Kinetics of Non-Isothermal and Isothermal Crystallization in a Liquid Crystal with Highly Ordered Smectic Phase as Reflected by Differential Scanning Calorimetry, Polarized Optical Microscopy and Broadband Dielectric Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Broadband Dielectric Spectroscopy (BDS)
2.3. Differential Scanning Calorimetry (DSC)
2.4. Polarizing Optical Microscopy (POM)
3. Results and Discussion
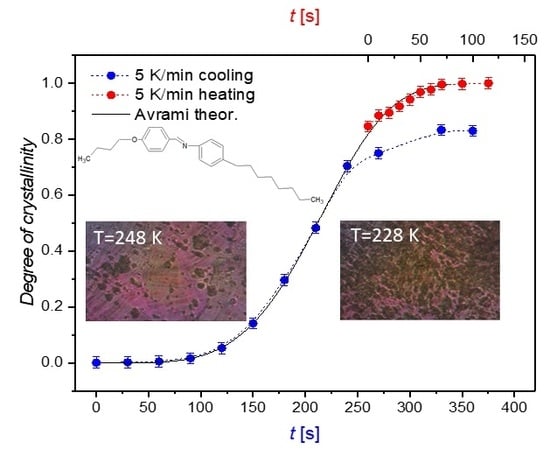
3.1. Non-Isothermal Crystallization Processes as Followed Using DSC and POM
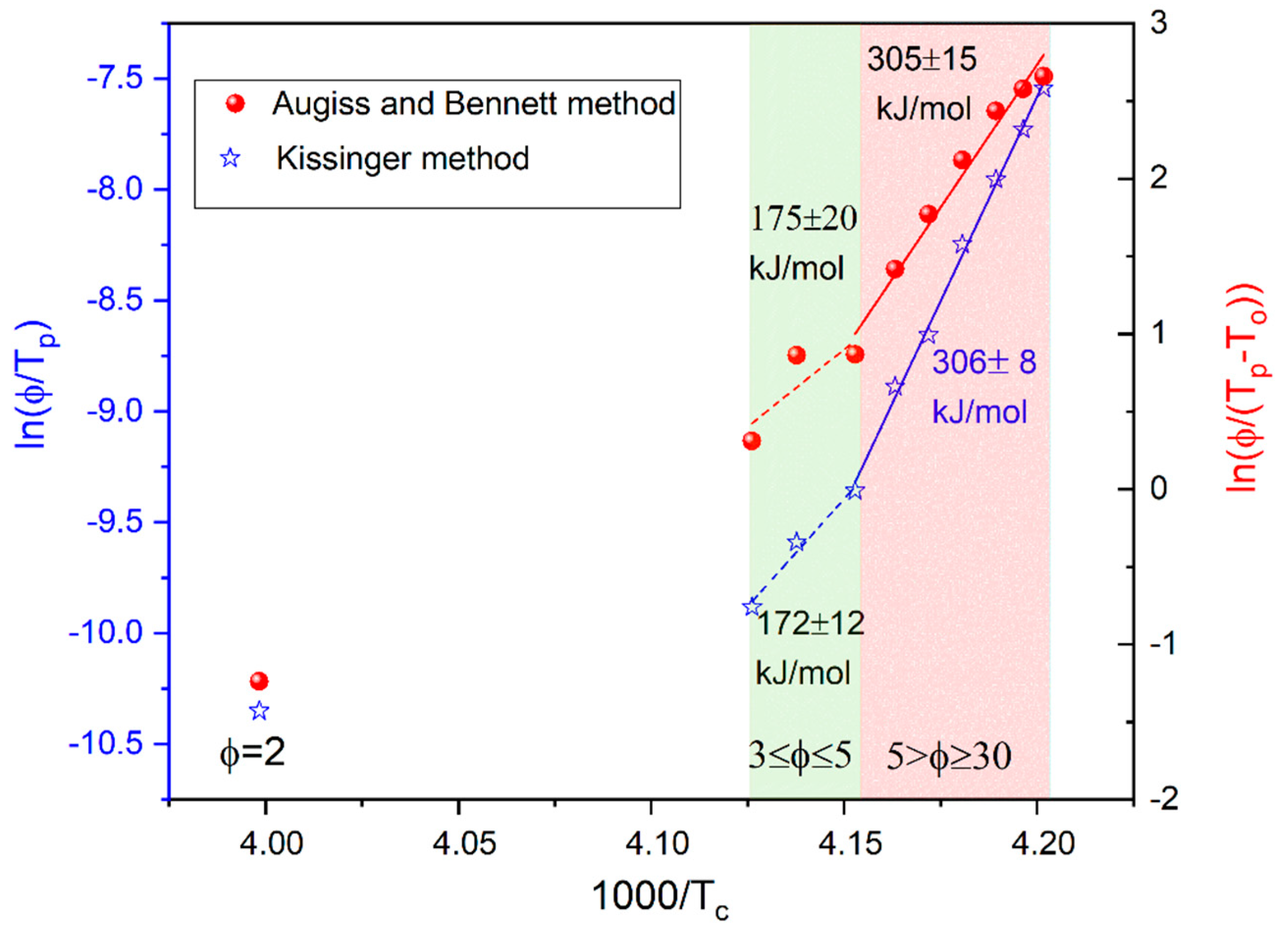
3.2. Analysis of Non-Isothermal Melt Crystallization Kinetics Observed by DSC
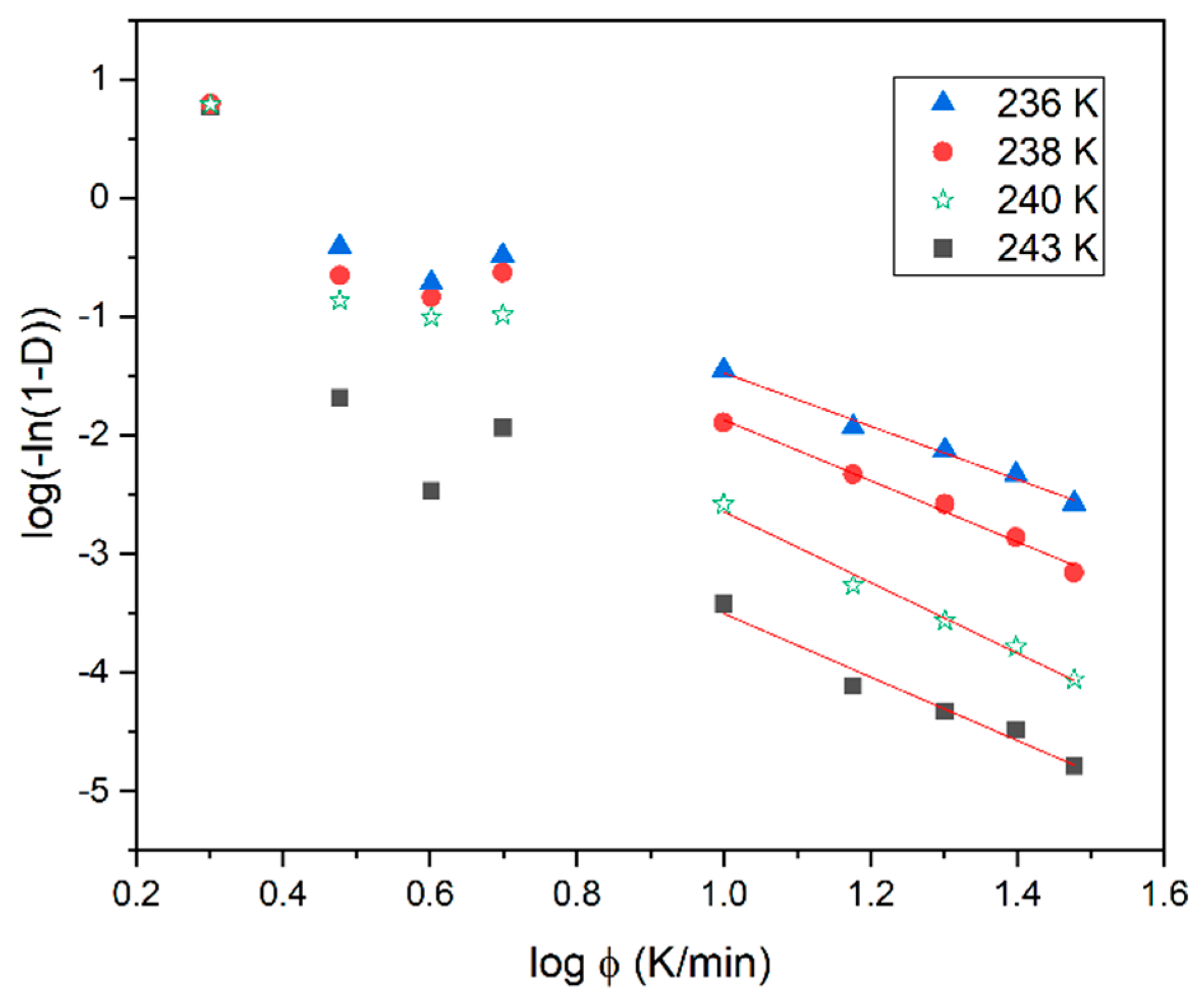
3.3. Isothermal Melt Crystallization Kinetics of SmBcr Studied by BDS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ediger, M.D.; Harrowell, P.; Yu, L. Crystal growth kinetics exhibit a fragility-dependent decoupling from viscosity. J. Chem. Phys. 2008, 128, 034709. [Google Scholar] [CrossRef] [PubMed]
- Tung, H.H.; Paul, E.L.; Midler, M.; McCauley, J.A. Crystallization of Organic Compounds: An Industrial Perspective; John Wiley & Sons, Inc.: Hoboken, New Jersey, USA, 2009; ISBN 978-0-471-46780-9. [Google Scholar]
- Xue, D.; Li, K.; Liu, J.; Sun, C.; Chen, K. Crystallization and functionality of inorganic materials. Mater. Res. Bull. 2012, 47, 2838–2842. [Google Scholar] [CrossRef]
- Gutzow, I.S.; Schmelzer, J.W.P. The Vitreous State: Thermodynamics, Structure, Rheology, and Crystallization, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1995; ISBN 978-3-642-34633-0. [Google Scholar]
- Jackson, K.A. Kinetic Processes; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; ISBN 9783527603893. [Google Scholar]
- Szklarz, G.; Adrjanowicz, K.; Paluch, M. Cooling-Rate versus Compression-Rate Dependence of the Crystallization in the Glass-Forming Liquid, Propylene Carbonate. Cryst. Growth Des. 2018, 18, 2538–2544. [Google Scholar] [CrossRef]
