Self-Assembly Motifs of Water in Crystals of Palladium β-Amino Acid Complexes Influenced by Methyl Substitution on the Amino Acid Backbone
Abstract
:1. Introduction
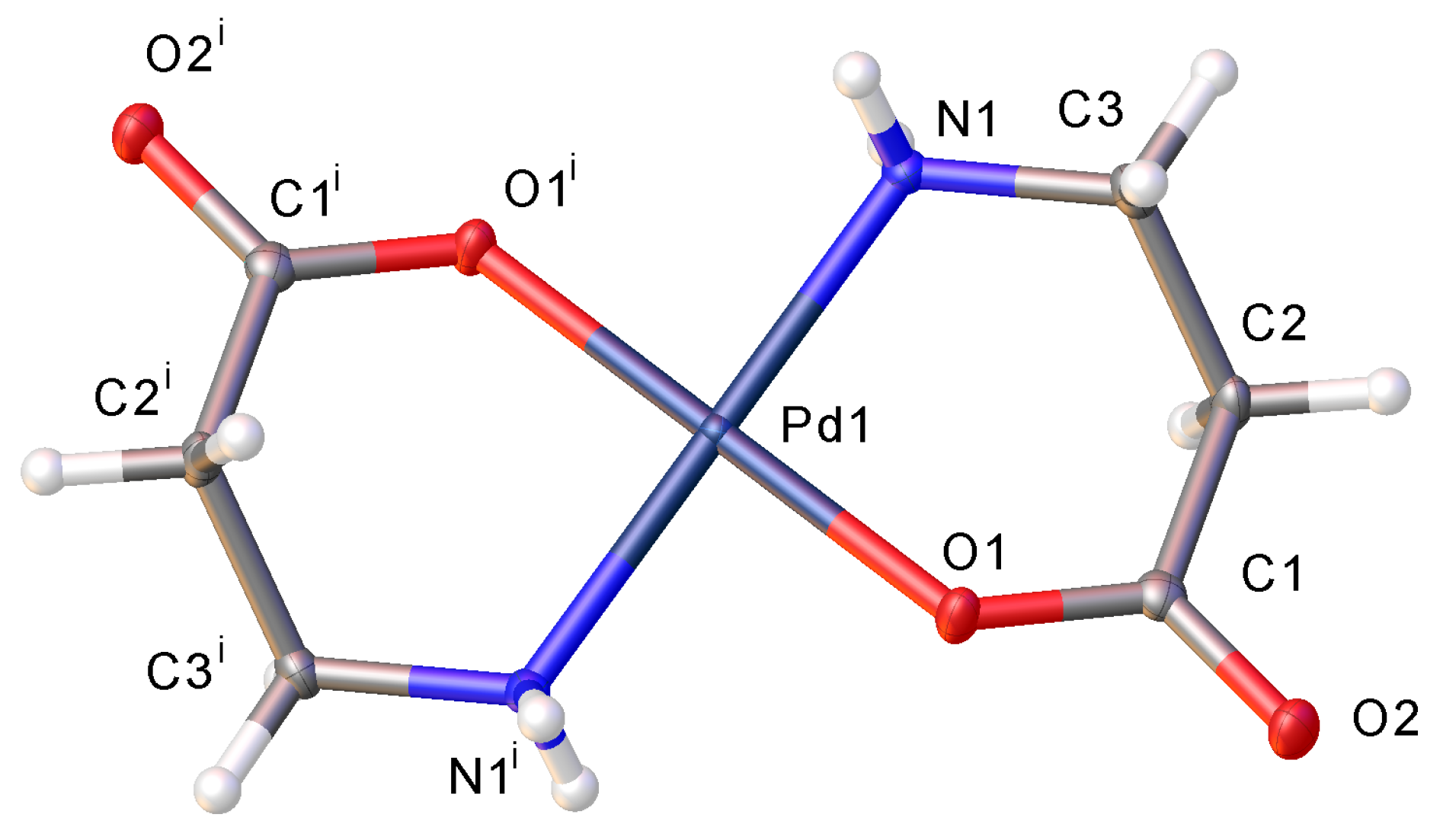
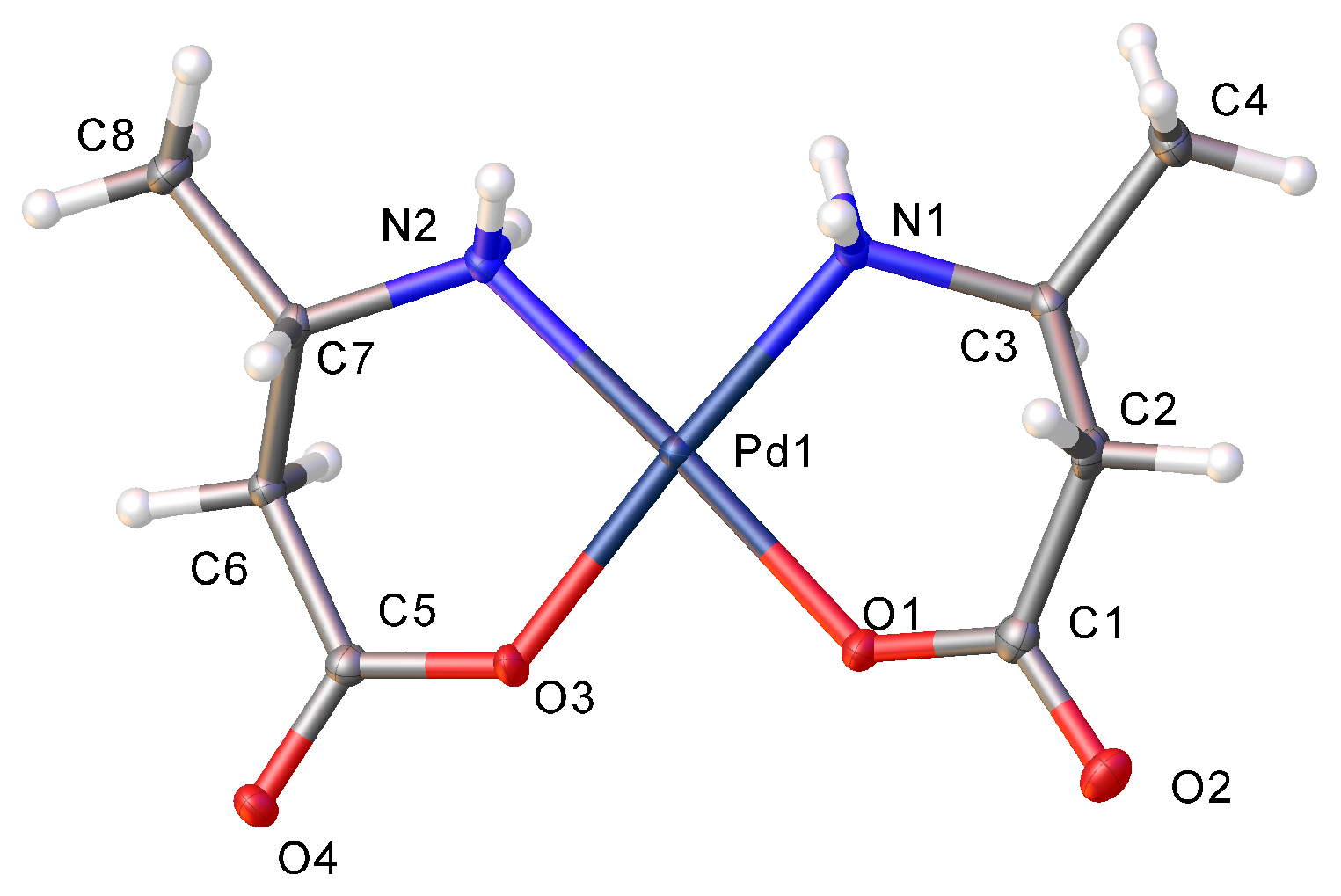
2. Results and Discussion
2.1. Complex Syntheses
2.2. Hydrogen Bonding Motifs
3. Conclusions
4. Experimental
4.1. General Synthetic Techniques
4.2. Synthesis of Complexes
4.2.1. Synthesis of trans-bis-(3-aminopropionato)palladium(II)
4.2.2. Synthesis of cis-bis-((S)-3-aminobutanoato)palladium(II)
4.2.3. Synthesis of trans-bis-(3-amino-3-methylbutanoato)palladium(II)
4.3. X-ray Crystallography
5. Associated Content
Accession Codes
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Severin, K.; Bergs, R.; Beck, W. Bioorganometallic Chemistry ± Transition Metal Complexes with. Angew. Chem. Int. Ed. Engl. 1998, 37, 1634–1654. [Google Scholar] [CrossRef]
- Carmona, D.; Pilar Lamata, M.; Viguri, F.; San José, E.; Mendoza, A.; Lahoz, F.J.; García-Orduña, P.; Atencio, R.; Oro, L.A. Half-sandwich organometallic complexes with stereogenic metal centres: Synthesis and characterization of diastereomeric [(η-ring)M(Aa)X] (Aa = α-amino carboxylate) compounds. J. Organomet. Chem. 2012, 717, 152–163. [Google Scholar] [CrossRef]
- Biancalana, L.; Abdalghani, I.; Chiellini, F.; Zacchini, S.; Pampaloni, G.; Crucianelli, M.; Marchetti, F. Ruthenium Arene Complexes with α-Aminoacidato Ligands: New Insights into Transfer Hydrogenation Reactions and Cytotoxic Behaviour. Eur. J. Inorg. Chem. 2018, 2018, 3041–3057. [Google Scholar] [CrossRef]
- Roy, C.P.; Huff, L.A.; Barker, N.A.; Berg, M.A.G.; Merola, J.S. Iridium(III) hydrido amino acid compounds: Chiral complexes and a helical extended lattice. J. Organomet. Chem. 2006, 691, 2270–2276. [Google Scholar] [CrossRef]
- Merola, J.S.; Slebodnick, C.; Berg, M.; Ritchie, M.K. mer-Hydridotris(trimethylphosphane-κP)(d-valinato-κ2 N,O)iridium hexafluoridophosphate dichloromethane 0.675-solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, m82. [Google Scholar] [CrossRef]
