Investigation of HfO2 Thin Films on Si by X-ray Photoelectron Spectroscopy, Rutherford Backscattering, Grazing Incidence X-ray Diffraction and Variable Angle Spectroscopic Ellipsometry
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. X-ray Photoelectron Spectroscopy (XPS)
3.1.1. XPS Survey Scan
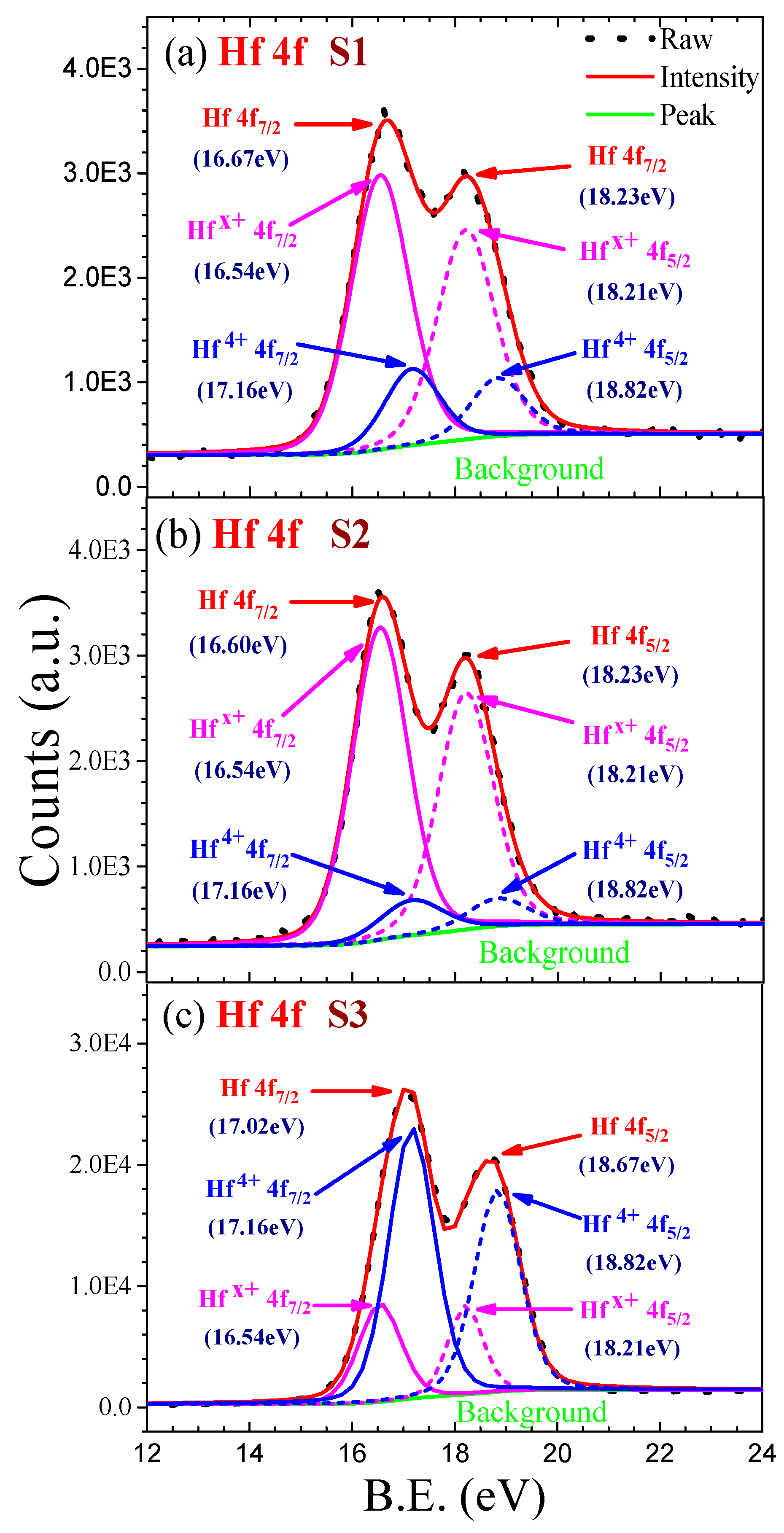
3.1.2. XPS Fitting and the Elements Composition Calculating
3.1.3. Energy Bandgaps of HfO2 Deduced from XPS
3.2. Rutherford Backscattering Spectrometry (RBS)
3.3. Grazing Incidence X-ray Diffraction (GIXRD)
3.4. The Variable Angle Spectroscopic Ellipsometry (VASE)
4. Conclusions
- Through XPS measurements and analyses on the Hf 4f peaks and the change of intensity ratio between Hf4+ and Hfx+ peaks, it was revealed that at the lower oxygen flow rate of 500 sccm, the insufficient chemical reaction leaded to more Hf suboxide bonds and non-lattice oxygen produced at HfO2 film surface; while, as the oxygen flow rate increased to 800 sccm, the quality of the surface films had been significantly improved.