- Sanz, A.; Niss, K. Coupling between Molecular Mobility and Kinetics of Crystal Growth in a Hydrogen-Bonded Liquid. Cryst. Growth Des. 2017, 17, 4628–4636. [Google Scholar] [CrossRef]
- Swallen, S.F.; Ediger, M.D. Self-diffusion of the amorphous pharmaceutical indomethacin near Tg. Soft Matter 2011, 7, 10339–10344. [Google Scholar] [CrossRef]
- Descamps, M.; Dudognon, E. Crystallization from the amorphous state: Nucleation-growth decoupling, polymorphism interplay, and the role of interfaces. J. Pharm. Sci. 2014, 103, 2615–2628. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, G.G.Z.; Law, D.; Grant, D.J.W.; Schmitt, E.A. Thermodynamics, molecular mobility and crystallization kinetics of amorphous griseofulvin. Mol. Pharm. 2008, 5, 927–936. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S. Crystallization of glass-forming liquids: Specific surface energy. J. Chem. Phys. 2016, 145, 064512. [Google Scholar] [CrossRef]
- Pełka, R.; Yamamura, Y.; Jasiurkowska, M.; Massalska-Arodź, M.; Saito, K. Rich polymorphism in 4-propyl-4′-thiocyanato-1,1′-biphenyl (3TCB) revealed by adiabatic calorimetry. Liq. Cryst. 2008, 35, 179–186. [Google Scholar] [CrossRef]
- Massalska-Arodź, M.; Williams, G.; Smith, I.K.; Conolly, C.; Anthony Aldridge, G.; Dabrowski, R. Molecular dynamics and crystallization behaviour of isopentyl cyanobiphenyl as studied by dielectric relaxation spectroscopy. J. Chem. Soc. Faraday Trans. 1998, 94, 387–394. [Google Scholar] [CrossRef]
- Massalska-Arodź, M.; Williams, G.; Thomas, D.K.; Jones, W.J.; Dabrowski, R. Molecular Dynamics and Crystallization Behavior of Chiral Isooctyloxycyanobiphenyl as Studied by Dielectric Relaxation Spectroscopy. J. Phys. Chem. B 1999, 103, 4197–4205. [Google Scholar] [CrossRef]
- Rozwadowski, T.; Massalska-Arodź, M.; Kolek, Ł.; Grzybowska, K.; Bąk, A.; Chłędowska, K. Kinetics of Cold Crystallization of 4-Cyano-3-fluorophenyl 4-Butylbenzoate (4CFPB) Glass Forming Liquid Crystal. I. Nonisothermal Process As Studied by Microscopic, Calorimetric, and Dielectric Methods. Cryst. Growth Des. 2015, 15, 2891–2900. [Google Scholar] [CrossRef]
