Raman Spectra and Microstructure of Zinc Oxide irradiated with Swift Heavy Ion
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
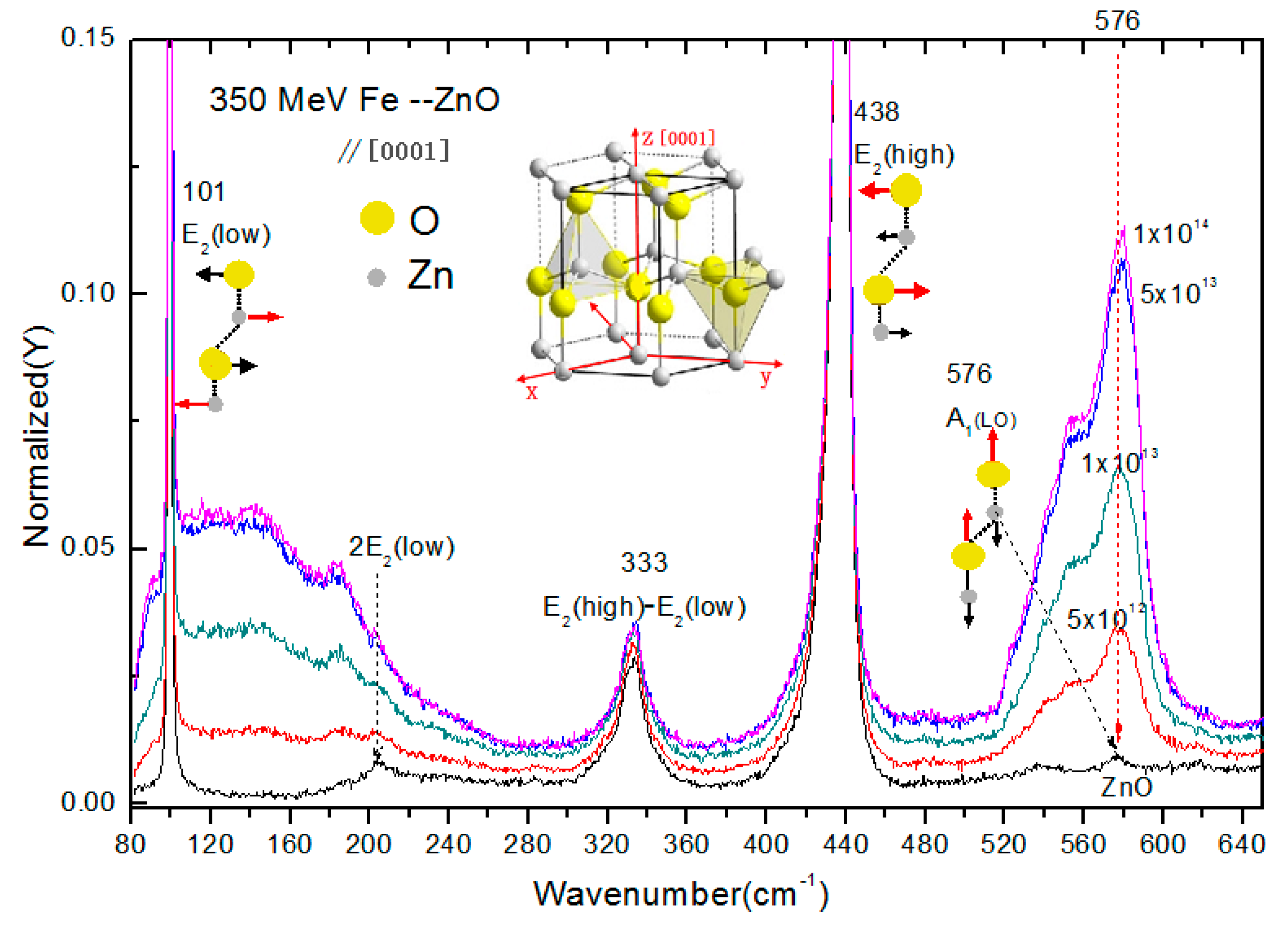
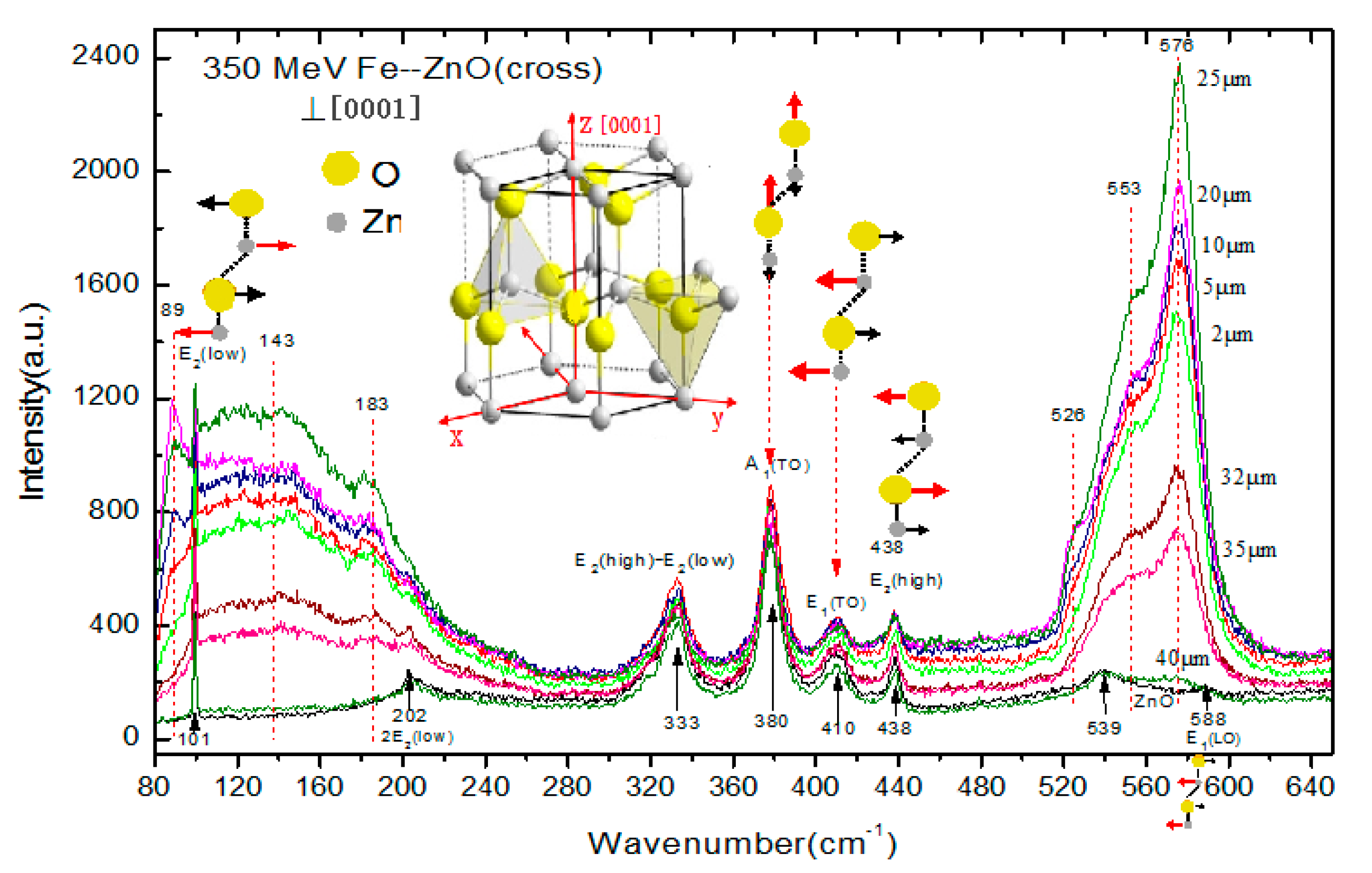
3.1. Raman Spectra of 56Fe21+ Ion-Irradiated ZnO
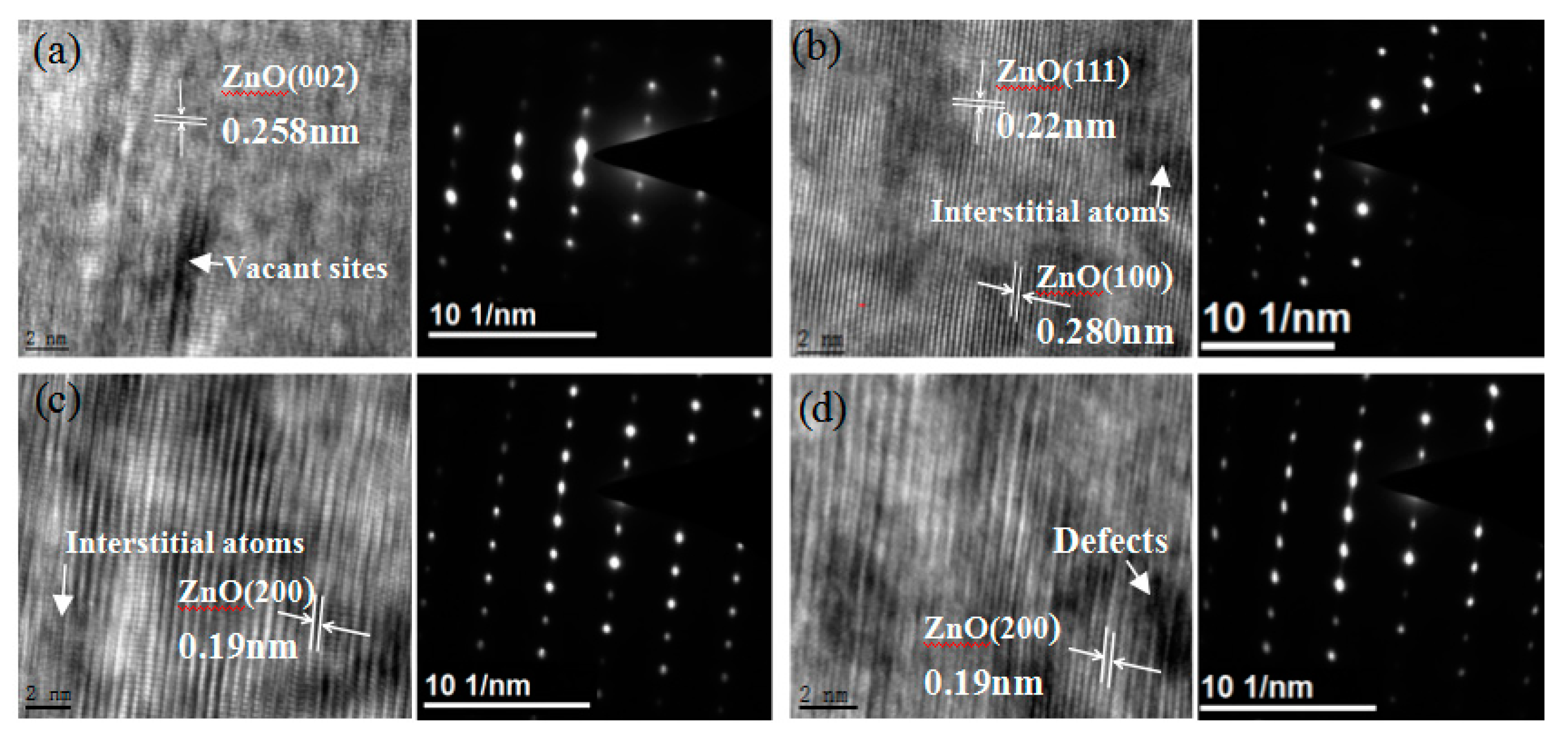
3.2. TEM of ZnO Irradiated with 56Fe21+ Ions
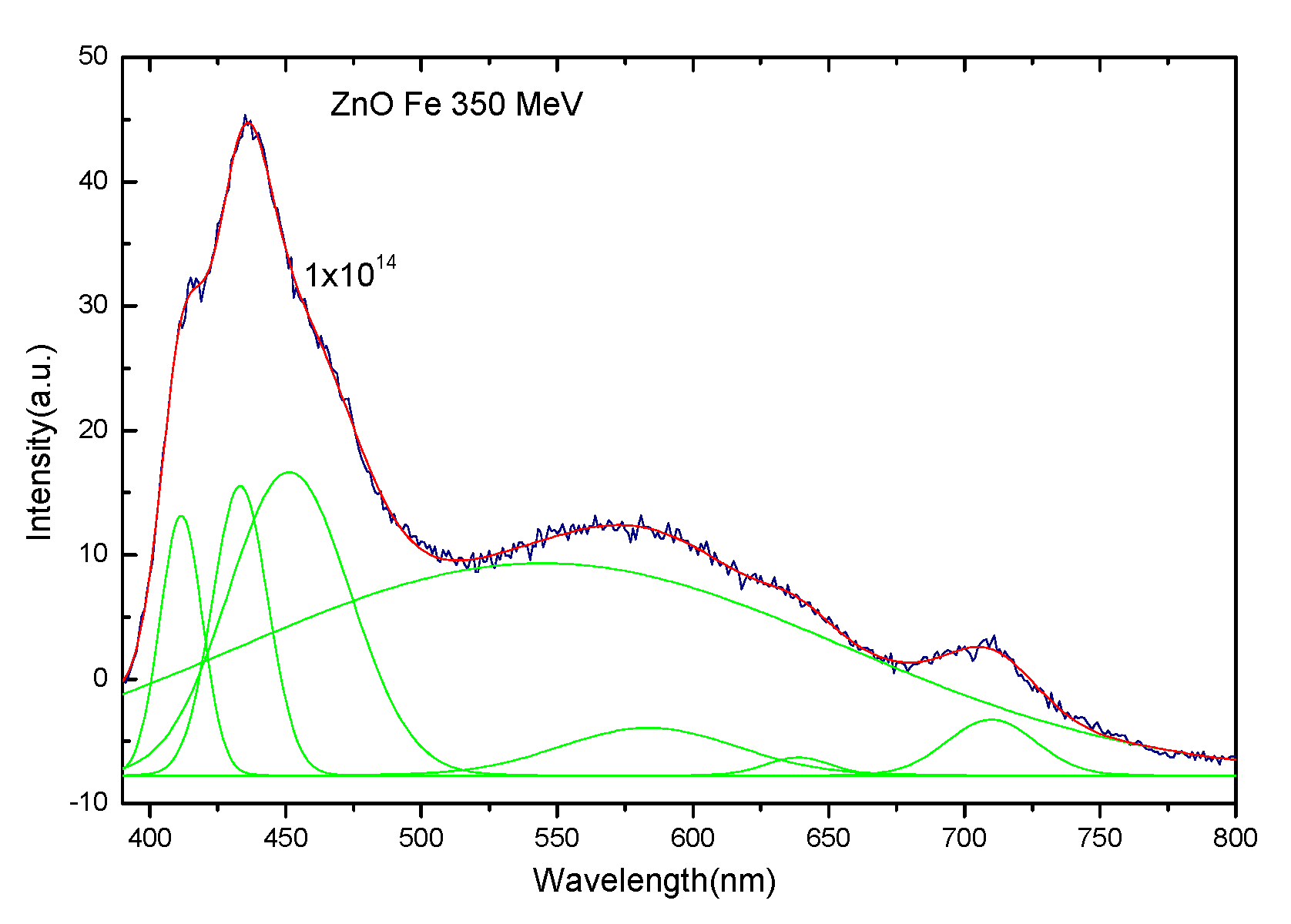
3.3. Photoluminescence Spectra of ZnO Irradiated with 56Fe21+ Ions
3.4. Theoretical Calculation and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter. 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Ozgur, U.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.J.; Morkoçet, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 41301. [Google Scholar] [CrossRef]
- Pearton, S.J.; Norton, D.P.; Tien, L.C.; Guo, J. Modeling and Fabrication of ZnO Nanowire Transistors. IEEE Trans. Electron Devices 2008, 55, 3012–3019. [Google Scholar] [CrossRef]
- Wu, C.L.; Guo, J.H.; Yao, H.B. Study on the photoluminescence properties of ZnOsinglecrystal. Spectrosc. Spectr. Anal. 2016, 6, 1700–1702. [Google Scholar]