- The deposited HfO2 films on Si were amorphous in nature at the low deposition temperature of 400 °C by AVD, while at higher deposition temperature, polycrystalline HfO2 films were achieved.
- At the initial stage of film growth, the monoclinic, tetragonal, and orthorhombic phases co-existed. As the film grew thicker, the tetragonal and orthorhombic phases gradually weakened until the monoclinic phase dominated. The crystallite size of HfO2 film became smaller from interface to surface, confirmed using varied angle GIXRD.
- It was found that for HfO2 film, higher crystallization and more ordered structure correspond to a smaller bandgap, determined from VASE and XPS, close to single crystal HfO2.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lu, Q.; Huang, R.; Lan, X.; Chi, X.; Lu, C.; Li, C.; Wu, Z.; Li, J.; Han, G.; Yan, P. Amazing diffusion depth of ultra-thin hafnium oxide film grown on n-type silicon by lower temperature atomic layer deposition. Mater. Lett. 2016, 169, 164–167. [Google Scholar] [CrossRef]
- Gao, L.; Yalon, E.; Chew, A.R.; Deshmukh, S.; Salleo, A.; Pop, E.; Demkov, A.A. Effect of oxygen vacancies and strain on the phonon spectrum of HfO2 thin films. J. Appl. Phys. 2017, 121, 224101. [Google Scholar] [CrossRef]
- Fan, X.; Liu, H.; Zhong, B.; Fei, C.; Wang, X.; Wang, Q. Optical characteristics of H2O-based and O3-based HfO2 films deposited by ALD using spectroscopy ellipsometry. Appl. Phys. A 2015, 119, 957–963. [Google Scholar] [CrossRef]
- Shandalov, M.; McIntyre, P.C. Size-dependent polymorphism in HfO2 nanotubes and nanoscale thin films. J. Appl. Phys. 2009, 106, 084322. [Google Scholar] [CrossRef]
- Kim, J.C.; Heo, J.S.; Cho, Y.S.; Moon, S.H. Atomic layer deposition of an HfO2 thin film using Hf(O-iPr)4. Thin Solid Films 2009, 517, 5695–5699. [Google Scholar] [CrossRef]
- Monaghan, S.; Hurley, P.K.; Cherkaoui, K.; Negara, M.A.; Schenk, A. Determination of electron effective mass and electron affinity in HfO2 using MOS and MOSFET structures. Solid-State Electron. 2009, 53, 438–444. [Google Scholar] [CrossRef]
- Cosnier, V.; Dabertrand, K.; Blonkowski, S.; Lhostis, S.; Zoll, S.; Morand, Y.; Descombes, S.; Guillaumot, B.; Hobbs, C.; Rochat, N.; et al. Atomic Vapour Deposition (AVD™) Process for High Performance HfO2 Dielectric Layers. MRS Proc. 2004, 811, 287–292. [Google Scholar] [CrossRef]
- Schumacher, M.; Baumann, P.K.; Seidel, T. AVD and ALD as Two Complementary Technology Solutions for Next Generation Dielectric and Conductive Thin-Film Processing. Chem. Vapor Depos. 2006, 12, 99–108. [Google Scholar] [CrossRef]
- Leu, C.-C.; Lin, C.-H.; Chien, C.-H.; Yang, M.-J. Effects of HfO2 buffer layer thickness on the properties of Pt/SrBi2Ta2O9/HfO2/Si structure. J. Mater. Res. 2008, 23, 2023–2032. [Google Scholar] [CrossRef]
- Lin, C.-P.; Tsui, B.-Y.; Yang, M.-J.; Huang, R.-H.; Chien, C.-H. High-performance poly-silicon TFTs using HfO2 gate dielectric. IEEE Electron Device Lett. 2006, 27, 360–363. [Google Scholar] [CrossRef]