- Jasiurkowska-Delaporte, M.; Rozwadowski, T.; Dmochowska, E.; Juszyńska-Gałązka, E.; Kula, P.; Massalska-Arodź, M. Interplay between Crystallization and Glass Transition in Nematic Liquid Crystal 2,7-Bis(4-pentylphenyl)-9,9-diethyl-9H-fluorene. J. Phys. Chem. B 2018, 122, 10627–10636. [Google Scholar] [CrossRef]
- Georgopoulos, D.; Kripotou, S.; Argyraki, E.; Kyritsis, A.; Pissis, P. Study of Isothermal Crystallization Kinetics of 5CB with Differential Scanning Calorimetry and Broadband Dielectric Spectroscopy. Mol. Cryst. Liq. Cryst. 2015, 611, 197–207. [Google Scholar] [CrossRef]
- Mastrangelo, J.C.; Blanton, T.N.; Chen, S.H. Crystallization upon thermal annealing of a glass-forming liquid crystal in the nematic regime. Appl. Phys. Lett. 1995, 66, 2212–2214. [Google Scholar] [CrossRef]
- Juszyńska, E.; Jasiurkowska, M.; Massalska-Arodź, M.; Takajo, D.; Inaba, A. Phase Transition and Structure Studies of a Liquid Crystalline Schiff-Base Compound (4O.8). Mol. Cryst. Liq. Cryst. 2011, 540, 127–134. [Google Scholar] [CrossRef]
- Dierking, I. Textures of Liquid Crystals; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; ISBN 3527307257. [Google Scholar]
- Jasiurkowska-Delaporte, M.; Napolitano, S.; Leys, J.; Juszyńska-Gałązka, E.; Wübbenhorst, M.; Massalska-Arodź, M. Glass Transition Dynamics and Crystallization Kinetics in the Smectic Liquid Crystal 4-n-Butyloxybenzylidene-4′-n′-octylaniline (BBOA). J. Phys. Chem. B 2016, 120, 12160–12167. [Google Scholar] [CrossRef] [PubMed]
- Górecka, E.; Chen, L.; Pyżuk, W.; Krówczyński, A.; Kumar, S. X-ray studies of the hexatic phase in liquid crystals with a crystal-B–hexatic-B–smectic-A phase sequence. Phys. Rev. E 1994, 50, 2863–2867. [Google Scholar] [CrossRef]