- Xu, S.; Qin, Y.; Xu, C.; Wei, Y.; Yang, R.; Wang, Z.L. Self-powered nanowire devices. Nat. Nanotechnol. 2010, 5, 366–373. [Google Scholar] [CrossRef]
- Leong, E.S.P.; Yu, S.F.; Lau, S.P. Directional edge-emitting UV random laser diodes. Appl. Phys. Lett. 2006, 89, 221109. [Google Scholar] [CrossRef]
- Guo, R.; Nishimura, J.; Matsumoto, M.; Higashihata, M.; Nakamura, D.; Okada, T. Electroluminescence from ZnO nanowire-based p-GaN/n-ZnO heterojunction light-emitting diodes. Appl. Phys. B 2009, 94, 33–38. [Google Scholar] [CrossRef]
- Duta, M.; Perniu, D.; Duta, A. Photocatalytic zinc oxide thin films obtained by surfactant assisted spray pyrolysis deposition. Appl. Surf. Sci. 2014, 306, 80–88. [Google Scholar] [CrossRef]
- Pal, S.; Sarkar, A.; Chattopadhyay, S.; Chakrabarti, M.; Sanyal, D.; Kumar, P.; Kanjilal, D.; Rakshitf, T.; Ray, S.K.; Jana, D. Defects in 700 keV oxygen ion irradiated ZnO. Nucl. Instrum. Methods Phys. Res. B 2013, 311, 20–26. [Google Scholar] [CrossRef]
