Abstract
TldD and TldE proteins interact and form a complex to degrade unfolded peptides. The gene Tk0499 from Thermococcus kodakarensis encoded a putative modulator of gyrase (TkTldE). Although TldE genes were common in bacteria and archaea, the structural basis on the evolution of proteins remained largely unknown. Here, the three-dimensional structure of TkTldE was determined by X-ray diffraction. Crystals were acquired by the sitting-drop vapor-diffusion method. X-ray diffraction data from crystals were collected at 2.35 Å. The space group and unit-cell parameters suggested that there were two molecules in the asymmetric unit. Our results showed that TkTldE forms a homodimer, which contained anti-parallel β-strands and a pair of α-helices. Comparison of the structures of TldE and TldD showed that despite their high sequence similarity, TldE lacked the conserved HExxxH and GxC motif in which two His and a Cys residues bound a metal ion. Taken together, these results provided insight into the structural information of this class of TldE/TldD.
1. Introduction
The TldD and TldE genes, which encoded a putative protease complex, were common in archaebacteria and eubacteria [1]. It was reported that TldD/TldE were involved in degrading unfolded peptides by two pathways—i.e., antibiotic peptide microcin B17 (MccB17) production and CcdA antidote degradation—in prokaryotes to ensure the survival of prokaryotic cells [2,3]. MccB17 was secreted by Escherichia coli strains containing the plasmid pMccB17 [3]. MccB17 production and immunity required seven genes (mcbA, B, C, D, E, F, and G) [4,5]. The mcbA gene encoding the MccB17 synthetase synthesized a precursor peptide of 69 amino acids [6]. The products of McbBCD genes modified the precursor peptide into an active antibiotic (MccB17), transforming the cysteine and serine residues of the peptide into thiazole and oxazole [7]. The products of the mcbE and mcbF genes took part in the export of mature MccB17, and that of mcbG conferred immunity upon MccB17 [8,9]. MccB17 inhibited DNA gyrase, which was a prokaryotic type-II topoisomerase, leading to the induction of the SOS repair system, double-strand breaks, and ultimately, cell death [10,11,12,13]. A chromosomal gene called pmbA (or TldE) was involved in the cleavage of the MccB17 leader peptide in the final step and secretion of MccB17 [3]. It has been reported that in pmbA (TldE) or its homologous TldD mutant, pro-MccB17 cannot be released from cells, and so inhibited DNA replication [1,3]. Although the TldD and TldE proteins had high sequence similarity, only TldD contained a conserved metalloprotease-like HExxxH sequence motif that might contribute to catalysis [14]. Ghilarov reported that a heterodimeric TldD/TldE complex was needed for the cleavage of the N-terminal of the modified MccB17 precursor peptide to yield the mature antibiotic [15]. In the CcdA/CcdB poison-antipoison system, CcdA interacted with CcdB to inhibit the cytotoxic activity of CcdB [16]. CcdB inhibited the DNA supercoiling activity of gyrase and was lethal to bacteria [17]. Both TldD and TldE (pmbA) were involved in CcdA antidote degradation [1].
Recently, a few studies have focused on archaeal TldD/TldE proteins. TldD/TldE from archaebacteria were different to those from eubacteria. The structure of a putative modulator of DNA gyrase (TldE) from Thermotoga maritima was reported, but shed little light on its functions as a modulator of DNA gyrase [18]. Sso0660 from Sulfolobus solfataricus, a TldD homologue, could function alone as a metalloprotease, as it could proteolytically degrade azocasein and FITC–BSA substrates with a zinc ion as its cofactor [14]. The function of these homologous TldD/TldE proteins in Thermococcus kodakarensis has not been reported. Whether or not the TldE encoded a metalloprotease remains to be explored in further biological studies.
Here, we cloned a TldE gene from the Thermococcus kodakarensis genome [19] and reported its crystal structure. Multiple sequence alignment with other organisms TldDs/TldEs and structure comparisons with homologues revealed that they shared a conserved fold with a family of the metalloprotease class. However, detailed comparisons showed that TkTldE bore no conserved HExxxH motifs in the vicinity of the corresponding active pocket of EcTldD. These results provide a structural basis for further investigation of the function of these proteins.
2. Materials and Methods
2.1. Macromolecule Production
The full-length TkTldE consists of 1323 bp encoding 441 amino acid residues. Gene fragments were amplified and inserted into expression vector pHAT2 with NcoI and XhoI, including N-terminal 6xHis, using sticky-end cloning [20] (Table 1). The correctness of the recombinant plasmids was confirmed by sequencing. The prepared TldE construct was transformed into E. coli strain BL21(DE3) cells for protein expression. Cells with the recombinant plasmids were grown at 310 K (optical density 0.6–0.8), and expression was induced overnight with 0.4 mM IPTG at 289 K. After harvesting, the cells were resuspended in buffer (20 mM Tris, 150 mM NaCl, pH 7.5) and lysed by sonication. The His-tagged protein in filtered supernatant was obtained by centrifugation and applied to an Ni-NTA agarose column (GE Healthcare) pre-equilibrated with buffer (20 mM Tris, 150 mM NaCl, pH 7.5). The column was washed with four column volumes of lysis buffer containing 20 mM imidazole, and the protein was eluted using 500 mM imidazole. The protein eluate was concentrated in a centrifugal concentrator (Millipore) and applied to a Superdex 200 Increase gel filtration column pre-equilibrated with 20 mM Tris and 150 mM NaCl at pH 7.5 (Table 2). The collected protein was concentrated to 10 mg/mL for crystal screening.

