The Problem of Formation of Mixed Crystals and High-Efficiency K2(Co, Ni)(SO4)2 • 6H2O Optical Filters
Abstract
:1. Introduction
2. Morphological Effects in Liquid-Phase Epitaxy
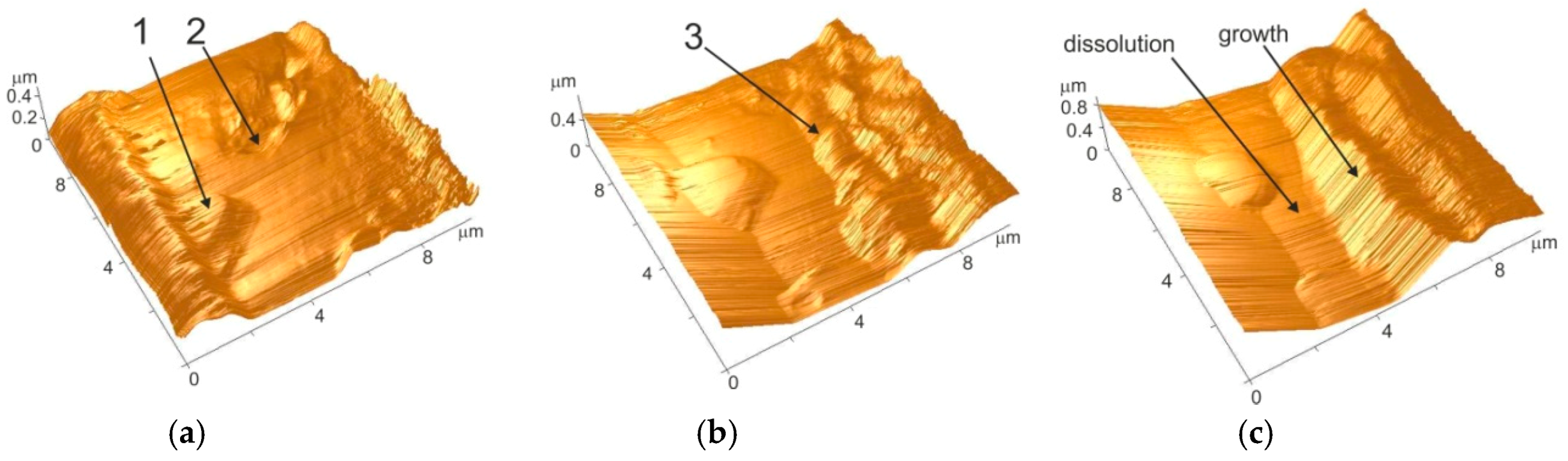
2.1. In situ Atomic Force Microscopy [14]
2.2. Morphological Effect of the Reaction of Isomorphous Replacement [14]
2.3. Volume Effect of the Reaction of Isomorphous Replacement
2.4. Micromosaic Inhomogeneity of Mixed Crystals
2.5. Study of Quasi-Equilibrium States
2.6. The Mechanism of Stress Relaxation at Crystal Growth from Low-temperature Solutions
3. Growth of High-Perfect K2NixCo1-x(SO4)2·6H2O Mixed Crystals for UV Light Applications
3.1. General Characteristic of the Defect Structure of KCNSH Mixed Crystals
3.2. Experimental Setup
3.3. Calculation of the Solution Feeding Modes [41]
3.3.1. Non-Stationary Feeding Mode
3.3.2. Stationary Feeding Mode
3.4. Characterization of the Grown KCNSH Crystals
4. Conclusions
- The general mechanism of reaction, i.e., isomorphous replacement, was determined. The process of interaction of a crystal with a foreign solution of isomorphic component includes the following stages: primary crystal dissolution, the formation of a highly supersaturated solution of mixed composition in the subsurface layer, crystallization of new phase islands of the composition close to equilibrium with the solution, and tangential growth of the islands. The possibility of island accretion is determined by the volume effect of the reaction ω, which depends on the ratio of the volume solubilities of the initial salts. When ω > 1, the islands grow together, forming a continuous epitaxial layer; when ω < 1, there is no splicing, and simultaneously with the growth of the islands, the initial crystal dissolves in the spaces between them. Thus, the crystal surface turns into a mosaic of local areas where multidirectional processes (growth and dissolution) simultaneously proceed. This results in the formation of a mosaic microinhomogeneity, a new kind of composition inhomogeneity which is characteristic only of multicomponent crystals. It is shown that the primary dissolution of the substrate can be suppressed by creating a certain supercooling in the system. An analytical description of the process is given and expressions for the volume effect of reaction are obtained.
