05O | 374 | 429 | 275 | 226 | 218 | 344 |
| Zn0.80Mn0.10Co0.10O | 205 | 258 | 145 | 244 | 280 | 196 |
| Zn0.70Mn0.15Co0.15O | 219 | 160 | 094 | 088 | 399 | 152 |
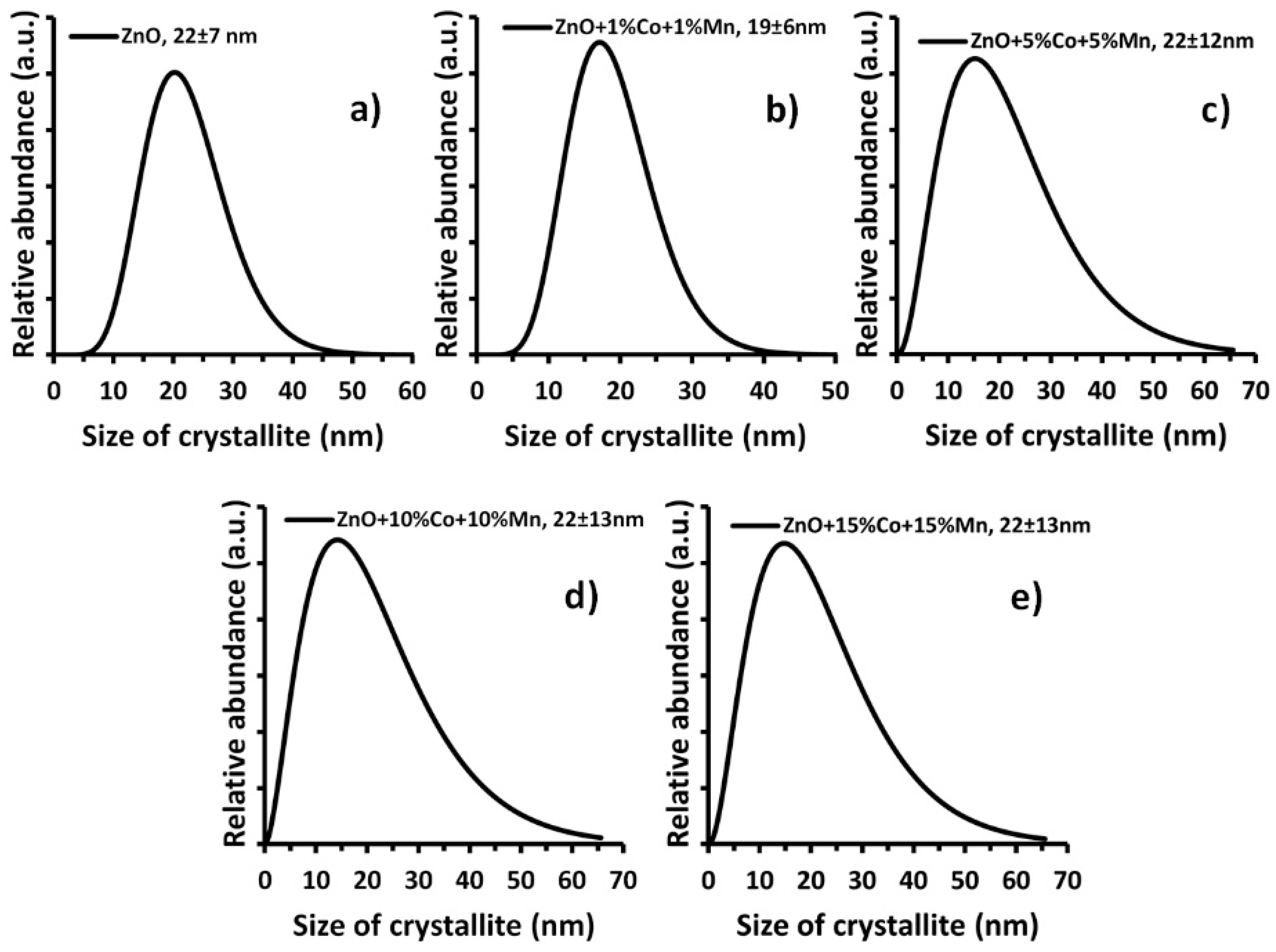
| Sample | Specific Surface Area, as ± σ (m2/g) | Skeleton Density, ρs ± σ (g/cm3) | Average Particle Size from SSA BET, d ± σ (nm) | Average Crystallite Size from Nanopowder XRD Processor Demo, d ± σ (nm) | Average crystallite Size, Scherrer’s Formula, da, dc (nm) |
|---|---|---|---|---|---|
| ZnO | 39.8 ± 0.1 | 5.25 ± 0.02 | 29 ± 1 | 22 ± 7 | 20a, 24c |
| Zn0.98Mn0.01Co0.01O | 46.4 ± 0.1 | 5.24 ± 0.02 | 24 ± 1 | 19 ± 6 | 16a, 18c |
| Zn0.90Mn0.05Co0.05O | 45.8 ± 0.1 | 5.23 ± 0.03 | 25 ± 1 | 22 ± 12 | 15a, 14c |
| Zn0.80Mn0.10Co0.10O | 50.6 ± 0.1 | 5.15 ± 0.03 | 23 ± 1 | 22 ± 13 | 14a, 11c |
| Zn0.70Mn0.15Co0.15O | 56.4 ± 0.1 | 5.06 ± 0.02 | 21 ± 1 | 22 ± 13 | 15a, 11c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojnarowicz, J.; Omelchenko, M.; Szczytko, J.; Chudoba, T.; Gierlotka, S.; Majhofer, A.; Twardowski, A.; Lojkowski, W. Structural and Magnetic Properties of Co?Mn Codoped ZnO Nanoparticles Obtained by Microwave Solvothermal Synthesis. Crystals 2018, 8, 410. https://doi.org/10.3390/cryst8110410
Wojnarowicz J, Omelchenko M, Szczytko J, Chudoba T, Gierlotka S, Majhofer A, Twardowski A, Lojkowski W. Structural and Magnetic Properties of Co?Mn Codoped ZnO Nanoparticles Obtained by Microwave Solvothermal Synthesis. Crystals. 2018; 8(11):410. https://doi.org/10.3390/cryst8110410
Chicago/Turabian StyleWojnarowicz, Jacek, Myroslava Omelchenko, Jacek Szczytko, Tadeusz Chudoba, Stanisław Gierlotka, Andrzej Majhofer, Andrzej Twardowski, and Witold Lojkowski. 2018. "Structural and Magnetic Properties of Co?Mn Codoped ZnO Nanoparticles Obtained by Microwave Solvothermal Synthesis" Crystals 8, no. 11: 410. https://doi.org/10.3390/cryst8110410
APA StyleWojnarowicz, J., Omelchenko, M., Szczytko, J., Chudoba, T., Gierlotka, S., Majhofer, A., Twardowski, A., & Lojkowski, W. (2018). Structural and Magnetic Properties of Co?Mn Codoped ZnO Nanoparticles Obtained by Microwave Solvothermal Synthesis. Crystals, 8(11), 410. https://doi.org/10.3390/cryst8110410