Table 1.
Macromoleculeproduction information.

Table 2.
Crystallization information.
2.2. Crystallization, Data Collection, and Processing
Initially, crystallization conditions were obtained from commercially-available crystallization screening kits from Hampton Research (Aliso Viejo, CA, USA), and screening was performed at 291 K by the sitting-drop diffusion method with an Oryx4 robot (Douglas Instruments Ltd. Berkshire, UK). Drops consisting of 0.25 µL protein solution and 0.25 µL reservoir solution were equilibrated against a 35 µL reservoir solution. Initial crystals appeared for several conditions of three kits (PEGRX1, PEG/ION SCREEN, and PEG/ION2 SCREEN). The reservoir solution (0.02 M calcium chloride, 0.1 M sodium acetate, pH 4.6, 30% v/v MPD), which gave the best initial crystal morphology, was further optimized by changing the precipitant concentration and introducing additives. The best crystals were obtained after 7 days. Crystallization details are shown in Table 2.
Crystals were soaked in a well solution supplemented with 20% glycerol, and then flash-cooled in liquid nitrogen before data collection. X-ray diffraction data were collected using an ADSC Q315 CCD detector on beamline BL17U1 at the Shanghai Synchrotron Radiation Facility. The data sets were indexed, integrated, and scaled using the HKL-2000 package [21]. Data collection and processing statistics are shown in Table 3.

Table 3.
Data collection and processing.
2.3. Structure Solution and Refinement