- Manikanthababu, N.; Dhanunjaya, M.; Nageswara Rao, S.V.S.; Pathak, A.P. SHI induced effects on the electrical and optical properties of HfO2 thin films deposited by RF sputtering. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2016, 379, 230–234. [Google Scholar] [CrossRef]
- He, G.; Jiang, S.; Li, W.; Zheng, C.; He, H.; Li, J.; Sun, Z.; Liu, Y.; Liu, M. Interface chemistry and electronic structure of ALD-derived HfAlO/Ge gate stacks revealed by X-ray photoelectron spectroscopy. J. Alloys Compd. 2017, 716, 1–6. [Google Scholar] [CrossRef]
- Cho, Y.J.; Nguyen, N.V.; Richter, C.A.; Ehrstein, J.R.; Lee, B.H.; Lee, J.C. Spectroscopic ellipsometry characterization of high-k dielectric HfO2 thin films and the high-temperature annealing effects on their optical properties. Appl. Phys. Lett. 2002, 80, 1249–1251. [Google Scholar] [CrossRef]
- Takeuchi, H.; Ha, D.; King, T.-J. Observation of bulk HfO2 defects by spectroscopic ellipsometry. J. Vac. Sci. Technol. A Vac. Surf. Films 2004, 22, 1337–1341. [Google Scholar] [CrossRef]
- Buiu, O.; Lu, Y.; Mitrovic, I.Z.; Hall, S.; Chalker, P.; Potter, R.J. Spectroellipsometric assessment of HfO2 thin films. Thin Solid Films 2006, 515, 623–626. [Google Scholar] [CrossRef]
- Sancho-Parramon, J.; Modreanu, M.; Bosch, S.; Stchakovsky, M. Optical characterization of HfO2 by spectroscopic ellipsometry: Dispersion models and direct data inversion. Thin Solid Films 2008, 516, 7990–7995. [Google Scholar] [CrossRef]
- Pang, H.; Deng, N. A Forming-Free Bipolar Resistive Switching in HfOx -Based Memory with a Thin Ti Cap. Chin. Phys. Lett. 2014, 31, 107303. [Google Scholar] [CrossRef]
- Sharath, S.U.; Bertaud, T.; Kurian, J.; Hildebrandt, E.; Walczyk, C.; Calka, P.; Zaumseil, P.; Sowinska, M.; Walczyk, D.; Gloskovskii, A.; et al. Towards forming-free resistive switching in oxygen engineered HfO2−x. Appl. Phys. Lett. 2014, 104, 063502. [Google Scholar] [CrossRef]
- Tan, T.; Guo, T.; Wu, Z.; Liu, Z. Charge transport and bipolar switching mechanism in a Cu/HfO2/Pt resistive switching cell. Chin. Phys. B 2016, 25, 117306. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, J.-Z.; Cao, Z.-Y.; Li, A.-D.; Wang, L.-G.; Zhu, L.; Li, X.; Cao, Y.-Q.; Wu, D. Bipolar Resistive Switching Characteristics of HfO2/TiO2/HfO2 Trilayer-Structure RRAM Devices on Pt and TiN-Coated Substrates Fabricated by Atomic Layer Deposition. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Kondaiah, P.; Shaik, H.; Mohan Rao, G. Studies on RF magnetron sputtered HfO2 thin films for microelectronic applications. Electron. Mater. Lett. 2015, 11, 592–600. [Google Scholar] [CrossRef]
- Bagus, P.S.; Illas, F.; Pacchioni, G.; Parmigiani, F. Mechanisms responsible for chemical shifts of core-level binding energies and their relationship to chemical bonding. J. Electron Spectrosc. Relat. Phenom. 1999, 100, 215–236. [Google Scholar] [CrossRef]
- Kim, M.-S.; Ko, Y.-D.; Yun, M.; Hong, J.-H.; Jeong, M.-C.; Myoung, J.-M.; Yun, I. Characterization and process effects of HfO2 thin films grown by metal-organic molecular beam epitaxy. Mater. Sci. Eng. B 2005, 123, 20–30. [Google Scholar] [CrossRef]
