Effect of Solid Forms on Physicochemical Properties of Valnemulin
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
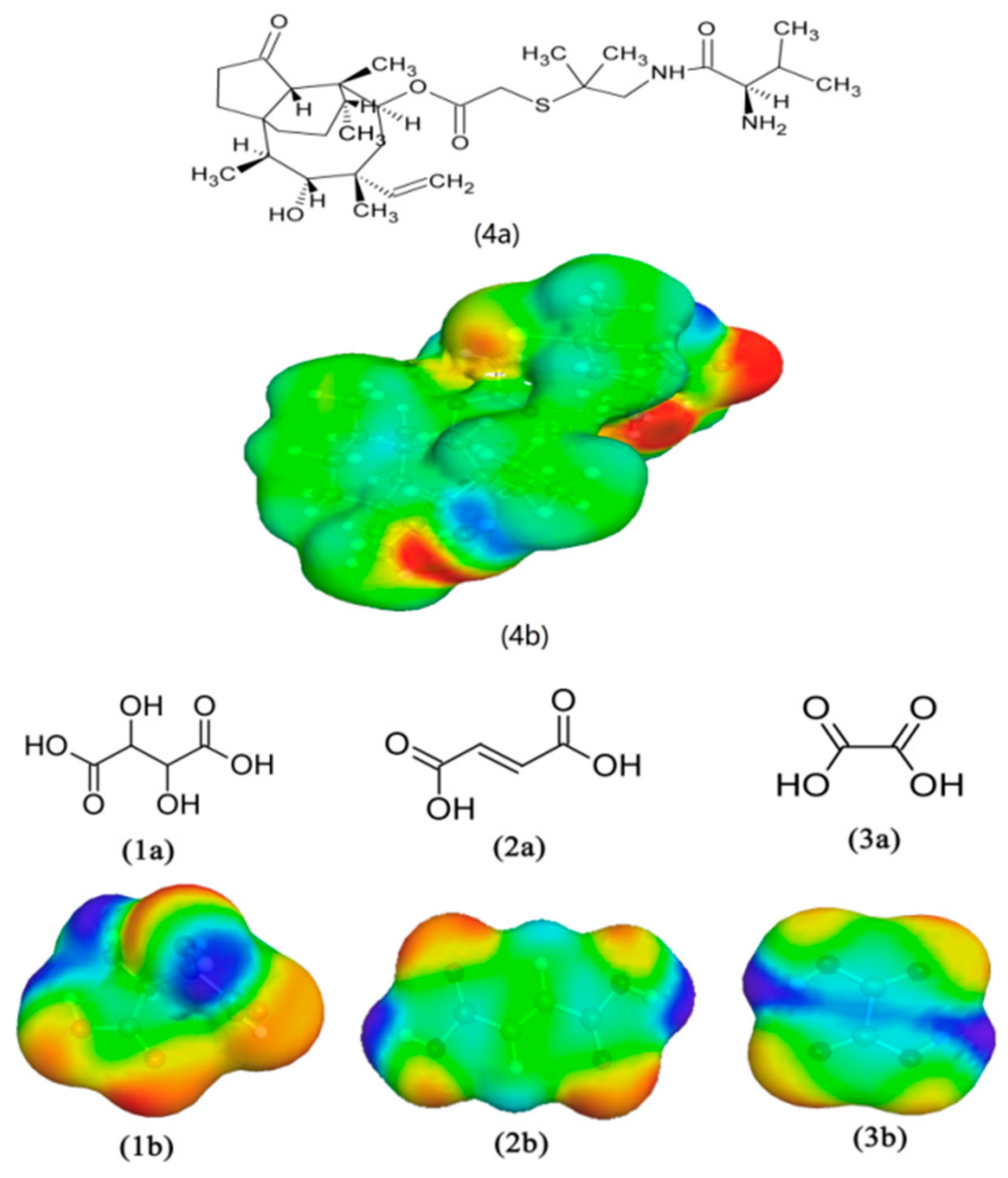
2.2. COSMO-RS Calculation
2.3. Preparation of VLM Multi-Component Solids.
2.4. X-Ray Powder Diffraction
2.5. Thermal Analysis
2.6. FT-IR Spectroscopy
2.7. Dynamic Vapor Sorption (DVS)
2.8. Intrinsic Dissolution Rate
3. Results and Discussion
3.1. COSMO-RS Calculation
3.2. XRPD/DSC/SEM
3.3. FT-IR Spectroscopy
3.4. Dynamic Vapor Sorption (DVS) Analysis
3.5. Intrinsic Dissolution Rate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Leeson, P. Drug discovery: Chemical beauty contest. Nature 2012, 481, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.B.; Wang, J.K.; Wang, Y.L.; Yin, Q.X.; Hong, H.X. Thermodynamic study on dynamic water and organic vapor sorption on amorphous valnemulin hydrochloride, Front. Chem. Sci. Eng. 2015, 9, 94–104. [Google Scholar]
- Schultheiss, N.; Newman, A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yoshihashi, Y.; Yonemochi, E.; Fujii, K.; Uekusa, H.; Terada, K. Cocrystallization and amorphization induced by drug–excipient interaction improves the physical properties of acyclovir. Int. J. Pharm. 2012, 422, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Morissette, S.L.; Almarsson, Ö.; Peterson, M.L.; Remenar, J.F.; Read, M.J.; Lemmo, A.V.; Ellis, S.; Cima, M.J.; Gardner, C.R. High-throughput crystallization: Polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv. Drug Deliver Rev. 2004, 56, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Carina, D.; Sergey, V.D.; Michael, S.; István, F. Polymer Swelling. Drug Mobilization and Drug Recrystallization in Hydrating Solid Dispersion Tablets Studied by Multinuclear NMR Microimaging and Spectroscopy. Mol. Pharm. 2011, 8, 1247–1256. [Google Scholar]
- Geetha, B.; Ashiwini, N. Clofazimine Mesylate: A High Solubility Stable Salt. Cryst. Growth Des. 2012, 12, 6250–6259. [Google Scholar]
- Flavia, A.M.; Mihaela, M.P.; Gheorghe, B.; Xenia, F.; Irina, K. Ketoconazole Salt and Co-crystals with Enhanced Aqueous Solubility. Cryst. Growth Des. 2013, 13, 4295–4304. [Google Scholar]
- Gardner, D.R.; Almarsson, O.; Chen, H.; Morrisette, S.; Peterson, M.; Zhang, Z.; Wang, S.; Lemmo, A.; Gonzales-Zugasti, J.; Monagle, J.; et al. Application of high throughput technologies to drug substance and drug product development. J. Comput. Chem. Eng. 2004, 28, 943–953. [Google Scholar] [CrossRef]
- Ballach, S.; Korn, C. Pharmaceutical evaluation of early development candidates “the 100 mg-approach”. Int. J. Pharm. 2004, 275, 1–12. [Google Scholar] [CrossRef]
