Choosing the Method of Crystallization to Obtain Optimal Results
Abstract
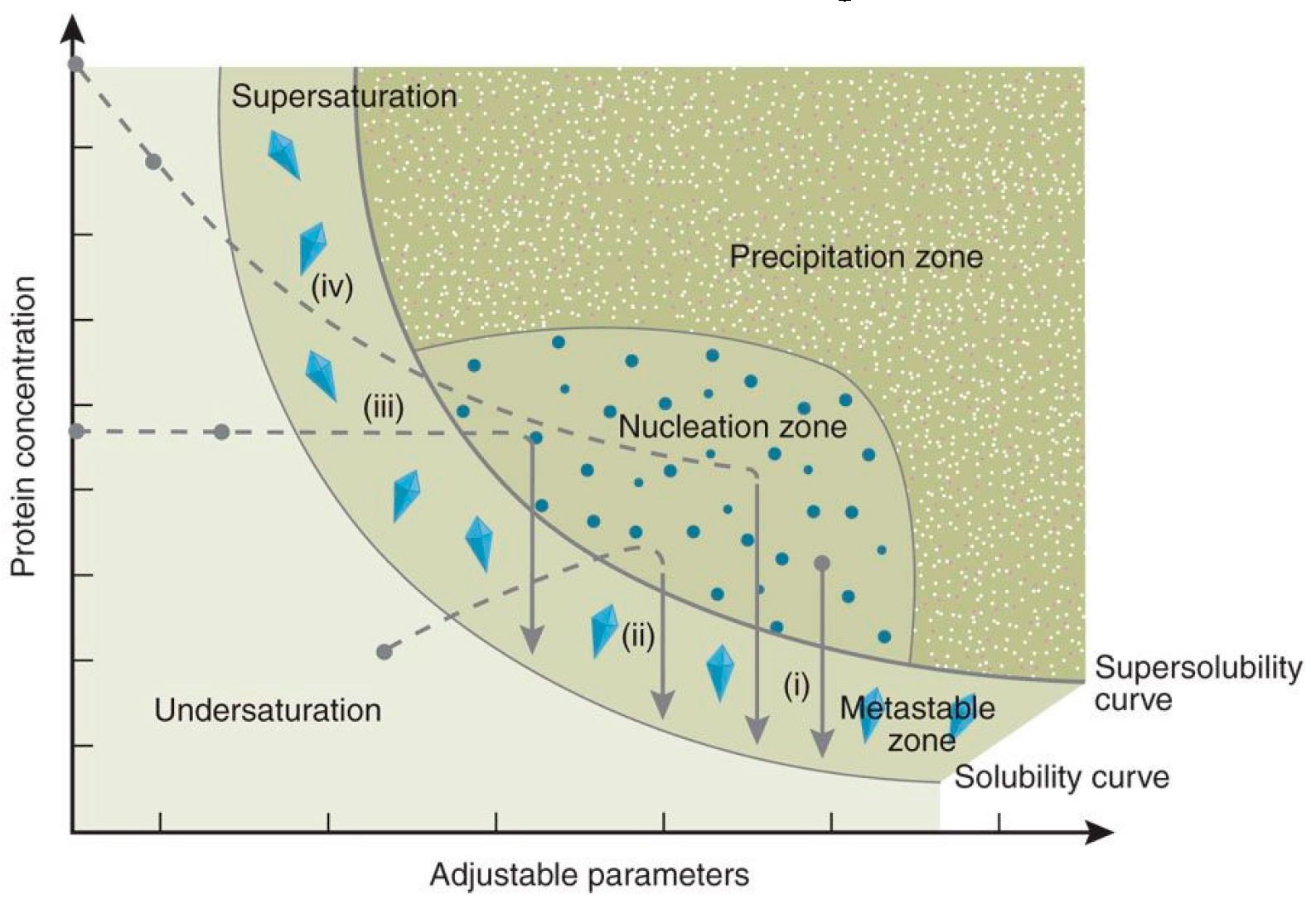
:1. Introduction
- Obtaining no crystals at all.
- Obtaining showers of small crystals which are not suitable for diffraction.
- Obtaining large crystals or twinned crystals that do not diffract.
- Obtaining reasonable crystals that require improvement.