- Rao, N.V.S.; Potukuchi, D.M.; Pisipati, V.G.K.M. Phase Transitions in N(p-n-Bu toxybenzylidene) p-n-Alkyl Anilines: Density and Refractive Index Studies. Mol. Cryst. Liq. Cryst. 1991, 196, 71–87. [Google Scholar] [CrossRef]
- Haines, P.J. Thermal Methods of Analysis: Principles, Applications and Problems; Springer: Dordrecht, Netherlands, 1995; ISBN 978-0751400502. [Google Scholar]
- Suzuki, Y.; Yamamura, Y.; Sumita, M.; Yasuzuka, S.; Saito, K. Neat Liquid Consisting of Hydrogen-Bonded Tetramers: Dicyclohexylmethanol. J. Phys. Chem. B 2009, 113, 10077–10080. [Google Scholar] [CrossRef]
- Henderson, D.W. Thermal analysis of non-isothermal crystallization kinetics in glass forming liquids. J. Non-Cryst. Solids 1979, 30, 301–315. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of Peak Temperature With Heating Rate In Differential Thermal Analysis. J. Res. Natl. Bur. Stand. (1934) 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Augis, J.A.; Bennett, J.E. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J. Therm. Anal. Calorim. 1978, 13, 283–292. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; He, Y.; Li, S. Nonisothermal Crystallization Kinetics of Poly(b-hydroxybutyrate). J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1305–1312. [Google Scholar]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal Melt and Cold Crystallization Kinetics of Poly(Ary1 Ether Ether Ketone Ketone). Polym. Eng. Sci. 1997, 33, 568–575. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Tarnacka, M.; Kamińska, E.; Dulski, M.; Kamiński, K.; Paluch, M. Crystallization Kinetics under Confinement. Manipulation of the Crystalline Form of Salol by Varying Pore Diameter. Cryst. Growth Des. 2016, 16, 1218–1227. [Google Scholar] [CrossRef]
- Napolitano, S.; Wübbenhorst, M. Slowing Down of the Crystallization Kinetics in Ultrathin Polymer Films: A Size or an Interface Effect? Macromolecules 2006, 39, 5967–5970. [Google Scholar] [CrossRef]






| Ozawa Model | ||
|---|---|---|