- Crupi, I.; Boscarino, S.; Strano, V.; Mirabella, S.; Simone, F.; Terrasi, A. Optimization of ZnO:Al/Ag/ZnO:Al structures for ultra-thin high-performance transparent conductive electrodes. Thin Solid Films 2012, 520, 4432–4435. [Google Scholar] [CrossRef]
- Choi, K.H.; Nam, H.J.; Jeong, J.A.; Cho, S.W.; Kim, H.K.; Kang, J.W.; Kim, D.G.; Cho, W.J. Highly flexible and transparent InZnSnOx/Ag/InZnSnOx multilayer electrode for flexible organic light emitting diodes. Appl. Phys. Lett. 2008, 92, 223302–223303. [Google Scholar] [CrossRef]
- Martín-Tovar, E.A.; Castro-Rodríguez, R.; Daza, L.G.; Méndez-Gamboa, J.; Medina-Esquivel, R.; Perez- Quintana, I.; Iribarren, A. Structural and optical properties of ZnO thin films prepared by laser ablation using target of ZnO powder mixture with glue. Bull. Mater. Sci. 2017, 40, 467–471. [Google Scholar] [CrossRef][Green Version]
- Jaramillo, A.F.; Baez-Cruz, R.; Montoya, L.F. Estimation of the surface interaction mechanism of ZnO nanoparticles modified with organosilane groups by Raman Spectroscopy. Ceram. Int. 2017, 43, 11838–11847. [Google Scholar] [CrossRef]