The structure was solved by molecular replacement using Phaser with 1VL4 as a search model [22], and the model was rebuilt interactively using Coot [23] and phenix.refine until the free R factor converged. The final model was validated using MolProbity [24]. The details of the data collection and refinement statistics are presented in Table 4.

Table 4.
Sturcture refinement.
3. Results and Discussion
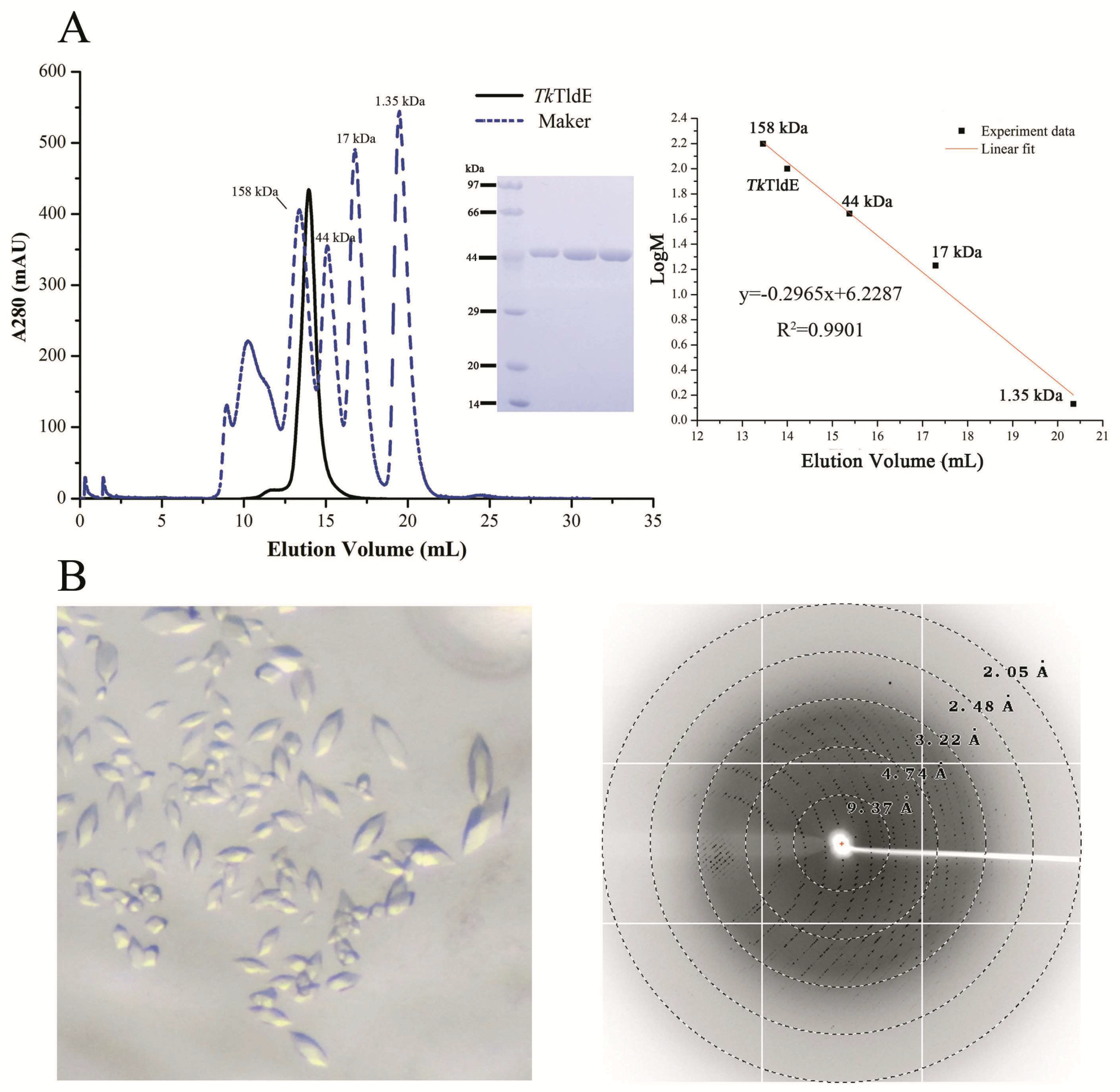
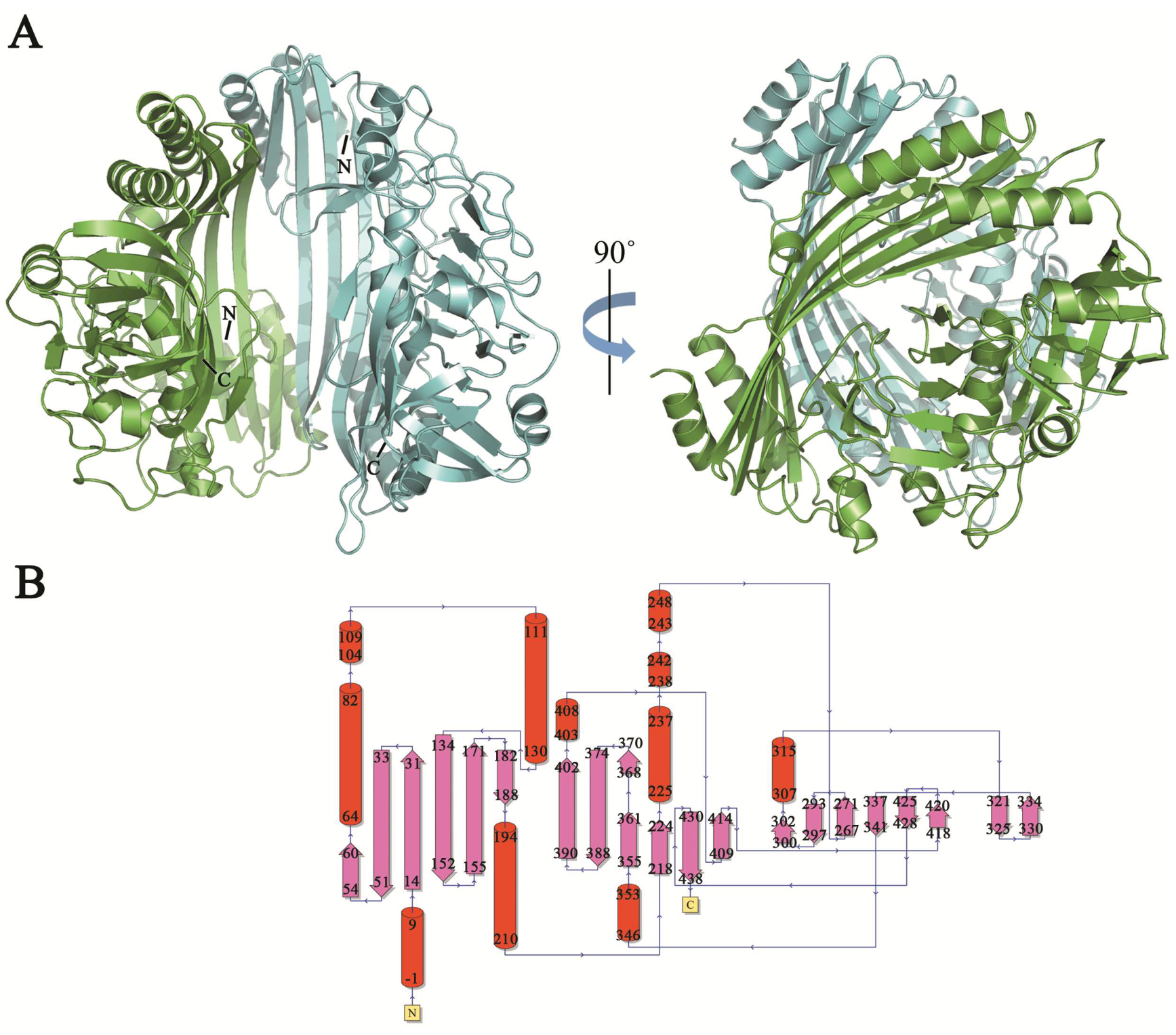
TldE crystals were obtained by the sitting-drop diffusion method and belonged to P43212, with unit cell parameters a = 104.50 Å, b = 104.50 Å, and c = 253.93 Å (Figure 1B). The structure was solved by the molecular replacement method using 1VL4 (a putative modulator of a DNA gyrase) as the initial model. There were two molecules in the asymmetric unit, with a solvent content of 46.89% (Figure 2A). The overall structure of TldE (Protein Data Bank (PDB): 6J6A) was shown in the topology diagram in Figure 2B. TldE subunits formed a hollow core homodimer, which was consistent with the gel filtration results (Figure 1A). The dimer interface consisted of 20 hydrogen bonds and two salt bridges (K77 and D113) (Figure S1). Like other protease family members, one subunit of TkTldE contained 11 α-helices and 21 β-strands (Figure 2B). As described for PmbA (PDB: 1VL4) [18] and E. coli TldD and TldE (PDB: 5NJ9) [15], one subunit of TkTldE contained two domains. The N-terminal domain was composed of six anti-parallel β-strands and five α-helices distributing on the outer surface of β-sheet. The C-terminal domain consisted of a six-stranded anti-parallel β barrel, seven β-strands, and five α-helices, which were strongly twisted (Figure 2).