- Karpin, G.; Merola, J.; Falkinham, J., III. Transition metal-α-amino acid complexes with antibiotic activity against Mycobacterium spp. Antimicrob. Agents Chemother. 2013, 57. [Google Scholar] [CrossRef]
- Morris, D.M.; McGeagh, M.; De Peña, D.; Merola, J.S. Extending the range of pentasubstituted cyclopentadienyl compounds: The synthesis of a series of tetramethyl(alkyl or aryl)cyclopentadienes (Cp*R ), their iridium complexes and their catalytic activity for asymmetric transfer hydrogenation. Polyhedron 2014, 84, 120–135. [Google Scholar] [CrossRef]
- Wagner-Schuh, B.; Beck, W. Metal Complexes of Biologically Important Ligands, CLXXVII. Dichlorido Platinum(II) and Palladium(II) Complexes with Long Chain Amino Acids and Amino Acid Amides. Z. Anorg. Allg. Chem. 2017, 643, 632–635. [Google Scholar] [CrossRef]
- Cai, J.; Hu, X.; Feng, X.; Ji, L.; Bernal, I. Structures of three cis-β1 and three cis-β2 isomers of [Co(trien)(aminoacidato)]2+ complexes. Acta Crystallogr. Sect. B Struct. Sci. 2001, B57, 45–53. [Google Scholar] [CrossRef]
- Ng, C.H.; Ong, H.K.A.; Ngai, K.S.; Tan, W.T.; Lim, L.P.; Teoh, S.G.; Chong, T.S. mer-Tris(β-alaninato)cobalt(III): Crystal structure, solution properties and its DNA cleavage in the presence of ascorbic acid. Polyhedron 2005, 24, 1503–1509. [Google Scholar] [CrossRef]
- Garg, B.S.; Dwivedi, P. Solution studies on ternary complex formation of bivalent metal ions with cephalexine and amino acids, β-alanine and cysteine. J. Indian Chem. Soc. 2004, 81, 239–241. [Google Scholar]
- Gaizer, F.; Gondos, G.; Gera, L. Protonation and complex formation of the cis and trans isomers of alicyclic β-amino acids. Polyhedron 1986, 5, 1149–1156. [Google Scholar] [CrossRef]
- Juranic, N.; Prelesnik, B.; Manojlovic-Muir, L.; Andjelkovic, K.; Niketic, S.R.; Celap, M.B. Geometrical isomerism of mixed tris(amino carboxylato)cobalt(III) complexes containing glycinato and β-alaninato ligands. Crystal structure of trans(O5)-(β-alaninato)bis(glycinato)cobalt(III) trihydrate. Inorg. Chem. 2005, 29, 1491–1495. [Google Scholar] [CrossRef]