- Guo, T.; Tan, T.; Liu, Z. Resistive switching behavior of HfO2 film with different Ti doping concentrations. J. Phys. D Appl. Phys. 2016, 49, 045103. [Google Scholar] [CrossRef]
- Miyazaki, S.; Narasaki, M.; Ogasawara, M.; Hirose, M. Characterization of ultrathin zirconium oxide films on silicon using photoelectron spectroscopy. Microelectron. Eng. 2001, 59, 373–378. [Google Scholar] [CrossRef]
- David, D.; Godet, C. Derivation of dielectric function and inelastic mean free path from photoelectron energy-loss spectra of amorphous carbon surfaces. Appl. Surf. Sci. 2016, 387, 1125–1139. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Chen, H.-Y.; Her, J.-L.; Ko, F.-H.; Pan, T.-M. Effect of Ti doping concentration on resistive switching behaviors of Yb2O3 memory cell. Appl. Phys. Lett. 2012, 101, 083506. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, Y.; Ahn, S.E.; Kang, B.S.; Lee, C.B.; Kim, K.H.; Xianyu, W.X.; Yoo, I.K.; Lee, J.H.; Chung, S.J.; et al. Comparative structural and electrical analysis of NiO and Ti doped NiO as materials for resistance random access memory. J. Appl. Phys. 2008, 103, 013706. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Zhang, Z.M.; Ding, Z.J.; Yoshikawa, H. Deriving Effective Energy Loss Function for Silver from XPS Spectrum. Phys. Procedia 2012, 32, 165–172. [Google Scholar] [CrossRef]
- Miyazaki, S. Photoemission study of energy-band alignments and gap-state density distributions for high-k gate dielectrics. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2001, 19, 2212. [Google Scholar] [CrossRef]
- Miyazaki, S.; Narasaki, M.; Ogasawara, M.; Hirose, M. Chemical and electronic structure of ultrathin zirconium oxide films on silicon as determined by photoelectron spectroscopy. Solid-State Electron. 2002, 46, 1679–1685. [Google Scholar] [CrossRef]
- Xu, D.-P.; Yu, L.-J.; Chen, X.-D.; Chen, L.; Sun, Q.-Q.; Zhu, H.; Lu, H.-L.; Zhou, P.; Ding, S.-J.; Zhang, D.W. In Situ Analysis of Oxygen Vacancies and Band Alignment in HfO2/TiN Structure for CMOS Applications. Nanoscale Res. Lett. 2017, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.; Chang, Y.C.; Chang, Y.H.; Lin, T.D.; Kwo, J.; Hong, M. Energy-band parameters of atomic layer deposited Al2O3 and HfO2 on InxGa1−xAs. Appl. Phys. Lett. 2009, 94, 052106. [Google Scholar] [CrossRef]
- Gaumer, C.; Martinez, E.; Lhostis, S.; Guittet, M.-J.; Gros-Jean, M.; Barnes, J.-P.; Licitra, C.; Rochat, N.; Barrett, N.; Bertin, F.; et al. Impact of the TiN electrode deposition on the HfO2 band gap for advanced MOSFET gate stacks. Microelectron. Eng. 2011, 88, 72–75. [Google Scholar] [CrossRef]
- Martínez, F.L.; Toledano-Luque, M.; Gandía, J.J.; Cárabe, J.; Bohne, W.; Röhrich, J.; Strub, E.; Mártil, I. Optical properties and structure of HfO2 thin films grown by high pressure reactive sputtering. J. Phys. D Appl. Phys. 2007, 40, 5256–5265. [Google Scholar] [CrossRef]