- Obtaining good crystals that are unreproducible or difficult to repeat
2. Obtaining No Crystals or Crystals that Do Not Diffract in the Screening Stage of Crystallization
2.1. Adopting A Variety of Crystallization Methods
2.2. Concentration of Drops that Would Remain Clear Indefinitely
3. Obtaining Showers of Small Crystals
3.1. Rigorous Filtration
3.2. Slowing Down the Crystallization Process
3.3. Separation of the Nucleation and Growth Stages of Crystallization
Volume Adjustments during Crystallization
4. The Application of Light-Scattering Techniques
5. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Saridakis, E.; Chayen, N.E. Systematic improvement of protein crystals by determining the supersolubility curves of phase diagrams. Biophys. J. 2003, 84, 1218–1222. [Google Scholar] [CrossRef]
- Chayen, N.E.; Saridakis, E. Protein crystallization: From purified protein to diffraction-quality crystal. Nat. Methods 2008, 5, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Giegé, R.; Dock, A.C.; Kern, D.; Lorber, B.; Thierry, J.C.; Moras, D. The role of purification in the crystallization of proteins and nucleic acids. J. Cryst. Growth 1986, 76, 554–561. [Google Scholar] [CrossRef]
- Bergfors, T.M. Protein Crystallization. Techniques, Strategies, and Tips; Bergfors, T.M., Ed.; International University Line: San Diego, CA, USA, 1999. [Google Scholar]
- McPherson, A. Crystallization of Biological Macromolecules; Cold Spring Harbor Lab Press: Cold Spring Harbor, NY, USA, 1999. [Google Scholar]
- Ducruix, A.; Giegé, R. Crystallization of Nucleic Acids and Proteins: A Practical Approach, 2nd ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Ericsson, U.B.; Hallberg, B.M.; DeTitta, G.T.; Dekker, N.; Nordlund, P. Thermofluor-based high-throughput stability optimization of proteins for structural studies. Anal. Biochem. 2006, 357, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Baldock, P.; Mills, V.; Stewart, P.S. A comparison of microbatch and vapour diffusion for initial screening of crystallization conditions. J. Cryst. Growth 1996, 168, 170–174. [Google Scholar] [CrossRef]
- Hansen, C.L.; Skordalakes, E.; Berger, J.M.; Quake, S.R. A robust and scalable microfluidic metering method that allows protein crystal growth by free interface diffusion. Proc. Natl. Acad. Sci. USA 2002, 99, 16531. [Google Scholar] [CrossRef] [PubMed]
- Sauter, C.; Dhouib, K.; Lorber, B. From macrofluidics to microfluidics for the crystallization of biological macromolecules. Cryst. Growth Des. 2007, 7, 2247–2250. [Google Scholar] [CrossRef]
- Abdallah, B.G.; Roy-Chowdhury, S.; Fromme, R.; Fromme, P.; Ros, A. Protein Crystallization in an Actuated Microfluidic Nanowell Device. Cryst. Growth Des. 2016, 16, 2074–2082. [Google Scholar] [CrossRef] [Green Version]
- Otálora, F.; Gavira, J.A.; Ng, J.D.; García-Ruiz, J.M. Counterdiffusion methods applied to protein crystallization. Prog. Biophys. Mol. Biol. 2009, 101, 26–37. [Google Scholar] [CrossRef]
- Khurshid, S.; Govada, L.; Chayen, N.E. Dynamic Screening Experiments to Maximize Hits for Crystallization. Cryst. Growth Des. 2007, 7, 2171–2175. [Google Scholar] [CrossRef]
- Govada, L.; Chayen, N.E. Crystallization by Controlled Evaporation Leading to High Resolution Crystals of the C1 Domain of Cardiac Myosin Binding Protein-C (cMyBP-C). Cryst. Growth Des. 2009, 9, 1729–1732. [Google Scholar] [CrossRef]
- Chayen, N.E. The role of oil in macromolecular crystallization. Structure 1997, 5, 1269–1274. [Google Scholar] [CrossRef] [Green Version]
- Chayen, N.E. Rigorous filtration for protein crystallization. J. Appl. Crystallogr. 2009, 42, 743–744. [Google Scholar] [CrossRef]
- Chayen, N.E. A novel technique to control the rate of vapour diffusion, giving larger protein crystals. J. Appl. Crystallogr. 1997, 30, 198–202. [Google Scholar] [CrossRef] [Green Version]
- Mandelman, D.; Gonzalo, P.; Lavergne, J.P.; Corbier, C.; Reboud, J.P.; Haser, R. Crystallization and preliminary X-ray study of an N-terminal fragment of rat liver ribosomal P2 protein. Acta Crystallogr. D 2002, 58, 668–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, H.V.; Tabachnikov, O.; Feinberg, H.; Govada, L.; Chayen, N.E.; Shoham, Y.; Shoham, G. Crystallization and preliminary crystallographic analysis of GanB, a GH42 intracellular beta-galactosidase from Geobacillus stearothermophilus. Acta Crystallogr. F 2013, 69, 1114–1119. [Google Scholar] [CrossRef]
- Saridakis, E.E.G.; Stewart, P.D.S.; Lloyd, L.F.; Blow, D.M. Phase-Diagram and Dilution Experiments in the Crystallization of Carboxypeptidase-G(2). Acta Crystallogr. D 1994, 50, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Chayen, N.E. Methods for separating nucleation and growth in protein crystallisation. Prog. Biophys. Mol. Biol. 2005, 88, 329–337. [Google Scholar] [CrossRef]