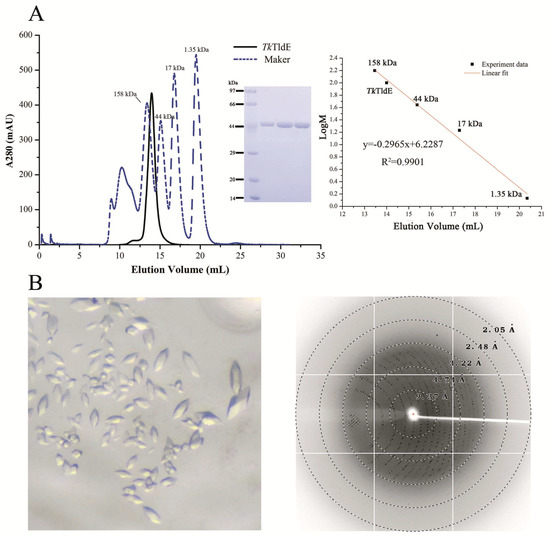
Figure 1.
Size-exclusion chromatogram and crystals of TkTldE. (A) Sodium dodecyl sulfate polyacrylamide gel electrophoresis profile of protein samples after size-exclusion chromatogram. Four experiment data points (13.46 mL/158 kDa, 15.38 mL/44 kDa, 17.29 mL/17kDa, 20.35 mL/1.35 kDa) from the Gel Filtration Standard (Bio-Rad, Cat: 1511901) was used for the column calibration. TkTldE was eluted at about 14.18 mL with superdex 200 column and the molecular weight was calculated as approximate 105 kDa in solution. (B) Crystal image and the representative X-ray diffraction image of a TkTldE crystal. Diffraction resolutions were labelled as circles.
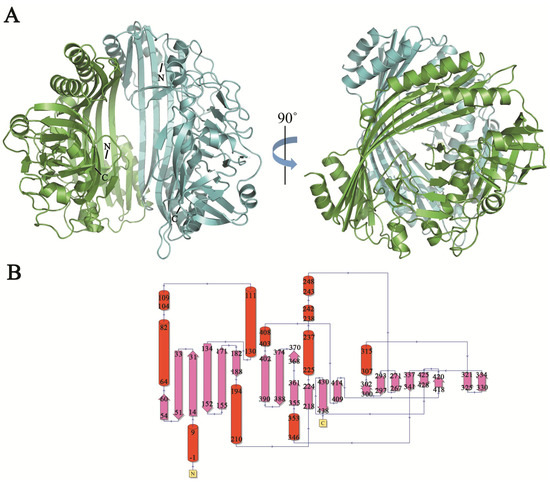
Figure 2.
Overall structure of TldE homodimer. (A) Two molecules were shown as blue and green cartoons. N- and C-termini were labelled in each molecule. (B) Corresponding topology diagrams of the TldE structure. The 21 β-strands were shown as arrows and 11 α-helices as cylinders.