- Chae, K. Electronic structures of a Zn vacancy on the ZnOsurface: Density functional theory calculations. J. Korean Phys. Soc. 2013, 62, 508–512. [Google Scholar] [CrossRef]
- Yao, Z.R.; Tang, K.; Xu, Z.H.; Ye, J.D.; Zhu, S.M.; Gu, S.L. The luminescent inhomogeneity and the distribution of zinc vacancy-related acceptor-like defects in N-Doped ZnOmicrorods. Nanoscale Res. Lett. 2016, 11, 511. [Google Scholar] [CrossRef][Green Version]
- Yao, Y.H.; Cao, Q.X. First-principles study on infrared absorptions of transition metal-doped ZnO with oxygen vacancy. Acta. Metall. Sin. (Engl. Lett.) 2013, 26, 467–472. [Google Scholar] [CrossRef]
- Li, W.L.; Hou, Q.Y.; Jia, X.F.; Xu, Z.C. Effects of la doping and Zn or O vacancy on the magnetic property of ZnO. J. Supercond. Nov. Magn. 2018, 31. [Google Scholar] [CrossRef]
- Ding, Y.; Pradel, K.C.; Wang, Z.L. In situ transmission electron microscopy observation of ZnO polar and non-polar surfaces structure evolution under electron beam irradiation. J. Appl. Phys. 2016, 119, 015305. [Google Scholar] [CrossRef]
- Zeng, H.B.; Duan, G.T.; Li, Y.; Yang, S.K.; Xu, X.X.; Cai, W.P. Blue luminescence of ZnO nanoparticles based on non-equilibrium processes defect origins and emission controls. Adv. Funct. Mater. 2010, 20, 561. [Google Scholar] [CrossRef]
- Duan, L.B.; Zhao, X.R.; Wang, Y.J.; Geng, W.C.; Zhang, F.L. Structural and optical properties of (Mg, Al) –codoped ZnO nanoparticles synthesized by the auto combustion method. Ceram. Int. 2015, 41, 6373–6380. [Google Scholar] [CrossRef]
- Serrano, J.; Romero, A.H.; Manjon, F.J.; Lauck, R.; Cardona, M.; Rubio, A. Pressure dependence of the lattice dynamics of ZnO: An ab initio approach. Phys. Rev. B 2004, 69, 094306. [Google Scholar] [CrossRef]
- Loudon, R. The Raman effect in crystals. Adv. Phys. 1964, 13, 423–482. [Google Scholar] [CrossRef]
- Tell, B.; Damen, T.C.; Porto, S.P.S. Raman Effect in cadmium sulfide. Phy. Rev. 1966, 142, 570–576. [Google Scholar] [CrossRef]