- Saridakis, E.; Chayen, N.E. Improving protein crystal quality by decoupling nucleation and growth in vapor diffusion. Protein Sci. 2000, 9, 755–757. [Google Scholar] [CrossRef]
- Krengel, U.; Dey, R.; Sasso, S.; Okvist, M.; Ramakrishnan, C.; Kast, P. Preliminary X-ray crystallographic analysis of the secreted chorismate mutase from Mycobacterium tuberculosis: A tricky crystallization problem solved. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 441–445. [Google Scholar] [CrossRef]
- Nield, J.; Rizkallah, P.; Barber, J.; Chayen, N.E. The 1.45 Angstrom three-dimensional structure of C-phycocyanin from the thermophilic cyanobacterium Synechococcus elongatus. J. Struct. Biol. 2003, 141, 149–155. [Google Scholar] [CrossRef]
- Saridakis, E.; Dierks, K.; Moreno, A.; Dieckmann, M.W.M.; Chayen, N.E. Separating nucleation and growth in protein crystallization using dynamic light scattering. Acta Crystallogr. D 2002, 58, 1597–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shmitz, S. An introduction to Dynamic Light Scattering by Macromoelcules. Phys. Today 1990, 44, 66. [Google Scholar] [CrossRef]
- Dierks, K.; Meyer, A.; Einspahr, H.; Betzel, C. Dynamic Light Scattering in Protein Crystallization Droplets: Adaptations for Analysis and Optimization of Crystallization Processes. Cryst. Growth Des. 2008, 8, 1628–1634. [Google Scholar] [CrossRef]
- Proteau, A.; Shi, R.; Cygler, M. Application of Dynamic Light Scattering in Protein Crystallization. Curr. Protoc. Protein Sci. 2010, 61, 10–17. [Google Scholar]
- Rosenberger, F.; Howard, S.B.; Sowers, J.W.; Nyce, T.A. Temperature dependence of protein solubility—Determination and application to crystallization in X-ray capillaries. J. Cryst. Growth 1993, 129, 1–12. [Google Scholar] [CrossRef]
- Chayen, N.E.; Dieckmann, M.; Dierks, K. Use of Dynamic Light Scattering in a Method for Producing Macromolecular Crystals. WO 03080901 A1, 23 May 2002. [Google Scholar]
- Collingsworth, P.; Bray, T.; K Christopher, G. Crystal Growth via Computer Controlled Vapor Diffusion. J. Cryst. Growth 2000, 219, 283–289. [Google Scholar] [CrossRef]
- Meyer, A.; Dierks, K.; Hilterhaus, D.; Klupsch, T.; Muhlig, P.; Kleesiek, J.; Schopflin, R.; Einspahr, H.; Hilgenfeld, R.; Betzel, C. Single-drop optimization of protein crystallization. Acta Crystallogr. Sect. F 2012, 68, 994–998. [Google Scholar] [CrossRef]
- Schubert, R.; Meyer, A.; Baitan, D.; Dierks, K.; Perbandt, M.; Betzel, C. Real-Time Observation of Protein Dense Liquid Cluster Evolution during Nucleation in Protein Crystallization. Cryst. Growth Des. 2017, 17, 954–958. [Google Scholar] [CrossRef]
- Moreno, A.; Saridakis, E.; Chayen, N.E. Combination of oils and gels for enhancing the growth of protein crystals. J. Appl. Crystallogr. 2002, 35, 140–142. [Google Scholar] [CrossRef] [Green Version]
- Gavira, J.A.; Hernandez-Hernandez, M.A.; Gonzalez-Ramirez, L.A.; Briggs, R.A.; Kolek, S.A.; Shaw Stewart, P.D. Combining Counter-Diffusion and Microseeding to Increase the Success Rate in Protein Crystallization. Cryst. Growth Des. 2011, 11, 2122–2126. [Google Scholar] [CrossRef]
- Bolanos-Garcia, V.M.; Chayen, N.E. New directions in conventional methods of protein crystallization. Prog. Biophys. Mol. Biol. 2009, 101, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A. Protein Crystallization under the Presence of an Electric Field; MDPI Books: Basel, Switzerland, 2018. [Google Scholar]
- Johansson, L.C.; Stauch, B.; Ishchenko, A.; Cherezov, V. A Bright Future for Serial Femtosecond Crystallography with XFELs. Trends Biochem. Sci. 2017, 42, 749–762. [Google Scholar] [CrossRef]
- McSweeney, S.; Fromme, P. Crystallography: Sources of inspiration. Nature 2014, 505, 620–621. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govada, L.; Chayen, N.E. Choosing the Method of Crystallization to Obtain Optimal Results. Crystals 2019, 9, 106. https://doi.org/10.3390/cryst9020106
Govada L, Chayen NE. Choosing the Method of Crystallization to Obtain Optimal Results. Crystals. 2019; 9(2):106. https://doi.org/10.3390/cryst9020106
Chicago/Turabian StyleGovada, Lata, and Naomi E. Chayen. 2019. "Choosing the Method of Crystallization to Obtain Optimal Results" Crystals 9, no. 2: 106. https://doi.org/10.3390/cryst9020106
APA StyleGovada, L., & Chayen, N. E. (2019). Choosing the Method of Crystallization to Obtain Optimal Results. Crystals, 9(2), 106. https://doi.org/10.3390/cryst9020106




