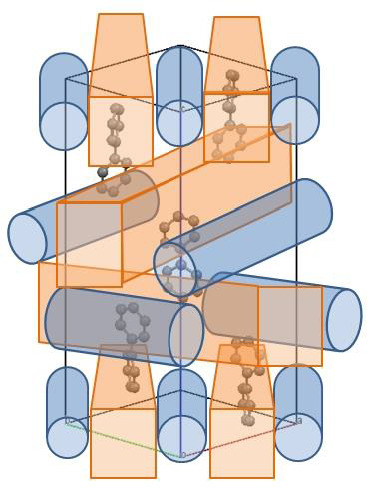
Threefold Spiral Structure Constructed by 1D Chains of [[M(NCS)2(bpa)2]·biphenyl]n (M = Fe, Co; bpa = 1,2-bis(4-pyridyl)ethane)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Gütlich, P.; Bill, E.; Trautwein, A.X. Mössbauer Spectroscopy and Transition Metal Chemistry; Springer-Verlag: Berlin/Heidelberg, Germany, 2011; pp. 391–476. ISBN 978-3-540-88427-9. [Google Scholar]
- Kahn, O.; Kröber, J.; Jay, C. Spin transition molecular materials for displays and data recording. Adv. Mater. 1992, 4, 718–728. [Google Scholar] [CrossRef]
- Gentili, D.; Demitri, N.; Schäfer, B.; Liscio, F.; Bergenti, I.; Ruani, G.; Ruben, M.; Cavallini, M. Multi-modal sensing in spin crossover compounds. J. Mater. Chem. C 2015, 3, 7836–7844. [Google Scholar] [CrossRef]
- Nakashima, S.; Yamamoto, Y.; Asada, Y.; Koga, N.; Okuda, T. New assembled Fe-trans-1,2-Bis(4-pyridyl)ethylene-NCS(NCSe) complexes—Hydrogen bonded and π-π interacted structure and grid structure enclathrating ligand. Inorg. Chim. Acta 2005, 358, 257–264. [Google Scholar] [CrossRef]
- Kitazawa, T.; Gomi, Y.; Takahashi, M.; Takeda, M.; Enomoto, M.; Miyazaki, A.; Enoki, T. Spin-crossover behaviour of the coordination polymer FeII(C5H5N)2NiII(CN)4. J. Mater. Chem. 1996, 6, 119–121. [Google Scholar] [CrossRef]
- Niel, V.; Martinez-Agudo, J.M.; Muñoz, M.C.; Gaspar, A.B.; Real, J.A. Cooperative Spin Crossover Behavior in Cyanide-Bridged Fe(II)−M(II) Bimetallic 3D Hofmann-like Networks (M = Ni, Pd, and Pt). Inorg. Chem. 2001, 40, 3838–3839. [Google Scholar] [CrossRef]
- Muñoz, M.C.; Gaspar, A.B.; Galet, A.; Real, J.A. Spin-Crossover Behavior in Cyanide-Bridged Iron(II)–Silver(I) Bimetallic 2D Hofmann-like Metal-Organic Frameworks. Inorg. Chem. 2007, 46, 8182–8192. [Google Scholar] [CrossRef]
- Agustí, G.; Muñoz, M.C.; Gaspar, A.B.; Real, J.A. Spin-Crossover Behavior in Cyanide-bridged Iron(II)−Gold(I) Bimetallic 2D Hofmann-like Metal-Organic Frameworks. Inorg. Chem. 2008, 47, 2552–2561. [Google Scholar] [CrossRef]
- Rodriguez-Velamazan, J.A.; Carbonera, C.; Castro, M.; Palacios, E.; Kitazawa, T.; Letard, J.-F.; Burriel, R. Two-step thermal spin transition and LIESST relaxation of the polymeric spin-crossover compounds Fe(X-py)2[Ag(CN)2]2 (X = H, 3-Methyl, 4-Methyl, 3,4-Dimethyl, 3-Cl). Chem. Eur. J. 2010, 16, 8785–8796. [Google Scholar] [CrossRef]
- Dîrtu, M.M.; Naik, A.D.; Rotaru, A.; Spinu, L.; Poelman, D.; Garcia, Y. FeII Spin Transition Materials Including an Amino–Ester 1,2,4-Triazole Derivative, Operating at, below, and above Room Temperature. Inorg. Chem. 2016, 55, 4278–4295. [Google Scholar] [CrossRef]
- Roubeau, O. Triazole-Based One-Dimensional Spin-Crossover Coordination Polymers. Chem. Eur. J. 2012, 18, 15230–15244. [Google Scholar] [CrossRef] [Green Version]