| T (K) | no | log (Z (K/min)no) |
| 236 | 2.24 ± 0.12 | 0.77 ± 0.16 |
| 238 | 2.56 ± 0.14 | 0.69 ± 0.17 |
| 240 | 2.98 ± 0.19 | 0.34 ± 0.24 |
| 243 | 2.67 ± 0.28 | −0.82 ± 0.35 |
| T (K) | nA | lnK | lnτcr |
|---|---|---|---|
| 274 | 2.3 ± 0.1 | −12.5 ± 0.2 | 5.5 ± 0.2 |
| 276 | 2.8 ± 0.1 | −17.3 ± 0.2 | 6.1 ± 0.2 |
| 278 | 2.5 ± 0.1 | −18.0 ± 0.2 | 6.9 ± 0.2 |
| 281 | 2.6 ± 0.2 | −19.7 ± 0.2 | 7.7 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasiurkowska-Delaporte, M.; Rozwadowski, T.; Juszyńska-Gałązka, E. Kinetics of Non-Isothermal and Isothermal Crystallization in a Liquid Crystal with Highly Ordered Smectic Phase as Reflected by Differential Scanning Calorimetry, Polarized Optical Microscopy and Broadband Dielectric Spectroscopy. Crystals 2019, 9, 205. https://doi.org/10.3390/cryst9040205
Jasiurkowska-Delaporte M, Rozwadowski T, Juszyńska-Gałązka E. Kinetics of Non-Isothermal and Isothermal Crystallization in a Liquid Crystal with Highly Ordered Smectic Phase as Reflected by Differential Scanning Calorimetry, Polarized Optical Microscopy and Broadband Dielectric Spectroscopy. Crystals. 2019; 9(4):205. https://doi.org/10.3390/cryst9040205
Chicago/Turabian StyleJasiurkowska-Delaporte, Małgorzata, Tomasz Rozwadowski, and Ewa Juszyńska-Gałązka. 2019. "Kinetics of Non-Isothermal and Isothermal Crystallization in a Liquid Crystal with Highly Ordered Smectic Phase as Reflected by Differential Scanning Calorimetry, Polarized Optical Microscopy and Broadband Dielectric Spectroscopy" Crystals 9, no. 4: 205. https://doi.org/10.3390/cryst9040205
APA StyleJasiurkowska-Delaporte, M., Rozwadowski, T., & Juszyńska-Gałązka, E. (2019). Kinetics of Non-Isothermal and Isothermal Crystallization in a Liquid Crystal with Highly Ordered Smectic Phase as Reflected by Differential Scanning Calorimetry, Polarized Optical Microscopy and Broadband Dielectric Spectroscopy. Crystals, 9(4), 205. https://doi.org/10.3390/cryst9040205