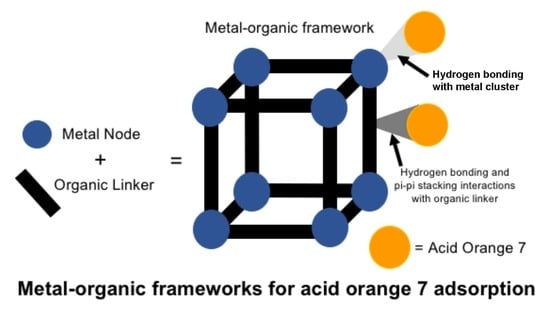
Removal of Acid Orange 7 from Aqueous Solution by Metal-Organic Frameworks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syntheses
2.2.1. Syntheses of UiO-66 Derivatives
2.2.2. Syntheses of ZIF Derivatives
2.2.3. Nitrogen Isotherms
2.2.4. UV-Vis Absorption Measurements
2.2.5. IR Absorption Measurements
2.2.6. Reusability Measurements
3. Results
3.1. Structural Characterization
3.2. Screening of MOFs for Adsorption of AO7
Adsorption of AO7 by MOFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial Decolorization of Textile-Dye-Containing Effluents: A Review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar] [CrossRef]
- Clarke, E.A.; Anliker, R. Organic Dyes and Pigments. In Handbook of Environmental Chemistry; Springer: New York, NY, USA, 1980. [Google Scholar]
- Wang, J.; Huang, C.P.; Allen, H.E.; Cha, D.K.; Kim, D.W. Adsorption Characteristics of Dye onto Sludge Particulates. J. Colloid Interface Sci. 1998, 208, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Hameed, B.H. Effect of preparation conditions of activated carbon from bamboo waste for real textile wastewater. J. Hazard. Mater. 2010, 173, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.J.; Arias, C.; Brillas, E.; Hernández-Ramírez, A.; Peralta-Hernández, J.M. Mineralization of Acid Yellow 36 azo dye by electro-Fenton and solar photoelectro-Fenton processes with a boron-doped diamond anode. Chemosphere 2011, 82, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.F.; Liao, C.H.; Po, S.T. Decolorization of textile wastewater by photo-fenton oxidation technology. Chemosphere 2000, 41, 1287–1294. [Google Scholar] [CrossRef]
- Kang, S.F.; Liao, C.H.; Chen, M.C. Pre-oxidation and coagulation of textile wastewater by the Fenton process. Chemosphere 2002, 46, 923–928. [Google Scholar] [CrossRef]
- Malik, P.K. Dye removal from wastewater using activated carbon developed from sawdust: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2004, 113, 81–88. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Malik, R.; Ramteke, D.S.; Wate, S.R. Adsorption of malachite green on groundnut shell waste based powdered activated carbon. Waste Manag. 2007, 27, 1129–1138. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Equilibrium, kinetics and mechanism of malachite green adsorption on activated carbon prepared from bamboo by K2CO3 activation and subsequent gasification with CO2. J. Hazard. Mater. 2008, 157, 344–351. [Google Scholar] [CrossRef]
- Bello, O.S.; Ahmad, M.A. Coconut (Cocos nucifera) shell based activated carbon for the removal of malachite green dye from aqueous solutions. Sep. Sci. Technol. 2011, 47, 903–912. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A. Analysis of the adsorption equilibrium for the system dilute aqueous solution of dissociating organic substance-activated carbon. Langmuir 1993, 9, 2344–2350. [Google Scholar] [CrossRef]
- Audu, C.O.; Nguyen, H.G.T.; Chang, C.Y.; Katz, M.J.; Mao, L.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. The dual capture of As V and As III by UiO-66 and analogues. Chem. Sci. 2016, 7, 6492–6498. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Katz, M.J.; Wang, T.C.; Platero-Prats, A.E.; Chapman, K.W.; Hupp, J.T.; Farha, O.K. High efficiency adsorption and removal of selenate and selenite from water using metal-organic frameworks. J. Am. Chem. Soc. 2015, 137, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeCoste, J.B.; Peterson, G.W.; Jasuja, H.; Glover, T.G.; Huang, Y.-G.; Walton, K.S. Stability and degradation mechanisms of metal-organic frameworks containing the Zr6O4(OH)4 secondary building unit. J. Mater. Chem. A 2013, 1, 5642. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.J.; Planas, N.; Semrouni, D.; Gagliardi, L.; Hupp, J.T.; Farha, O.K. Are Zr6-based MOFs water stable? Linker hydrolysis vs. capillary-force-driven channel collapse. Chem. Commun. 2014, 50, 8944–8946. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Yang, C.-X.; Yan, X.-P. Zeolitic imidazolate framework-8 for fast adsorption and removal of benzotriazoles from aqueous solution. ACS Appl. Mater. Interfaces 2013, 5, 9837–9842. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal-Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef]