- Quesada, M.; Koojiman, H.; Gomez, P.; Costa, J.S.; Koningsbruggen, P.J.; Weinberger, P.; Reissner, M.; Spek, A.L.; Haasnoot, J.G.; Reedjik, J. [Fe(μ-btzmp)2(btzmp)2](ClO4)2: A doubly-bridged 1D spin-transition bistetrazole-based polymer showing thermal hysteresis behavior. Dalton. Trans. 2007, 5434–5440. [Google Scholar] [CrossRef] [PubMed]
- Moliner, N.; Muñoz, M.C.; Létard, S.; Salmon, L.; Tuchgues, J.P.; Boussksou, A.; Real, J.A. Mass Effect on the Equienergetic High-Spin/Low-Spin States of Spin-Crossover in 4,4‘-Bipyridine-Bridged Iron(II) Polymeric Compounds: Synthesis, Structure, and Magnetic, Mössbauer, and Theoretical Studies. Inorg. Chem. 2002, 41, 6997–7005. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Asada, Y.; Okuda, T.; Nakashima, S. Isomerism of assembled iron complex bridged by 1,2-di(4-pyrisyl)ethane and its solid-to-solid transformation accompanied by a change of electronic state. Bull Chem. Soc. Jpn. 2006, 79, 738–744. [Google Scholar] [CrossRef]
- Morita, T.; Nakashima, S.; Yamada, K.; Inoue, K. Occurrence of the spin-crossover phenomenon of assembled complexes, Fe(NCX)2(bpa)2 (X= S, BH3; bpa= 1,2-bis(4-pyridyl)ethane) by enclathrating organic guest molecule. Chem. Lett. 2006, 35, 1042–1043. [Google Scholar] [CrossRef]
- Nakashima, S.; Morita, T.; Inoue, K. Spin-crossover phenomenon of the assembled iron complexes with 1,2-bis(4-pyridyl)ethane as bridging ligand studied by Mössbauer spectroscopy. Hyperfine Interact. 2009, 188, 107–111. [Google Scholar] [CrossRef]
- Dote, H.; Kaneko, M.; Inoue, K.; Nakashima, S. Synthesis of Anion-Mixed Crystals of the Assembled Complexes Bridged by 1,2-Bis(4-pyridyl)ethane and Ligand Field of Fe(NCS)(NCBH3) Unit. Bull. Chem. Soc. Jpn. 2018, 91, 71–81. [Google Scholar] [CrossRef]
- Atsuchi, M.; Higashikawa, H.; Yoshida, Y.; Nakashima, S.; Inoue, K. Novel 2D Interpenetrated Structure and Occurrence of the Spin-crossover Phenomena of Assembled Complexes, Fe(NCX)2(bpp)2 (X = S, Se, BH3; bpp = 1,3-Bis(4-pyridyl)propane. Chem. Lett. 2006, 36, 1064–1065. [Google Scholar] [CrossRef]
- Atsuchi, M.; Inoue, K.; Nakashima, S. Reversible structural change of host framework triggered by desorption and adsorption of guest benzene molecules in Fe(NCS)2(bpp)2·2(benzene) (bpp = 1,3-bis(4-pyridyl)propane). Inorg. Chim. Acta 2011, 370, 82–88. [Google Scholar] [CrossRef]
- Yoshinami, K.; Kaneko, M.; Yasuhara, H.; Nakashima, S. Effect of methyl substituent on the spin state of iron(II) assembled complex using 1,4-bis(4-pyridyl)benzene. Radioisotopes 2017, 66, 625–632. [Google Scholar] [CrossRef]
- Iwai, S.; Yoshinami, K.; Nakashima, S. Structure and Spin State of Iron(II) Assembled Complexes using 9,10-Bis(4-pyridyl)anthracene as Bridging Ligand. Inorganics 2017, 5, 61. [Google Scholar] [CrossRef]
- Kaneko, M.; Tokinobu, S.; Nakashima, N. Density Functional Study on Spin-crossover Phenomena of Assembled Complexes, [Fe(NCX)2(bpa)2]n (X = S, Se, BH3; bpa = 1,2-bis(4-pyridyl)ethane). Chem. Lett. 2013, 42, 1432–1434. [Google Scholar] [CrossRef]