- Jangmi, L.; Suzie, P.; Seon, J.Y.; Yong, W.J.; Youngjoo, B.; Soon, H.Y.; Min, K.J.; Sung, K.K.; Eun, H.L. Multicomponent System of NPS-1034, an Orally Administered Lung Cancer Drug Candidate, with Sulfonic Acids and Solid State Characterization. Cryst. Growth Des. 2013, 13, 3958–3968. [Google Scholar]
- Ouyang, J.B.; Na, B.; Zhou, L.M.; Xiao, S.J.; Xiong, G.X.; Jin, T.X. Crystal structures and phase transformation of two novel solvates of valnemulin hydrochloride. CrystEngComm 2018, 20, 563–569. [Google Scholar] [CrossRef]
- Guo, M.S.; Wang, K.; Qiao, N.; Yardley, V.; Li, M.Z. Investigating Permeation Behavior of Flufenamic Acid Cocrystals Using a Dissolution and Permeation System. Mol. Pharm. 2018, 15, 4257–4272. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.B.; Na, B.; Liu, Z.R.; Zhou, L.M.; Hao, H.X. Determination of Solubility and Nucleation Kinetics of Valnemulin Hydrochloride Solvate. J. Solut. Chem. 2019, 48, 413–426. [Google Scholar] [CrossRef]
- Yun, H.; Katarzyna, G.; Andrea, E.; Patrick, M. Mechanochemical Reaction of Sulfathiazole with Carboxylic Acids: Formation of a Cocrystal, a Salt, and Coamorphous Solids. Cryst. Growth Des. 2014, 14, 803–813. [Google Scholar]
- Hathwar, V.R.; Pal, R.; Guru Row, T.N. Charge Density Analysis of Crystals of Nicotinamide with Salicylic Acid and Oxalic Acid: An Insight into the Salt to Cocrystal Continuum. Cryst. Growth Des. 2010, 10, 3306–3310. [Google Scholar]
- Wouters, J.; Quere, L.; Thurston, D.E. Pharmaceutical Salts and Cocrystals; Royal Society of Chemistry: Cambridge, UK, 2011. [Google Scholar]
- Cecília, C.P.S.; Rebeka, D.O.; Juan, C.T.; Sara, B.H.; Alejandro, P.A.; Javier, E. The Continuum in 5-Fluorocytosine. Toward Salt Formation. Cryst. Growth Des. 2013, 13, 4315–43225. [Google Scholar]
- Sharmarke, M.; Derek, A.T.; Martin, V.; Panagiotis, G.K.; Sarah, L.P. Salt or Cocrystal? A New Series of Crystal Structures Formed from Simple Pyridines and Carboxylic Acids. Cryst. Growth Des. 2009, 9, 2881–2889. [Google Scholar]
- Pramod, K.G.; Ram, T.; Arunachalam, R. Multiple Crystal Forms of p-Aminosalicylic Acid: Salts, Salt Co-Crystal Hydrate, Co-Crystals, and Co-Crystal Polymorphs. Cryst. Growth Des. 2013, 13, 360–366. [Google Scholar]
- Schultheiss, N.; Smit, J.P.; Hanko, J.A. Three isostructural solvates of finasteride and their solid-state characterization. Eur J Pharm Sci. 2009, 38, 498–503. [Google Scholar] [CrossRef]
- Myerson, A. Handbook of Industrial Crystallization, 2nd ed.; Butterworth-Heinemann: Boston, USA, 2002. [Google Scholar]