- Gutov, O.V.; Bury, W.; Gomez-Gualdron, D.A.; Krungleviciute, V.; Fairen-Jimenez, D.; Mondloch, J.E.; Sarjeant, A.A.; Al-Juaid, S.S.; Snurr, R.Q.; Hupp, J.T.; et al. Water-Stable Zirconium-Based Metal-Organic Framework Material with High-Surface Area and Gas-Storage Capacities. Chem. Eur. J. 2014, 20, 12389–12393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.C.; Bury, W.; Gómez-Gualdrón, D.A.; Vermeulen, N.A.; Mondloch, J.E.; Deria, P.; Zhang, K.; Moghadam, P.Z.; Sarjeant, A.A.; Snurr, R.Q.; et al. Ultrahigh Surface Area Zirconium MOFs and Insights into the Applicability of the BET Theory. J. Am. Chem. Soc. 2015, 137, 3585–3591. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the complex structure of UiO-66 metal organic framework: A synergic combination of experiment and theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Gándara, F.; Zhang, Y.B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water adsorption in porous metal–organic frameworks and related materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and Highly Tunable Missing-Linker Defects in Zirconium Metal-Organic Framework UiO-66 and Their Important Effects on Gas Adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef] [PubMed]
- Barea, E.; Montoro, C.; Navarro, J.A.R. Toxic gas removal-Metal-organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W. Metal-Organic Frameworks for Air Purification of Toxic Chemicals. Chem. Rev. 2014, 114, 5695–5727. [Google Scholar] [CrossRef]
- Zhang, K.D.; Tsai, F.C.; Ma, N.; Xia, Y.; Liu, H.L.; Zhan, X.Q.; Yu, X.Y.; Zeng, X.Z.; Jiang, T.; Shi, D.; et al. Adsorption behavior of high stable Zr-based MOFs for the removal of acid organic dye from water. Materials 2017, 10, 205. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244–245, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Dincă, M.; Long, J.R. Hydrogen Storage in Microporous Metal–Organic Frameworks with Exposed Metal Sites. Angew. Chem. Int. Ed. 2008, 47, 6766–6779. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Yazaydın, A.Ö.; Eryazici, I.; Malliakas, C.D.; Hauser, B.G.; Kanatzidis, M.G.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2010, 2, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.A.; Chang, H.A. Ultra-high adsorption capacity of zeolitic imidazole framework-67 (ZIF-67) for removal of malachite green from water. Chemosphere 2015, 139, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, C.; Fang, W.J.; Chen, C.; Yan, R.W.; Huai, H.X.; Wang, C.C. Surfactant-free synthesis of a Fe3O4@ZIF-8 core–shell heterostructure for adsorption of methylene blue. CrystEngComm 2014, 16, 3960–3964. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.; Lv, M.; Xu, Y.; Yang, H.; Liu, X. Selective adsorption of cationic dyes by UiO-66-NH2. Appl. Surf. Sci. 2015, 327, 77–85. [Google Scholar] [CrossRef]
- Anliker, R. Organic Colorants Interpretation of Mammalian-, Geno- and Ecotoxicity Data in Terms of Potential Risk. In Toxic Hazard Assessment of Chemicals; Richardson, M., Ed.; The Royal Society of Chemistry: London, UK, 1986; pp. 166–187. [Google Scholar]
- Knackmuss, H.J. Basic Knowledge and Perspectives of Bioelimination of Xenobiotic Compounds. J. Biotechnol. 1996, 51, 287. [Google Scholar] [CrossRef]
- Cartwright, R.A. Historical and Modern Epidemiological Studies on Populations Exposed to N-substituted Aryl Compounds. Environ. Health Perspect. 1983, 49, 13–19. [Google Scholar] [CrossRef]
- Tsai, F.C.; Xia, Y.; Ma, N.; Shi, J.J.; Jiang, T.; Chiang, T.C.; Zhang, Z.C.; Tsen, W.C. Adsorptive removal of acid orange 7 from aqueous solution with metal-organic framework material, iron (III) trimesate. Desalin. Water Treat. 2016, 57, 3218–3226. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Poormohammadi, A.; Fatemeh, N.; Ahmadian, M. Removal of acid orange 7 from aqueous solution using activated carbon and graphene as adsorbents. Fresenius Environ. Bull. 2015, 24, 1841–1851. [Google Scholar]
- Smaranda, C.; Bulgariu, D.; Gavrilescu, M. An investigation of the sorption of acid orange 7 from aqueous solution onto soil. Environ. Eng. Manag. J. 2009, 8, 1391–1402. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Izadyar, S.; Azadeh, E.; Abyaz, A.; Asadollahi, Y. Application of Canola Stalks Waste as Adsorbent of Acid Orange 7 from Aqueous Solution. Iran. J. Health Environ. 2011, 4, 49–56. [Google Scholar]