- Gridchin, S.N.; Shekhanov, R.F.; Bychkova, S.A. Stability constants of cobalt (II) complexes of taurine and β-alanine. Izv. Vyss. Uchebnykh Zaved. Khimiya I Khimicheskaya Tekhnol. 2016, 59, 95–96. [Google Scholar] [CrossRef]
- Sharma, V.S.; Mathur, H.B. Thermodynamic properties of coordination complexes of transition metal ions with amino acids. Indian J. Chem. 1965, 3, 475–479. [Google Scholar]
- Skoulika, S.; Michaelides, A.; Aubry, A. Habit modification of coordination compounds in the presence of additives; a study of the system Ni(β−ala)2·2H2O-glycine. J. Cryst. Growth 1991, 108, 285–289. [Google Scholar] [CrossRef]
- Böhm, A.; Seebach, D. Determination of Enantiomer Purity of β- and γ-Amino Acids by NMR Analysis of Diastereoisomeric Palladium Complexes. Helv. Chim. Acta 2000, 83, 3262–3278. [Google Scholar] [CrossRef]
- Cui, X.; Qin, T.; Wang, J.R.; Liu, L.; Guo, Q.X. Pd(N, N -Dimethyl β-alaninate) 2 as a High-Turnover-Number, Phosphine-Free Catalyst for the Suzuki Reaction. Synth. (Stuttg.) 2007, 2007, 393–399. [Google Scholar] [CrossRef]
- Krylova, L.F.; Kovtunova, L.M.; Romanenko, G.V. Pt(II) and Pd(II) Complexes with β-Alanine. Bioinorg. Chem. Appl. 2008, 2008, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, G.; Franchini-Angela, M. Survey of the geometry and environment of water molecules in crystalline hydrates studied by neutron diffraction. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 3572–3583. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Desiraju, G.R. Hydrogen bonding in organometallic crystals—A survey. J. Organomet. Chem. 1997, 548, 33–43. [Google Scholar] [CrossRef]
- Fucke, K.; Steed, J.W. X-ray and neutron diffraction in the study of organic crystalline hydrates. Water 2010, 2, 333–350. [Google Scholar] [CrossRef]