- Kaneko, M.; Nakashima, S. Computational Study on Thermal Spin-Crossover Behavior for Coordination Polymers Possessing trans-Fe(NCS)2(pyridine)4 Unit. Bull. Chen. Soc. Jpn. 2015, 88, 1164–1170. [Google Scholar] [CrossRef]
- Hernández, M.L.; Barandika, M.G.; Urtiaga, M.K.; Cortés, R.; Lezama, L.; Arriortuac, M.L.; Rojo, T. Structural analysis and magnetic properties of the 1-D compounds [M(NCS)2bpa2] [M = Fe, Co, Ni and bpa = 1,2-bis(4-pyridyl)ethane]. J. Chem. Soc. Dalton Trans. 1999, 1401–1406. [Google Scholar] [CrossRef]
- Nakashima, S.; Morita, T.; Inoue, K.; Hayami, S. Spiral assembly of the 1D chain sheet of Fe(NCBH3)2(bpa)2·(biphenyl) (bpa = 1,2-bis(4-pyridyl)ethane) and its stepwise spin-crossover phenomenon. Polymers 2011, 4, 880–888. [Google Scholar] [CrossRef]
- The SHELX Homepage. Available online: http://shelxl.uni-ac.gwdg.de/ (accessed on 14 February 2019).







| Guest | NCS | NCSe | NCBH3 | |
|---|---|---|---|---|
| Anion | ||||
| biphenyl | 2D grid | Linear | Linear | |
| 2-nitrobiphenyl | Interpenetrated | Interpenetrated | 2D grid | |
| 1,4-dichlorobenzene | Linear | Linear | Not included | |
| diphenylmethane | Interpenetrated | Interpenetrated | 2D grid | |
| Fe(NCS)2(bpa)2·biphenyl | |
|---|---|
| Temperature | RT |
| Space group | P3121 |
| a,b/Å | 10.234(5) |
| c/Å | 30.070(15) |
| α,β/° | 90 |
| γ/° | 120 |
| R1 | 0.0368 |
| wR2 | 0.0909 |
| Goodness of fit | 1.039 |
| Volume/Å3 | 2727(3) |
| Flack parameter | 0.022(26) |
| Flack parameter when using P3221 | 0.9764 |
| Co(NCS)2(bpa)2·biphenyl | |
|---|---|
| Temperature | 173K |
| Space group | P3121 |
| a,b/Å | 10.1607(4) |
| c/Å | 30.0262(13) |
| α,β/° | 90 |
| γ/° | 120 |
| R1 | 0.0232 |
| wR2 | 0.0572 |
| Goodness of fit | 1.113 |
| Volume/Å3 | 2684.6(2) |
| Flack parameter | 0.015(15) |
| Flack parameter when using P3221 | 0.9822 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokinobu, S.; Dote, H.; Nakashima, S. Threefold Spiral Structure Constructed by 1D Chains of [[M(NCS)2(bpa)2]·biphenyl]n (M = Fe, Co; bpa = 1,2-bis(4-pyridyl)ethane). Crystals 2019, 9, 97. https://doi.org/10.3390/cryst9020097
Tokinobu S, Dote H, Nakashima S. Threefold Spiral Structure Constructed by 1D Chains of [[M(NCS)2(bpa)2]·biphenyl]n (M = Fe, Co; bpa = 1,2-bis(4-pyridyl)ethane). Crystals. 2019; 9(2):97. https://doi.org/10.3390/cryst9020097
Chicago/Turabian StyleTokinobu, Satoshi, Haruka Dote, and Satoru Nakashima. 2019. "Threefold Spiral Structure Constructed by 1D Chains of [[M(NCS)2(bpa)2]·biphenyl]n (M = Fe, Co; bpa = 1,2-bis(4-pyridyl)ethane)" Crystals 9, no. 2: 97. https://doi.org/10.3390/cryst9020097
APA StyleTokinobu, S., Dote, H., & Nakashima, S. (2019). Threefold Spiral Structure Constructed by 1D Chains of [[M(NCS)2(bpa)2]·biphenyl]n (M = Fe, Co; bpa = 1,2-bis(4-pyridyl)ethane). Crystals, 9(2), 97. https://doi.org/10.3390/cryst9020097