- Alvarez, A.J.; Myerson, A.S. Continuous Plug Flow Crystallization of Pharmaceutical Compounds. Cryst. Growth Des. 2010, 10, 2219–2228. [Google Scholar] [CrossRef]
- Chen, J.; Sarma, B.; Evans, J.M.; Myerson, A.S. Pharmaceutical Crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Ouyang, J.B.; Wang, J.K.; Huang, X.; Gao, Y.; Bao, Y.; Wang, Y.; Yin, Q.; Hao, H. Gel Formation and Phase Transformation during the Crystallization of Valnemulin Hydrogen Tartrate. Ind. Eng. Chem. Res. 2014, 53, 16859–16863. [Google Scholar] [CrossRef]
- Wattanaphansak, S.; Singer, R.S.; Gebhart, C.J. In vitro antimicrobial activity against 10 North American and European Lawsonia intracellularis isolates. Vet. Microbiol. 2009, 134, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Oxberry, S.L.; Hampson, D.J. Antimicrobial susceptibility testing of Australian isolates of Brachyspira hyodysenteriae using a new broth dilution method. Vet. Microbiol. 2002, 84, 123–133. [Google Scholar] [CrossRef]
- Qiu, S.H.; Jian, C.L.; Li, J.X.; Xi, X.; Peng, D.; Jian, Z.S.; Shuang, Y.D. Residue depletion of valnemulin in swine tissues after oral administration. Anal. Chim. Acta. 2010, 664, 62–67. [Google Scholar]
- Klamt, A.; Eckert, F. COSMO-RS: A novel and efficient method for the a priori prediction of thermophysical data of liquids. Fluid Phase Equilib. 2000, 172, 43–72. [Google Scholar] [CrossRef]
- Klamt, A. The COSMO and COSMO-RS solvation models. Wiley Interdiscipl. Rev. Comput. Mol. Sci. 2011, 1, 699–709. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Christoph, L.; Andreas, K. Pharmaceutics, Preformulation and Drug Delivery: Rational Coformer or Solvent Selection for Pharmaceutical Cocrystallization or Desolvation. J. Pharm. Sci. 2012, 101, 3687–3697. [Google Scholar] [CrossRef]
- Klamt, A. COSMO-RS: From Quantum Chemistry to Fluid Phase Thermodynamics and Drug Design; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Mohamed, S.; Tocher, D.A.; Price, S.L. Computational prediction of salt and co-crystal structures—Does a proton position matter. Int. J. Pharm. 2011, 418, 187–198. [Google Scholar] [CrossRef]
- Musumeci, D.; Hunter, C.A.; Prohens, R.; Scuderi, S.; McCabe, J.F. Virtual co-crystal screening. Chem Sci. 2011, 2, 883–890. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, L.; Ye, Y.M.; Chen, L.F.; Qi, Z.W.; Freund, H.; Sundmacher, K. An Overview of Mutual Solubility of Ionic Liquids and Water Predicted by COSMO-RS. Ind. Eng. Chem. Res. 2012, 51, 6256–6264. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. Fast solvent screening via quantum chemistry: COSMO-RS approach. AIChE J. 2002, 48, 369–385. [Google Scholar] [CrossRef]