- A new mechanism for the relaxation of elastic mismatch stresses in heterocompositions of brittle crystals growing from low-temperature solutions, when the formation of misfit dislocations is impossible, was discovered. In this case, the process of relaxation of the mismatch stresses is due to the formation of numerous inclusions at the heterointerface, which (by analogy) can be called mismatch inclusions. The probability of the formation of inclusions decreases with decreasing lattice mismatches.
- According to the research results, the general concept of growing mixed crystals from solutions was formulated and implemented to ensure a high level of structural perfection:
- crystal growth in the shapers to eliminate sectoral inhomogeneity;
- feeding the solution according to a special law to eliminate the initial transient region;
- growth at a constant temperature to reduce zonal inhomogeneity, the supercooling of the solution should be sufficient to suppress exchange reactions in the system.
Funding
Conflicts of Interest
References
- Bolkhovityanov, Y.B. The peculiarities of isothermal contact of liquid and solid phase during the LPE of A3B5 compounds. J. Cryst. Growth. 1981, 55, 591–598. [Google Scholar] [CrossRef]
- Bolkhovityanov, Y.B. Contact phenomena at the stage of establishing an equilibrium between liquid and solid phases as applied to liquid-phase heteroepitaxy of III-V compounds. In Materiali Electronnoi Tekhniki. 1. Fiziko-Khimicheskie Osnovi Metodov Sinteza; Nauka, Sibirskoe Otdelenie: Novosibirsk, Russia, 1981; pp. 63–82. [Google Scholar]
- Bolkhovityanov, Y.B. The single mechanism for relaxation of non-equilibrium boundary at the liquid-solid front with liquid phase heteroepitaxial of III-V compounds. In Rost Kristallov, 18; Nauka: Moscow, Russia, 1990; pp. 158–172. [Google Scholar]
- Glikin, A.E.; Sinai, M.Y. Morphological and genetic classification of crystal substitution products. Zapiski Vsesoyus. Mineral. Obshch. 1991, 120, 3–17. [Google Scholar]
- Glikin, A.E.; Sinai, M.Y. Experimental study of the genesis of the single crystal pseudomorphs. Zapiski Vsesoyus. Mineral. Obshch. 1983, 112, 742–748. [Google Scholar]
- Glikin, A.E.; Leontyeva, O.A.; Sinai, M.Y. Mechanisms for the exchange of isomorphic components between crystal and solution and macrodefects in secondary crystals. Zhurnal Struct. Khim. 1994, 35, 79–83. [Google Scholar]
- Glikin, A.E. On the theory of formation of isomorphic mixed crystals. Zapiski Vseross. Mineral. Obshch. 1995, 124, 125–133. [Google Scholar]
- Glikin, A.E. On equilibrium supercooled solutions due to the formation of isomorphic mixed crystals. Zapiski Vseross. Mineral. Obshch. 1996, 125, 103–111. [Google Scholar]
- Voloshin, A.E.; Glikin, A.E.; Kovalev, S.I.; Rudneva, E.B.; Sinai, M.Y. The mechanisms of formation of mixed crystals in the system biphthalate potassium - rubidium biphthalate. In Proceedings of the IX National Conference on Crystal Growth, Moscow, Russia, 15–20 October 2000; p. 361. [Google Scholar]
- Voloshin, A.E.; Glikin, A.E.; Kovalev, S.I.; Rudneva, E.B.; Sinai, M.Y. The Mechanisms of Mixed Crystals Formation in KAP-RbAP and KDP-ADP Systems. In Proceedings of the ICCG-13, Kyoto, Japan, 30 July–4 August 2001; p. 481. [Google Scholar]
- Kryuchkova, L.Y.; Glikin, A.E.; Voloshin, A.E.; Kovalev, S.I. Kinetic-morphological phenomena of growth and isomorphic substitution of mixed crystals in solutions [by the example of series (Co,Ni)(NH4)2(SO4)2·6H2O]. Zapiski Vseross. Mineral. Obshch. 2002, 131, 62–77. [Google Scholar]
- Pina, C.M.; Putnis, C.; Pollok, K.; Glikin, A.; Putnis, A. Replacement reactions in the KBr– KCl–H2O system: experiments and modeling. In Proceedings of the 14th Kongsberg Seminar Spatio-Temporal Patterns in the Earth, Kongsberg, Norway, 7–10 May 2001; p. 31. [Google Scholar]
- Putnis, C.; Pina, C.M.; Pollok, K.; Glikin, A. Ineral replacement reactions in solid solution—Aqueous solution systems II. In Proceedings of the 11th Annual Goldschmidt Conference, Hot Springs, VA, USA, 20–24 May 2001; p. 3555. [Google Scholar]
- Voloshin, A.E.; Kovalev, S.I.; Rudneva, E.B.; Glikin, A.E. Phenomena and mechanisms of mixed crystal formation in solutions II. Mechanism of interface processes. J. Cryst. Growth. 2004, 261, 105–117. [Google Scholar] [CrossRef]
- Voloshin, A.E.; Kovalev, S.I.; Rudneva, E.B.; Shubnikov Institute of Crystallography of RAS, Moscow, Russia; Glikin, A.E.; Sankt-Petersburg State University, Sankt-Petersburg, Russia. Personal communication, 2004.