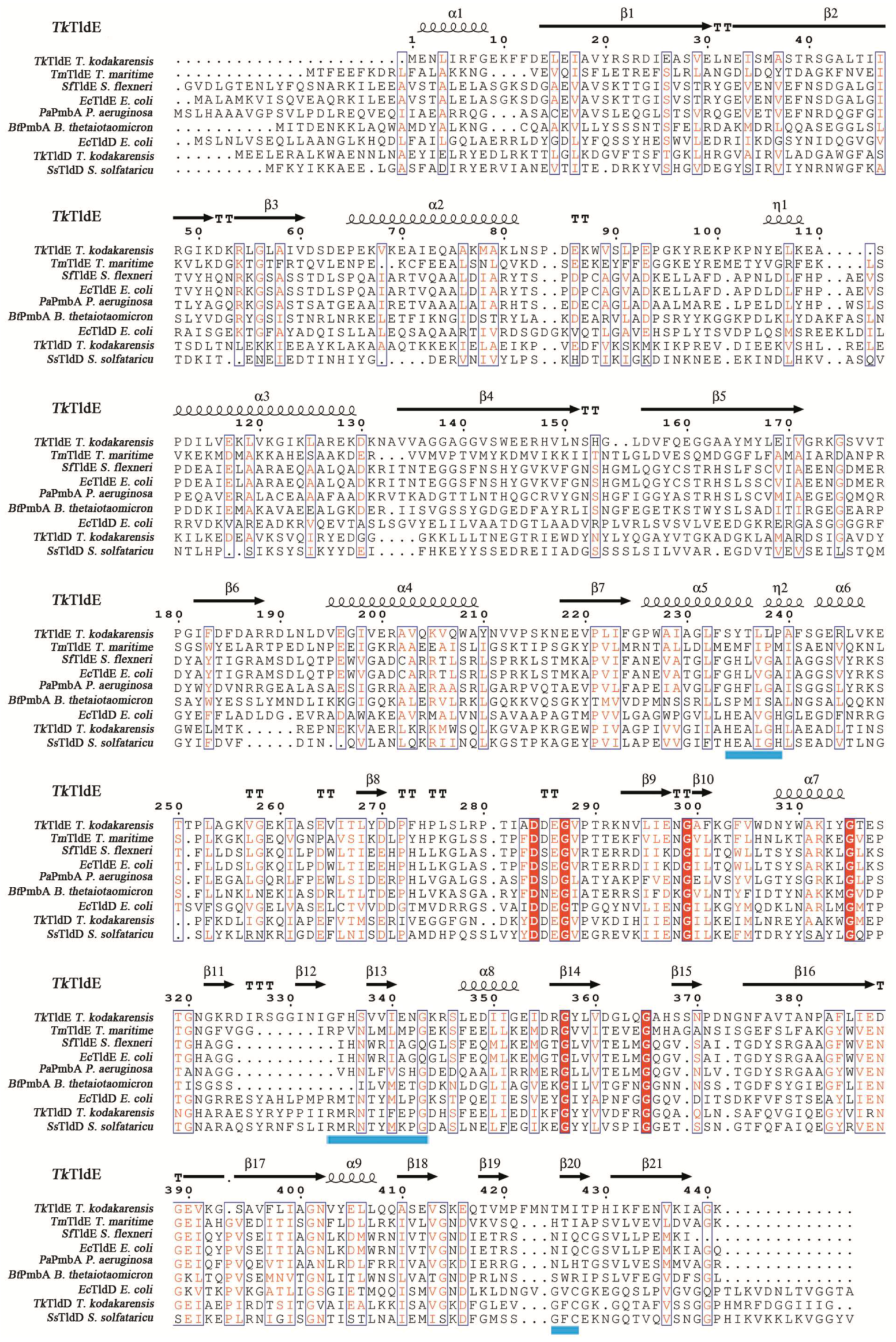
In a structural similarity search, only five structures similar to TkTldE deposited in the PDB were found: 5NJ9, 1VL4, 3TV9, 1VPB and 3QTD. All structures except for 5NJ9 formed spherical homodimers without any recognizable catalytic site. The hydrogen bond/salt bridge network contributed to the stability of all dimers. Multiple alignments based on secondary structure of eight representative sequences, TldE from T. kodakarensis (TkTldE, 6J6A), TldE and TldD from E. coli (EcTldE/TldD, 5NJ9), TldE from T. maritime (TmTIDE, 1VL4), TldE from Shigella flexneri (SsTldE, 3TV9), PmbA from Pseudomonas aeruginosa (PaPmbA, 3QTD), PmbA from Bacteroides thetaiotaomicron (BtPmbA, 1VPB), TldD from T. kodakarensis (TkTldD, tk0502), and TldD from S. solfataricu (SsTldD, SsO0660), revealed several conserved motifs including HExxxH, a metalloprotease-like motif, RMxNTxxxPG, and GxC in TldDs but TldEs [14] (Figure 3). The conserved residues in HExxxH and GxC motify coordinated a metal ion and was response for catalytically activity [14]. The Cα root mean square deviations between TkTldE and the other structural homologues EcTldE, EcTldD, 1VL4, 3TV9, 1VPB, and 3QTD were 2.6, 3.6, 2.1, 2.6, 2.5, and 2.4 Å, respectively, as determined using the DALI server (see Supplementary Materials Table S1) [25]. TldE from T. kodakarensis had 20–23% sequence identity and 42–53% positive matches with other TldD or TldE homologues (Table S1). Together with the fact that these proteins were found to have similar structures, this indicated that they might have similar functions.
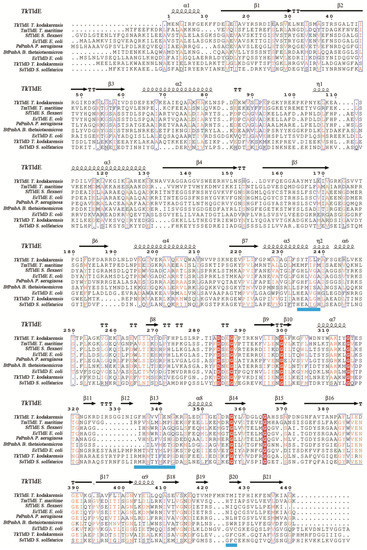
Figure 3.