- Infantes, L.; Motherwell, S. Water clusters in organic molecular crystals. CrystEngComm 2002, 4, 454–461. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F. Intermolecular interactions in nonorganic crystal engineering. Acc. Chem. Res. 2000, 33, 601–608. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydrogen Bridges in Crystal Engineering: Interactions without Borders. Acc. Chem. Res. 2002, 35, 565–573. [Google Scholar] [CrossRef]
- Brammer, L.; Mareque Rivas, J.C.; Reinaldo Atencio, J.; Fang, S.; Christopher Pigge, F. Combining hydrogen bonds with coordination chemistry or organometallic π-arene chemistry: Strategies for inorganic crystal engineering. J. Chem. Soc. Dalt. Trans. 2000, 3855–3867. [Google Scholar] [CrossRef]
- Pal, S.; Sankaran, N.B.; Samanta, A. Structure of a self-assembled chain of water molecules in a crystal host. Angew. Chem. Int. Ed. 2003, 42, 1741–1743. [Google Scholar] [CrossRef]
- Infantes, L.; Chisholma, J.; Motherwell, S. Extended motifs from water and chemical functional groups in organic molecular crystals. CrystEngComm 2003, 5, 480–486. [Google Scholar] [CrossRef]
- Mascal, M.; Infantes, L.; Chisholm, J. Water oligomers in crystal hydrates—What’s news and what isn’t? Angew. Chem. Int. Ed. 2005, 45, 32–36. [Google Scholar] [CrossRef]
- Galek, P.T.; Fábián, L.; Motherwell, W.D.; Allen, F.H.; Feeder, N. Knowledge-based model of hydrogen-bonding propensity in organic crystals. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Galek, P.T.; Fábián, L.; Allen, F.H. Truly prospective prediction: Inter- and intramolecular hydrogen bonding. CrystEngComm 2010, 12, 2091–2099. [Google Scholar] [CrossRef]
- Cruz Cabeza, A.J.; Day, G.M.; Motherwell, W.D.; Jones, W. Importance of molecular shape for the overall stability of hydrogen bond motifs in the crystal structures of various carbamazepine-type drug molecules. Cryst. Growth Des. 2007, 7, 100–107. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Hobart, D.B.; Berg, M.A.G.; Merola, J.S. Bis-glycinato complexes of palladium(II): Synthesis, structural determination, and hydrogen bonding interactions. Inorg. Chim. Acta 2014, 423, 21–30. [Google Scholar] [CrossRef]