- Sun, W.; Zhai, X.; Zhao, L. Synthesis of ZIF-8 and ZIF-67 nanocrystals with well-controllable size distribution through reverse microemulsions. Chem. Eng. J. 2016, 289, 59–64. [Google Scholar] [CrossRef]
- Zhao, X.; Bu, X.; Wu, T.; Zheng, S.-T.; Wang, L.; Feng, P. Selective anion exchange with nanogated isoreticular positive metal-organic frameworks. Nat. Commun. 2013, 4, 2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, X.-Z.; Song, Z.-W.; Shi, Q.; Dong, J.-X. Utilization of zeolite imidazolate framework as an adsorbent for the removal of dye from aqueous solution. Asian J. Chem. 2013, 25, 8324–8328. [Google Scholar] [CrossRef]
- Vlasova, E.A.; Shalunova, N.K.; Makarova, A.S.; Kudrik, E.V.; Makarov, S.V. Metal-Organic Frameworks Based on Terephthalic Acid: Sorbents of Organic Dyes. Russ. J. Appl. Chem. 2014, 87, 1065–1069. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Liu, Y.T.; Chen, S.Y. Adsorption of fluoride to UiO-66-NH2 in water: Stability, kinetic, isotherm and thermodynamic studies. Journal of colloid and interface science. J. Colloid Interface Sci. 2016, 461, 79–87. [Google Scholar] [CrossRef]
- Du, X.; Wang, C.; Liu, J.; Zhao, X.; Zhong, J.; Li, Y.; Li, J.; Wang, P. Extensive and selective adsorption of ZIF-67 towards organic dyes: Performance and mechanism. J. Colloid Interface Sci. 2017, 506, 437–441. [Google Scholar] [CrossRef]
- Jung, BK.; Jun, JW.; Hasan, Z.; Jhung, SH. Adsorptive removal of p-arsanilic acid from water using mesoporous zeolitic imidazolate framework-8. Chem. Eng. J. 2015, 267, 9–15. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Chang, H. Zeolitic Imidazole Framework-67 (ZIF-67) as a heterogeneous catalyst to activate peroxymonosulfate for degradation of Rhodamine B in water. J. Taiwan Inst. Chem. Eng. 2015, 53, 40–45. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, K.; He, M.; Yao, J. Synthesis of ZIF-8 and ZIF-67 using mixed base and their dye adsorption. Micropor. Mesopor. Mater. 2016, 234, 287–292. [Google Scholar] [CrossRef]
- Hasan, Z.; Tong, M.; Jung, B.K.; Ahmed, I.; Zhong, C.; Jhung, S.H. Adsorption of pyridine over amino-functionalized metal-organic frameworks: Attraction via hydrogen bonding versus base–base repulsion. J. Phys. Chem. C 2014, 118, 21049–21056. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, K.; Gao, Z.; Gao, H.; Xie, Z.; Du, X.; Huang, H. Reversing the dye adsorption and separation performance of metal-organic frameworks via introduction of –SO3H groups. Ind. Eng. Chem. Res. 2017, 56, 4496–4501. [Google Scholar] [CrossRef]
- Wang, M.M.; Wei, Z.; Xu, L.; Liu, B.; Jiao, H. Two Temperature-Controlled Zinc Coordination Polymers: Ionothermal Synthesis, Properties, and Dye Adsorption. Eur. J. Inorg. Chem. 2018, 7, 932–939. [Google Scholar] [CrossRef]
- Hahm, H.; Kim, S.; Ha, H.; Jung, S.; Kim, Y.; Yoon, M.; Kim, M. Charged functional group effects on a metal-organic framework for selective organic dye adsorptions. Cryst. Eng. Commun. 2015, 17, 8418–8422. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, G.; Gao, W.Y.; Niu, Z.; Wojtas, L.; Ma, S. Anionic Metal-Organic Framework for Selective Dye Removal and CO2 Fixation. Eur. J. Inorg. Chem. 2016, 27, 4373–4377. [Google Scholar] [CrossRef]







| pH | Adsorbed Amount (mg AO7/g Adsorbent) | |||
|---|---|---|---|---|
| UiO-66 | UiO-66-NH2 | ZIF-8 | ZIF-67 | |
| 5 | 133 | 98 | 3.3 | 63.4 |
| 6 | 110 | 95 | 7.4 | 126.9 |
| 7 | 106.5 | 84.9 | 16.9 | 272.6 |
| 8 | 9.83 | 7.88 | 13.4 | 738 |
| 9 | 11.5 | 6.8 | 7.4 | 400 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.; Calvo, J.J.; So, M.C. Removal of Acid Orange 7 from Aqueous Solution by Metal-Organic Frameworks. Crystals 2019, 9, 17. https://doi.org/10.3390/cryst9010017
Yoon S, Calvo JJ, So MC. Removal of Acid Orange 7 from Aqueous Solution by Metal-Organic Frameworks. Crystals. 2019; 9(1):17. https://doi.org/10.3390/cryst9010017
Chicago/Turabian StyleYoon, Sungwon, James J. Calvo, and Monica C. So. 2019. "Removal of Acid Orange 7 from Aqueous Solution by Metal-Organic Frameworks" Crystals 9, no. 1: 17. https://doi.org/10.3390/cryst9010017
APA StyleYoon, S., Calvo, J. J., & So, M. C. (2019). Removal of Acid Orange 7 from Aqueous Solution by Metal-Organic Frameworks. Crystals, 9(1), 17. https://doi.org/10.3390/cryst9010017