- Shah, J.C.; Chen, J.R.; Chow, D. Metastable Polymorph of Etoposide with Higher Dissolution Rate. Drug Dev. Ind. Pharm. 1999, 25, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Costa, P. An alternative method to the evaluation of similarity factor indissolution testing. Int. J. Pharm. 2001, 220, 77–83. [Google Scholar] [CrossRef]
- Lauretta, M.; Giovanna, B.; Mariarosa, M.; Andrea, C.; Andrea, C.; Ubaldo, C. II. Technological approaches to improve the dissolution behavior of nateglinide, a lipophilic insoluble drug: Co-milling. Int. J. Pharm. 2013, 454, 568–572. [Google Scholar]





| Co-Former | Hex (kcal/mol) |
|---|---|
| Oxalic acid | −6.89 |
| Tartaric acid | −5.31 |
| Fumaric acid | −4.90 |
| Succinic acid | −3.44 |
| Salicylic acid | −3.29 |
| Hexanedioic acid | −2.83 |
| Isonicotinic acid | −1.15 |
| Samples | Onset (°C) | Endset (°C) | Tm (°C) |
|---|---|---|---|
| TAR | 200 | 230 | 210 |
| FUM | 250 | 310 | 295 |
| OXA | 170 | 200 | 188 |
| VLM | No peak | No peak | No peak |
| VLM-TAR | 170 | 180 | 175 |
| VLM-FUM | 120 | 135 | 130 |
| VLM-OXA | 120 | 135 | 125 |
| Samples | vN-H | vC=O | Co-Formers-vCOOH | Multi-Component-vCOO− |
|---|---|---|---|---|
| VLM | 3350 | 1750/1650 | ||
| TAR | 1750/761 | |||
| FUM | 1675/650 | |||
| OXA | 1675/745 | |||
| VLM-TAR | 3355 | 1745/1645 | 761 | |
| VLM-FUM | 3435 | 1700/1650 | 650 | |
| VLM-OXA | 3400 | 1700/1635 | 745 |
| Samples | Q10min (%) | T50% (min) |
|---|---|---|
| VLM | 4.56 | ∞ |
| VLM-TAR | 17.69 | 60 |
| VLM-FUM | 20.54 | 40 |
| VLM-OXA | 30.54 | 25 |
| Samples Compared | f2 |
|---|---|
| VLM-TAR:VLM | 21.80 |
| VLM-FUM:VLM | 18.07 |
| VLM-OXA:VLM | 9.76 |
| VLM-FUM:VLM-TAR | 78.11 |
| VLM-OXA:VLM-FUM | 57.17 |
| VLM-OXA:VLM-TAR | 60.74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.; Chen, J.; Zhou, L.; Han, F.; Huang, X. Effect of Solid Forms on Physicochemical Properties of Valnemulin. Crystals 2019, 9, 675. https://doi.org/10.3390/cryst9120675
Ouyang J, Chen J, Zhou L, Han F, Huang X. Effect of Solid Forms on Physicochemical Properties of Valnemulin. Crystals. 2019; 9(12):675. https://doi.org/10.3390/cryst9120675
Chicago/Turabian StyleOuyang, Jinbo, Jian Chen, Limin Zhou, Fangze Han, and Xin Huang. 2019. "Effect of Solid Forms on Physicochemical Properties of Valnemulin" Crystals 9, no. 12: 675. https://doi.org/10.3390/cryst9120675
APA StyleOuyang, J., Chen, J., Zhou, L., Han, F., & Huang, X. (2019). Effect of Solid Forms on Physicochemical Properties of Valnemulin. Crystals, 9(12), 675. https://doi.org/10.3390/cryst9120675