- Hobart, D.B.; Merola, J.S.; Rogers, H.M.; Saghal, S.; Mitchell, J.; Florio, J.; Merola, J.W. Synthesis, Structure, and Catalytic Reactivity of Pd(II) Complexes of Proline and Proline Homologs. Catalysts 2019, 9, 515. [Google Scholar] [CrossRef]
- Dexter, C.S.; Jackson, R.F.W. Efficient synthesis of β- and γ-amino acid derivatives using new functionalized zinc reagents: Enhanced stability and reactivity of β-amido zinc reagents in dimethylformamide. Chem. Commun. 1998, 75–76. [Google Scholar] [CrossRef]
- Abdel-Magid, A.F.; Cohen, J.H.; Maryanoff, C.A. Chemical process synthesis of β-amino acids and esters. Curr. Med. Chem. 1999, 6, 955–970. [Google Scholar]
- Hoen, R.; Tiemersma-Wegman, T.; Procuranti, B.; Lefort, L.; de Vries, J.G.; Minnaard, A.J.; Feringa, B.L. Enantioselective synthesis of β2-amino acids using rhodium-catalyzed hydrogenation. Org. Biomol. Chem. 2007, 5, 267–275. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobart, D.B.; Patel, V.G.; Pendergrass, H.; Florio, J.; Merola, J.S. Self-Assembly Motifs of Water in Crystals of Palladium β-Amino Acid Complexes Influenced by Methyl Substitution on the Amino Acid Backbone. Crystals 2019, 9, 590. https://doi.org/10.3390/cryst9110590
Hobart DB, Patel VG, Pendergrass H, Florio J, Merola JS. Self-Assembly Motifs of Water in Crystals of Palladium β-Amino Acid Complexes Influenced by Methyl Substitution on the Amino Acid Backbone. Crystals. 2019; 9(11):590. https://doi.org/10.3390/cryst9110590
Chicago/Turabian StyleHobart, David B., Vraj G. Patel, Heather Pendergrass, Jacqueline Florio, and Joseph S. Merola. 2019. "Self-Assembly Motifs of Water in Crystals of Palladium β-Amino Acid Complexes Influenced by Methyl Substitution on the Amino Acid Backbone" Crystals 9, no. 11: 590. https://doi.org/10.3390/cryst9110590