- Grebenev, V.V.; Lyasnikova, M.S.; Kovalev, S.I.; Rudneva, E.B.; Manomenova, V.L.; Voloshin, A.E.; FSRC “Crystallography and Photonics” RAS, Moscow, Russia. Personal communication, 2019.
- Kryuchkova, L.Y.; Glikin, A.E. Variations of the mechanism of single crystal isomorphous replacement. In Proceedings of the Conference Fedorov Session 2010, St. Petersburg, Russia, 11–14 October 2010; p. 111. [Google Scholar]
- Glikin, A.E. Phase relations of mixed crystals with solutions. In Proceedings of the 18th International Workshop on Industrial Crystallization (BIWIC-2011), Delft, Netherlands, 7–9 September 2011; p. 233. [Google Scholar]
- Kryuchkova, L.Y.; Sinai, M.Y.; Glikin, A.E. Compositional distribution of isomorphic crystals during spontaneous crystallization. Acta Cryst. 2011, 67, 469. [Google Scholar] [CrossRef]
- Grigoryeva, M.S.; Vasilyeva, N.A.; Artemov, V.V.; Voloshin, A.E. Mosaic microinhomogeneity in crystals of (Co,Ni)K2(SO4)2·6H2O solid solutions. Cryst. Rep. 2014, 59, 276–283. [Google Scholar] [CrossRef]
- Vasilyeva, N.A.; Rudneva, E.B.; Manomenova, V.L.; Grigoriev, Y.V.; Masalov, V.M.; Zhokhov, A.A.; Emelchenko, G.A.; Voloshin, A.E. Study of radial inhomogenity and mosaic microinhomogenity in mixed KCNSH crystals. Cryst. Rep. 2019, 64. in press. [Google Scholar] [CrossRef]
- Milvidsky, M.G.; Osvensky, B.V. Structural Defects. In Monocrystals of Semiconductors; Metallurgy: Moscow, Russia, 1984; p. 256. (In Russian) [Google Scholar]
- Acoustic Crystals; Shaskolskaya, M.P. (Ed.) Nauka: Moscow, USSR, 1982; p. 632. [Google Scholar]
- Grigor’eva, M.S.; Voloshin, A.E.; Rudneva, E.B.; Manomenova, V.L.; Khakhanov, S.N.; Shklover, V.Y. A study of the mechanisms of defect formation in K2Ni(SO4)2·6H2O/K2Co(SO4)2·6H2O bicrystals grown from aqueous solutions. Cryst. Rep. 2009, 54, 637–644. [Google Scholar] [CrossRef]
- Roitburd, A.L. Theory of formation of a heterophase structure during phase transformations in the solid state. Usp. Fiz. Nauk 1974, 113, 69–194. (In Russian) [Google Scholar]
- Grebenev, V.V.; Grigor’eva, M.S.; Voloshin, A.E. Formation of Solution Inclusions in Bicrystals of Potassium–Cobalt/Potassium–Nickel and Ammonium–Cobalt/Ammonium–Nickel Sulfates. Cryst. Rep. 2010, 55, 887–891. [Google Scholar] [CrossRef]
- Kockelmann, W.; Schaefer, W.; Kirfel, A. Hydrogen Positions in KCo-, CsCo-, CsNi-, and CsCu-Tutton Salt Compounds Determined by Neutron Powder Diffraction. Mater. Sci. Forum 2001, 378, 274–281. [Google Scholar] [CrossRef]
- Petrashko, A.; Perekalina, Z.B.; Soboleva, L.V.; Kirpichnikova, L.F. Structure and some physical properties of K2Ni(SO4)2⋅6H2O crystals. Cryst. Rep. 2000, 45, 479–481. [Google Scholar] [CrossRef]
- Montgomery, H.; Chastain, R.V.; Natt, J.J. The crystal structure of Tutton’s salts. VI. Vanadium(II), iron(II) and cobalt(II) ammonium sulfate hexahydrates. Acta Cryst. 1967, 22, 775–780. [Google Scholar] [CrossRef]