Structure-based amino-acid sequence alignment of TkTldE with other homologous proteins from different organisms: EcTldE (PDB: 5NJ9) and EcTldD (PDB: 5NJ9) from E. coli, TmTldE (PDB: 1VL4) from T. maritime, SfTldE (PDB: 3TV9) from S. flexneri, PaPmbA (PDB: 3QTD) from P. aeruginosa, BtPmbA (PDB: 1VPB) from B. thetaiotaomicron, TmTldD (Genbank accession numbers: BAD84691) from T. kodakarensis, and SsTldD (Genbank accession numbers: AAK40965) from S. solfataricu. Blue shaded line represented metal-binding motif (HExxxH) and conserved motifs (RMxNTxxxPG and GxC), strictly conserved residues were indicated by red shaded boxes, and similar residues were in red. The secondary structure assignment was produced using ESPript [26].
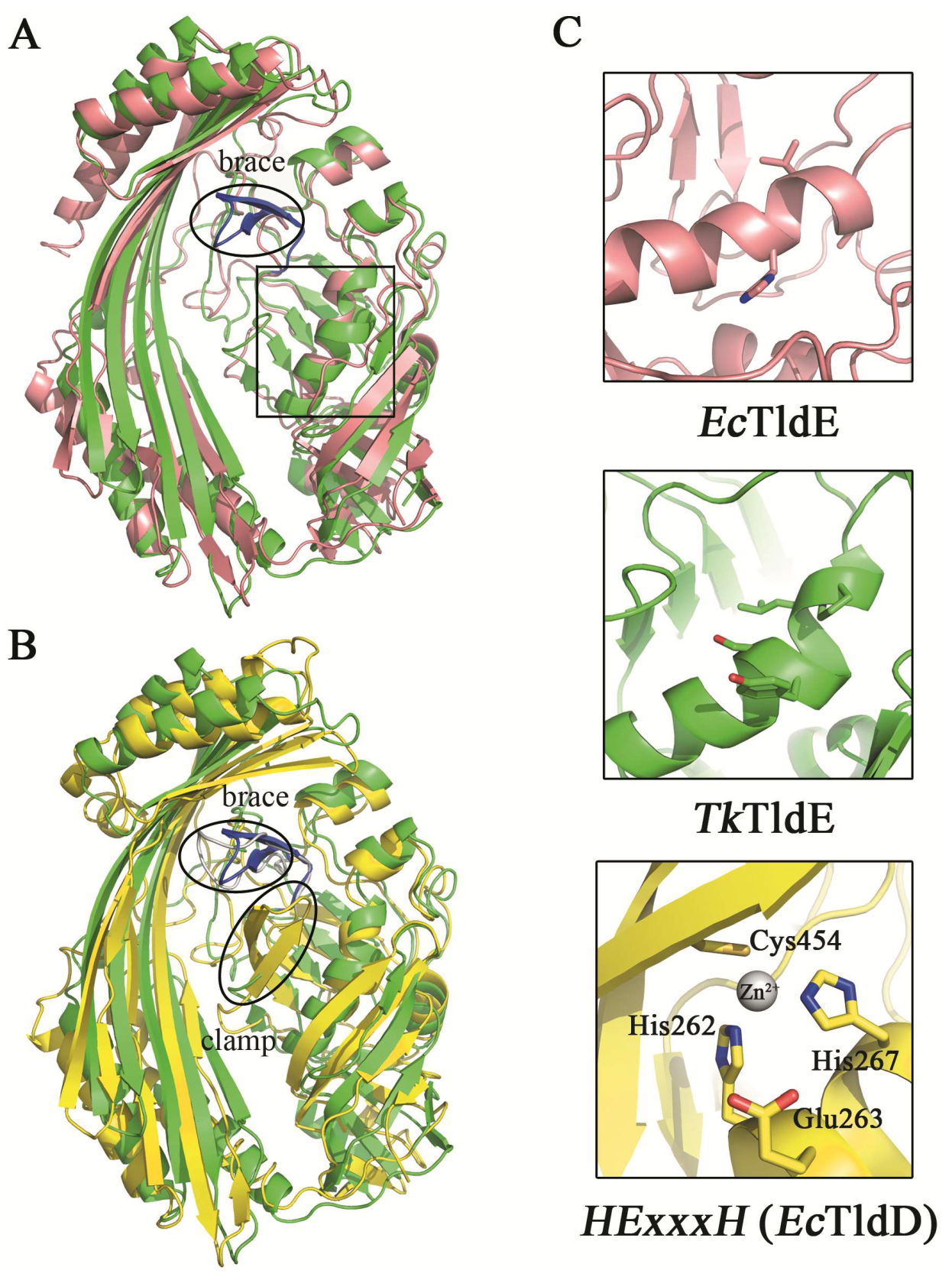
The function of EcTldD/TldE as a cleaving modified MccB17 precursor in vitro was reported [14]. The structural comparison between TkTldE and EcTldE/TldD indicated that the overall structures were similar, but there were additional folds termed the “clamp” in EcTldD, which made its β-strands longer than those of EcTldE; this clamp was also missing in TkTldE and other TldEs (Figure 4) [14]. Cys454 coordinating a Zn2+ ion with two histidine residues from HExxxH motif was located in the clamp motif of EcTldD (Figure 4C) [15]. In contrast, Cys416 in Sso0660, the equivalent of Cys454 in EcTldD was not necessary for the metal binding but the formation of protein dimer [14]. Cysteine was not found in TkTldE but existed in other TldE/TldDs. Though there was a cysteine in other TldEs at least, cysteines were not involved in coordinating a metal ion based on structural superposition. TkTldE had similar folds to the “brace” motif of EcTldD to support the inner face of β-strands [15]. We found that two β-sheets (residues 322–333) in TkTldE, instead of the “brace” of EcTldD, supported the inner surface of six anti-parallel β-strands, but these were not found in other TldEs (Figure 4B). Additionally, the helix α1 of TkTldE in the N-terminal structure was shorter than that of EcTldE/TldD. Together, the reason why TldE cannot bind with metal ions was that the HExxxH metal-ion-binding motif was missing from TkTldE and EcTldE (Figure 3).