- Cantas, A.; Aygun, G.; Basa, D.K. In-situ spectroscopic ellipsometry and structural study of HfO2 thin films deposited by radio frequency magnetron sputtering. J. Appl. Phys. 2014, 116, 083517. [Google Scholar] [CrossRef]
- Vargas, M.; Murphy, N.R.; Ramana, C.V. Structure and optical properties of nanocrystalline hafnium oxide thin films. Opt. Mater. 2014, 37, 621–628. [Google Scholar] [CrossRef]
- Blanchin, M.-G.; Canut, B.; Lambert, Y.; Teodorescu, V.S.; Barău, A.; Zaharescu, M. Structure and dielectric properties of HfO2 films prepared by a sol-gel route. J. Sol-Gel Sci. Technol. 2008, 47, 165–172. [Google Scholar] [CrossRef]
- Xue, K.-H.; Blaise, P.; Fonseca, L.R.C.; Nishi, Y. Prediction of Semimetallic Tetragonal Hf2O3 and Zr2O3 from First Principles. Phys. Rev. Lett. 2013, 110, 065502. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Rousselot, C.; Rondot, D.; Palmino, F.; Mercier, R. Microstructure modification of amorphous titanium oxide thin films during annealing treatment. Thin Solid Films 1997, 300, 113–121. [Google Scholar] [CrossRef]
- Aguirre, B.; Vemuri, R.S.; Zubia, D.; Engelhard, M.H.; Shutthananadan, V.; Bharathi, K.K.; Ramana, C.V. Growth, microstructure and electrical properties of sputter-deposited hafnium oxide (HfO2) thin films grown using a HfO2 ceramic target. Appl. Surf. Sci. 2011, 257, 2197–2202. [Google Scholar] [CrossRef]
- Ramzan, M.; Rana, A.M.; Ahmed, E.; Wasiq, M.F.; Bhatti, A.S.; Hafeez, M.; Ali, A.; Nadeem, M.Y. Optical characterization of hafnium oxide thin films for heat mirrors. Mater. Sci. Semicond. Process. 2015, 32, 22–30. [Google Scholar] [CrossRef]
- Pal, A.; Narasimhan, V.K.; Weeks, S.; Littau, K.; Pramanik, D.; Chiang, T. Enhancing ferroelectricity in dopant-free hafnium oxide. Appl. Phys. Lett. 2017, 110, 022903. [Google Scholar] [CrossRef]
- Polakowski, P.; Müller, J. Ferroelectricity in undoped hafnium oxide. Appl. Phys. Lett. 2015, 106, 232905. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, Y.H.; Kim, H.J.; Kim, Y.J.; Moon, T.; Kim, K.D.; Müller, J.; Kersch, A.; Schroeder, U.; Mikolajick, T.; et al. Ferroelectricity and Antiferroelectricity of Doped Thin HfO2 -Based Films. Adv. Mater. 2015, 27, 1811–1831. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.D.; Park, M.H.; Kim, H.J.; Kim, Y.J.; Moon, T.; Lee, Y.H.; Hyun, S.D.; Gwon, T.; Hwang, C.S. Ferroelectricity in undoped-HfO2 thin films induced by deposition temperature control during atomic layer deposition. J. Mater. Chem. C 2016, 4, 6864–6872. [Google Scholar] [CrossRef]
- Fu, W.-E.; Chang, Y.-Q.; Chen, Y.-C.; Secula, E.M.; Seiler, D.G.; Khosla, R.P.; Herr, D.; Michael Garner, C.; McDonald, R.; Diebold, A.C. Post-deposition annealing analysis for HfO2 thin films using GIXRR/GIXRD. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2009; Volume 1173, pp. 122–126. [Google Scholar]
- Pandey, S.; Kothari, P.; Sharma, S.K.; Verma, S.; Rangra, K.J. Impact of post deposition annealing in O2 ambient on structural properties of nanocrystalline hafnium oxide thin film. J. Mater. Sci. Mater. Electron. 2016, 27, 7055–7061. [Google Scholar] [CrossRef]