- Montgomery, H.; Lingafelter, E.C. The crystal structure of Tutton’s salts. II. Magnesium ammonium sulfate hexahydrate and nickel ammonium sulfate hexahydrate. Acta Cryst. 1964, 17, 1478–1479. [Google Scholar] [CrossRef]
- Glikin, A.E.; Kovalev, S.I.; Rudneva, E.B.; Kryuchkova, L.Y.; Voloshin, A.E. Phenomena and mechanisms of mixed crystal formation in solutions. I. General concept on the example of the system KHC8H4O4-RbHC8H4O4-H2O. J. Cryst. Growth. 2003, 255, 150–162. [Google Scholar] [CrossRef]
- Tanner, B.K. X-Ray Diffraction Topography; Pergamon Press: Oxford, UK, 1976. [Google Scholar]
- Manomenova, V.L.; Rudneva, E.B.; Voloshin, A.E. Crystals of the simple and complex nickel and cobalt sulfates as optical filters for the solar-blind technology. Russ. Chem. Rev. 2016, 85, 585–609. [Google Scholar] [CrossRef]
- Zhuang, X.; Genbo, S.; Youping, H.; Zheng, G. Growth and characterisation of potassium cobalt nickel sulfate hexahydrate for UV light filters. Cryst. Res. Technol. 2006, 41, 1031–1035. [Google Scholar] [CrossRef]
- Polovinco, I.I.; Rykhlyuk, S.V.; Koman, V.B.; Karbovnyk, I.D. Modification of the optical spectra of mixed K2CoxNi1-x(SO4)2·6H2O crystal. J. Appl. Spectrosc. 2009, 76, 116–120. [Google Scholar] [CrossRef]
- Vasilyeva, N.A.; Grigoryeva, M.S.; Grebenev, V.V.; Voloshin, A.E. Growth and properties of mixed K2NixCo1−x(SO4)2 6H2O crystals. Cryst. Rep. 2013, 58, 646–650. [Google Scholar] [CrossRef]
- Rudneva, E.B.; Manomenova, V.L.; Koldaeva, M.V.; Sorokina, N.I.; Voloshin, A.E.; Grebenev, V.V.; Verin, I.A.; Lyasnikova, M.S.; Masalov, V.M.; Zhokhov, A.A.; et al. Anomalies of properties in a series of K2CoxNi(1–x)(SO4)2·6H2O mixed crystals. Cryst. Rep. 2017, 62, 928–939. [Google Scholar] [CrossRef]
- Masalov, V.M.; Vasilyeva, N.A.; Manomenova, V.L.; Zhokhov, A.A.; Rudneva, E.B.; Voloshin, A.E.; Emelchenko, G.A. Growth of mixed K2(Ni, Co)(SO4)2·6H2O crystals under stationary conditions of supercooling and forced convection of the aqueous solution. J. Cryst. Growth. 2017, 475, 21–25. [Google Scholar] [CrossRef]
- Tiller, W.A.; Jackson, K.A.; Rutter, J.W.; Chalmers, B. The redistribution of solute atoms during the solidification of metals. Acta Met. 1953, 1, 428–437. [Google Scholar] [CrossRef]
- Voloshin, A.E. Study of the initial transient in the one-dimensional analytical models of impurity segregation during melt crystallization in the presence of convection. Cryst. Rep. 2013, 58, 939–947. [Google Scholar] [CrossRef]
- Voloshin, A.E.; Manomenova, V.L.; Rudneva, E.B.; Vasilyeva, N.A.; Masalov, V.M.; Zhokhov, A.A.; Emelchenko, G.A. Growth of high-perfect mixed K2NixCo1-x(SO4)2·6H2O crystals for fabrication of high-efficiency UV optical filters. J. Cryst. Growth. 2018, 500, 98–103. [Google Scholar] [CrossRef]