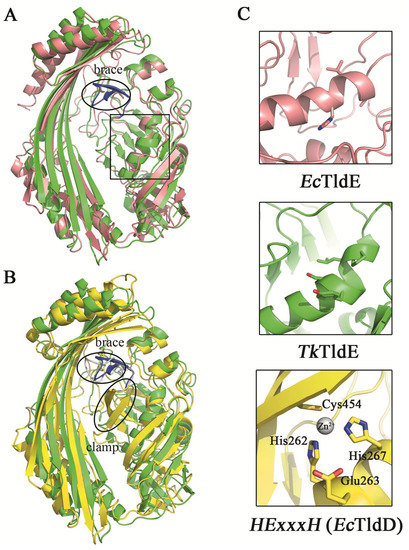
Figure 4.
Structural comparison between TkTldE and EcTldE/TldD. (A) The ribbon diagram of TkTldE (green) was superimposed on that of EcTldE (pink). (B) Superposition of TkTIDE and EcTldD (yellow). (C) Conserved residues consisting of the metal-binding motif in EcTldD. The metal-binding motif was shown to indicate the putative position of EcTldD (down), as distinguished from TkTldE (up) and EcTldE (middle). Side chains were shown as sticks.
TldD or TldE could form homodimers in solution, and the heterodimer of EcTldE/TldD represented an active state with the two proteins having co-evolved to directly regulate important enzymes by protein maturation or degradation [15]. The heterodimer of EcTldE/TldD was the active state, but the homodimer of TldD (Sso0660) from S. solfataricus was catalytically active [14,15]. We found that tk0502 which was located next to TldE in the T. kodakarensis genome encoded a TldD homologue (TkTldD) with the HExxxH and GxC motif (Figure 3). TkTldD may interact with TkTldE to form a heterodimer with similar function to that of EcTldE/TldD or form homodimer similar with Sso0660. Further study is needed to elucidate the connection between TkTldE and TkTldD.
In summary, the function of TldD and TldE was only investigated in the process of the maturation of MccB17 precursor and the degradation of CcdA antidote. We solved the crystal structure of TkTldE and compared it with the structures of other homologues. Despite their overall similarity, there were distinct differences between the partial motifs of TkTldE and those of other TldE/TldD homologues. To some degree, the brace formed from two β-sheets in TkTldE made the structure more stable than other TldE/TldDs. Based on multiple sequence alignments and structural superpositions, we speculated that TldE, which was absent at the catalytic site in archaeal and bacterial organisms, was likely not a protease, but that it would play a functional role as a partner of metalloprotease TldD. The biological functions of these proteins in archaebacteria remains to be further investigated.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4352/9/2/107/s1, Figure S1: The dimer interface of TkTldE. Table S1: DALI statistics on structural alignment of TkTIDE with and the other structural homologues.
Author Contributions
Conceptualization, X.Z., S.Z. and J.L.; methodology, X.Z. and Y.Z; software, X.Z. and X.C; validation, S.Z. and J.L.; formal analysis, X.Z., J.L.; investigation, Z.X. and Z.L.; resources, Z.L.; data curation, Y.L.; writing—original draft preparation, X.Z.; writing—review and editing, J.L.; visualization, J.L.; supervision, S.Z. and J.L.; project administration, J.L.; funding acquisition, J.L.
Funding
This research was funded by the National Key Research and Development Plan (Grant No. 2017YFD0201100).