- Ramadoss, A.; Krishnamoorthy, K.; Kim, S.J. Facile synthesis of hafnium oxide nanoparticles via precipitation method. Mater. Lett. 2012, 75, 215–217. [Google Scholar] [CrossRef]
- Ayyub, P.; Palkar, V.R.; Chattopadhyay, S.; Multani, M. Effect of crystal size reduction on lattice symmetry and cooperative properties. Phys. Rev. B 1995, 51, 6135–6138. [Google Scholar] [CrossRef]
- Matovic, B.; Pantic, J.; Lukovic, J.; Cebela, M.; Dmitrovic, S.; Mirkovic, M.; Prekajski, M. A novel reduction–oxidation synthetic route for hafnia. Ceram. Int. 2016, 42, 615–620. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; Ferguson, I.; Lin, T.; Wan, L.; Feng, Z.C.; Zhu, L.; Ye, Z. Spectroscopic ellipsometry studies on ZnCdO thin films with different Cd concentrations grown by pulsed laser deposition. Appl. Surf. Sci. 2017, 421, 383–388. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.X.; Wan, L.Y.; Kucukgok, B.; Ghafari, E.; Ferguson, I.T.; Zhang, X.; Wang, S.; Feng, Z.C.; Lu, N. Composition and temperature dependent optical properties of AlxGa1-x N alloy by spectroscopic ellipsometry. Appl. Surf. Sci. 2017, 421, 389–396. [Google Scholar] [CrossRef]
- Ding, L.; Friedrich, M.; Fronk, M.; Gordan, O.D.; Zahn, D.R.T.; Chen, L.; Wei Zhang, D.; Cobet, C.; Esser, N. Correlation of band gap position with composition in high-k films. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2014, 32, 03D115. [Google Scholar] [CrossRef]
- Di, M.; Bersch, E.; Diebold, A.C.; Consiglio, S.; Clark, R.D.; Leusink, G.J.; Kaack, T. Comparison of methods to determine bandgaps of ultrathin HfO2 films using spectroscopic ellipsometry. J. Vac. Sci. Technol. A Vac. Surf. Films 2011, 29, 041001. [Google Scholar] [CrossRef]
- Park, J.-W.; Lee, D.-K.; Lim, D.; Lee, H.; Choi, S.-H. Optical properties of thermally annealed hafnium oxide and their correlation with structural change. J. Appl. Phys. 2008, 104, 033521. [Google Scholar] [CrossRef]










| Sample | Structure | Growth Conditions | Thicknesses |
|---|---|---|---|
| S1 (Hf08a) | HfO2/Si | 400 °C, 5 mbar, O2: 500 sccm | 70 nm |
| S2 (Hf08b) | HfO2/Si | 500 °C, 5 mbar, O2: 500 sccm | 33 nm |
| S3 (Hf08c) | HfO2/Si | 500 °C, 5 mbar, O2: 800 sccm | 34 nm |
| Sample | Hfx+ 4f7/2 | Hfx+ 4f5/2 | Hf4+ 4f7/2 | Hf4+ 4f5/2 | O | O–Hf | ||
|---|---|---|---|---|---|---|---|---|
| S1 | Peak/eV | 16.54 | 18.21 | 17.16 | 18.82 | Peak/eV | 532.08 | 530.27 |
| FWHM | 1.34 | 1.32 | 1.27 | 1.25 | FWHM | 1.93 | 1.65 | |
| Area/ASF (2.05) | 4118.46 | 3088.84 | 1083.73 | 812.80 | Area/ASF (0.66) | 2400.19 | 3935.01 | |
| Atom | 0.14 | 0.11 | 0.04 | 0.03 | Atom | 0.26 | 0.42 | |
| Hf (%) | 31.63% | O (%) | 68.37% | |||||
| S2 | Peak/eV | 16.54 | 18.21 | 17.16 | 18.82 | Peak/eV | 532.08 | 530.28 |
| FWHM | 1.28 | 1.27 | 1.45 | 1.39 | FWHM | 1.96 | 1.57 | |
| Area/ASF (2.05) | 4425.81 | 3319.36 | 565.70 | 424.27 | Area/ASF (0.66) | 1822.13 | 3885.52 | |