- Zhokhov, A.A.; Masalov, V.M.; Zverkova, I.I.; Emelchenko, G.A.; Manomenova, V.L.; Rudneva, E.B.; Vasilieva, N.A.; Voloshin, A.E. Study of the K2Ni(SO4)2·6H2O–K2Co (SO4)2·6H2O–H2O diagram and determination of the conditions for growing K2(Ni, Co)(SO4)2·6H2O mixed crystals. Cryst. Rep. 2016, 61, 1027–1030. [Google Scholar] [CrossRef]























| Lattice Parameter | KAP | RbAP | Misfit, % |
|---|---|---|---|
| Space group | P21ab | ||
| a, Å | 6.47 | 6.58 | 1.7 |
| b, Å | 9.61 | 10.02 | 4.3 |
| c, Å | 13.26 | 12.99 | −2.1 |
| Lattice Parameter | ACSH/ANSH | KCSH/KNSH | ||||
|---|---|---|---|---|---|---|
| ACSH [27] | ANSH [28] | Misfit, % | KCSH [29] | KNSH [30] | Misfit, % | |
| Space group | P21/c | P21/c | ||||
| a, Å | 9.25 | 9.241 | 0.097 | 9.045 | 8.98 | 0.72 |
| b, Å | 12.52 | 12.544 | –0.192 | 12.193 | 12.159 | 0.275 |
| c, Å | 6.24 | 6.243 | –0.048 | 6.146 | 6.125 | 0.342 |
| β, deg | 107.1 | 106.97 | 0.121 | 104.31 | 105.6 | –0.734 |
| R | the normal crystal growth rate |
| x | the distance from the seed |
| the generalized equilibrium distribution coefficient of the components | |
| the generalized equilibrium distribution coefficient of the components in a stationary mode | |
| effective generalized coefficient of distribution of the components in the initial transient at the crystal length x | |
| the equilibrium distribution coefficient of KCSH | |
| the effective distribution coefficient of KCSH in the stationary mode | |
| the effective distribution coefficient of KCSH in the initial transient at the crystal length x | |
| C∞ | the KCSH mole fraction in the salt mass in the bulk of the solution |
| C0 | the KCSH mole fraction in the salt mass in the solution at the interface |
| c(x) | the average mole fraction of KCSH in the crystal cross section with the coordinate x |
| c0 | the initial mole fraction of KCSH in the crystal |
| c* | the KCSH mole fraction in the crystal in the stationary mode |
| δ | the thickness of the solution diffusion boundary layer |
| D | the diffusion coefficient in the solution |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voloshin, A.; Rudneva, E.; Manomenova, V.; Vasilyeva, N.; Kovalev, S.; Emelchenko, G.; Masalov, V.; Zhokhov, A. The Problem of Formation of Mixed Crystals and High-Efficiency K2(Co, Ni)(SO4)2 • 6H2O Optical Filters. Crystals 2019, 9, 390. https://doi.org/10.3390/cryst9080390
Voloshin A, Rudneva E, Manomenova V, Vasilyeva N, Kovalev S, Emelchenko G, Masalov V, Zhokhov A. The Problem of Formation of Mixed Crystals and High-Efficiency K2(Co, Ni)(SO4)2 • 6H2O Optical Filters. Crystals. 2019; 9(8):390. https://doi.org/10.3390/cryst9080390
Chicago/Turabian StyleVoloshin, Alexey, Elena Rudneva, Vera Manomenova, Natalie Vasilyeva, Sergey Kovalev, Gennadii Emelchenko, Vladimir Masalov, and Andrey Zhokhov. 2019. "The Problem of Formation of Mixed Crystals and High-Efficiency K2(Co, Ni)(SO4)2 • 6H2O Optical Filters" Crystals 9, no. 8: 390. https://doi.org/10.3390/cryst9080390