Acknowledgments
We thank the staff of the BL-17U1 beamline at the National Facility for Protein Science Shanghai, and the Shanghai Synchrotron Radiation Facility, Shanghai, China, for assistance during data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Allali, N.; Afif, H.; Couturier, M.; Van Melderen, L. The Highly Conserved TldD and TldE Proteins of Escherichia coli are Involved in Microcin B17 Processing and in CcdA Degradation. J. Bacteriol. 2002, 184, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Microcin B17: Posttranslational Modifications and their Biological Implications. Proc. Natl. Acad. Sci. USA 1994, 91, 4618–4620. [Google Scholar] [CrossRef]
- Rodríguez-Sáinz, M.C.; Hernández-Chico, C.; Moreno, F. Molecular Characterization of pmbA, an Escherichia coli Chromosomal Gene Required for the Production of the Antibiotic Peptide MccB17. Mol. Microbiol. 1990, 4, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Genilloud, O.; Moreno, F.; Kolter, R. DNA Sequence, Products, and Transcriptional Pattern of the Genes Involved in Production of the DNA Replication Inhibitor Microcin B17. J. Bacteriol. 1989, 171, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- San Millan, J.L.; Hernandez-Chico, C.; Pereda, P.; Moreno, F. Cloning and Mapping of the Genetic Determinants for Microcin B17 Production and Immunity. J. Bacteriol. 1985, 163, 275–281. [Google Scholar]
- Davagnino, J.; Herrero, M.; Furlong, D.; Moreno, F.; Kolter, R. The DNA Replication Inhibitor Microcin B17 is a Forty-Three-Amino-Acid Protein Containing Sixty Percent Glycine. Proteins 1986, 1, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Milne, J.C.; Madison, L.L.; Kolter, R.; Walsh, C.T. From Peptide Precursors to Oxazole and Thiazole-Containing Peptide Antibiotics: Microcin B17 Synthase. Science 1996, 274, 1188–1193. [Google Scholar] [CrossRef]
- Garrido, M.C.; Herrero, M.; Kolter, R.; Moreno, F. The Export of The DNA Replication Inhibitor Microcin B17 Provides Immunity for the Host Cell. EMBO J. 1988, 7, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- San Millán, J.L.; Kolter, R.; Moreno, F. Plasmid Genes Required for Microcin B17 Production. J. Bacteriol. 1985, 163, 1016–1020. [Google Scholar] [PubMed]
- Reece, R.J.; Maxwell, A. DNA Gyrase: Structure and Function. Crit. Rev. Biochem. Mol. Biol. 1991, 26, 335–375. [Google Scholar] [CrossRef]
- Herrero, M.; Moreno, F. Microcin B17 Blocks DNA Replication and Induces the SOS system in Escherichia coli. Microbiology 1986, 132, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Mayo, O.; Hernández-Chico, C.; Moreno, F. Microcin B17, A Novel Tool for Preparation of Maxicells: Identification of Polypeptides Encoded by the IncFII Minireplicon pMccB17. J. Bacteriol. 1988, 170, 2414–2417. [Google Scholar] [CrossRef]
- Vizan, J.L.; Hernández-Chico, C.; del Castillo, I.; Moreno, F. The Peptide Antibiotic Microcin B17 Induces Double-Strand Cleavage of DNA Mediated by E. coli DNA Gyrase. EMBO J. 1991, 10, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Peng, N.; Han, W.; Mei, Y.; Chen, Z.; Feng, X.; Liang, Y.X.; She, Q. An Archaeal Protein Evolutionarily Conserved in Prokaryotes is a Zinc-Dependent Metalloprotease. Biosci. Rep. 2012, 32, 609–618. [Google Scholar] [CrossRef]
- Ghilarov, D.; Serebryakova, M.; Stevenson, C.E.; Hearnshaw, S.J.; Volkov, D.S.; Maxwell, A.; Lawson, D.M.; Severinov, K. The Origins of Specificity in the Microcin-Processing Protease TldD/E. Structure 2017, 25, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Karoui, H.; Bex, F.; Dreze, P.; Couturier, M. Ham22, a Mini-F Mutation Which is Lethal to Host Cell and Promotes RecA-dependent Induction of Lambdoid Prophage. EMBO J. 1983, 2, 1863–1868. [Google Scholar] [CrossRef]
- Maki, S.; Takiguchi, S.; Miki, T.; Horiuchi, T. Modulation of DNA Supercoiling Activity of Escherichia coli DNA Gyrase by F Plasmid Proteins. Antagonistic Actions of LetA (CcdA) and LetD (CcdB) Proteins. J. Biol. Chem. 1992, 267, 12244–12251. [Google Scholar]
- Rife, C.; Schwarzenbacher, R.; McMullan, D.; Abdubek, P.; Ambing, E.; Axelrod, H.; Biorac, T.; Canaves, J.M.; Chiu, H.J.; Deacon, A.M.; et al. Crystal Structure of a Putative Modulator of DNA Gyrase (pmbA) from Thermotoga maritima at 1.95 Å Resolution Reveals a New Fold. Proteins Struct. Funct. Bioinf. 2005, 61, 444–448. [Google Scholar] [CrossRef]
- Fukui, T.; Atomi, H.; Kanai, T.; Matsumi, R.; Fujiwara, S.; Imanaka, T. Complete Genome Sequence of the Hyperthermophilic Archaeon Thermococcus kodakaraensis KOD1 and Comparison with Pyrococcus Genomes. Genome Res. 2005, 15, 352–363. [Google Scholar] [CrossRef]
- Pham, K.; LaForge, K.; Kreek, M. Sticky-end PCR: New Method for Subcloning. Biotechniques 1998, 25, 206–208. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [PubMed]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser Crystallographic Software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-Building Tools for Molecular Graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-Atom Structure Validation for Macromolecular Crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Holm LaRo, P. Dali Server: Conservation Mapping in 3D. Nucleic Acids Res. 2010, 38, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering Key Features in Protein Structures with the New ENDscript Server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).