| Atom | 0.17 | 0.13 | 0.02 | 0.02 | Atom | 0.21 | 0.46 | |
| Hf (%) | 33.01% | O (%) | 66.99% | |||||
| S3 | Peak/eV | 16.54 | 18.21 | 17.16 | 18.82 | Peak/eV | 532.08 | 530.33 |
| FWHM | 0.97 | 0.80 | 1.06 | 1.06 | FWHM | 1.38 | 1.67 | |
| Area/ASF (2.05) | 9172.99 | 6879.74 | 27,466.56 | 20,599.92 | Area/ASF (0.66) | 2901.01 | 32,328.96 | |
| Atom | 0.05 | 0.04 | 0.16 | 0.12 | Atom | 0.05 | 0.58 | |
| Hf (%) | 36.95% | O (%) | 63.05% | |||||
| Samples | Hf (Composition) | O (Composition) | Ratios (Hf:O) | |||
|---|---|---|---|---|---|---|
| RBS | XPS | RBS | XPS | RBS | XPS | |
| S1 (Hf08a) | 0.30 | 0.32 | 0.70 | 0.68 | 1:2.33 | 1:2.13 |
| S2 (Hf08b) | 0.34 | 0.33 | 0.66 | 0.67 | 1:1.94 | 1:2.03 |
| S3 (Hf08c) | 0.35 | 0.37 | 0.65 | 0.63 | 1:1.86 | 1:1.70 |
| Incident Angles | S2 | S3 | ||||
|---|---|---|---|---|---|---|
| (Degree) | 2θ (Degree) | β (Degree) | D (nm) | 2θ (Degree) | β (Degree) | D (nm) |
| 0.5° | 28.720 | 0.548 | 14.810 | |||
| 1° | 28.521 | 0.521 | 15.570 | 28.680 | 0.605 | 13.413 |
| 3° | 28.460 | 0.513 | 15.811 | 28.444 | 0.479 | 16.932 |
| 5° | 28.449 | 0.460 | 17.632 | 28.426 | 0.472 | 17.183 |
| Thickness (nm) | |||||||
|---|---|---|---|---|---|---|---|
| Samples | SE | RBS | HR-TEM | Grower | |||
| Roughness | HfO2 | Interface | HfO2 | HfO2 | Interface | HfO2 | |
| S1 | 4.71 ± 0.06 | 65.74 ± 0.08 | 1.90 ± 0.08 | 53.33 | 62.5 | 2.0 | 70 |
| S2 | 3.95 ± 0.05 | 32.14 ± 0.11 | 2.59 ± 0.11 | 32.67 | 33 | ||
| S3 | 4.01 ± 0.05 | 31.71 ± 0.10 | 3.37 ± 0.10 | 40.67 | 34 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Li, Y.; Yang, H.; Liang, Y.; He, K.; Sun, W.; Lin, H.-H.; Yao, S.; Lu, X.; Wan, L.; et al. Investigation of HfO2 Thin Films on Si by X-ray Photoelectron Spectroscopy, Rutherford Backscattering, Grazing Incidence X-ray Diffraction and Variable Angle Spectroscopic Ellipsometry. Crystals 2018, 8, 248. https://doi.org/10.3390/cryst8060248
Luo X, Li Y, Yang H, Liang Y, He K, Sun W, Lin H-H, Yao S, Lu X, Wan L, et al. Investigation of HfO2 Thin Films on Si by X-ray Photoelectron Spectroscopy, Rutherford Backscattering, Grazing Incidence X-ray Diffraction and Variable Angle Spectroscopic Ellipsometry. Crystals. 2018; 8(6):248. https://doi.org/10.3390/cryst8060248
Chicago/Turabian StyleLuo, Xuguang, Yao Li, Hong Yang, Yuanlan Liang, Kaiyan He, Wenhong Sun, Hao-Hsiung Lin, Shude Yao, Xiang Lu, Lingyu Wan, and et al. 2018. "Investigation of HfO2 Thin Films on Si by X-ray Photoelectron Spectroscopy, Rutherford Backscattering, Grazing Incidence X-ray Diffraction and Variable Angle Spectroscopic Ellipsometry" Crystals 8, no. 6: 248. https://doi.org/10.3390/cryst8060248
APA StyleLuo, X., Li, Y., Yang, H., Liang, Y., He, K., Sun, W., Lin, H. -H., Yao, S., Lu, X., Wan, L., & Feng, Z. (2018). Investigation of HfO2 Thin Films on Si by X-ray Photoelectron Spectroscopy, Rutherford Backscattering, Grazing Incidence X-ray Diffraction and Variable Angle Spectroscopic Ellipsometry. Crystals, 8(6), 248. https://doi.org/10.3390/cryst8060248