- Song, Y.; Gou, J.; Yang, Y.T.; Ding, Z.N.; Zhang, S.X.; Zhang, C.H. Microstructure property study of ZnO single crystal irradiated with 200 MeV Kr ions. Mater. Res. Express 2019, 6, 026203. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Zhang, S.; Zhang, C.; Yang, Y.; Lv, K. Raman Spectra and Microstructure of Zinc Oxide irradiated with Swift Heavy Ion. Crystals 2019, 9, 395. https://doi.org/10.3390/cryst9080395
Song Y, Zhang S, Zhang C, Yang Y, Lv K. Raman Spectra and Microstructure of Zinc Oxide irradiated with Swift Heavy Ion. Crystals. 2019; 9(8):395. https://doi.org/10.3390/cryst9080395
Chicago/Turabian StyleSong, Yin, Shengxia Zhang, Chonghong Zhang, Yitao Yang, and Kangyuan Lv. 2019. "Raman Spectra and Microstructure of Zinc Oxide irradiated with Swift Heavy Ion" Crystals 9, no. 8: 395. https://doi.org/10.3390/cryst9080395
APA StyleSong, Y., Zhang, S., Zhang, C., Yang, Y., & Lv, K. (2019). Raman Spectra and Microstructure of Zinc Oxide irradiated with Swift Heavy Ion. Crystals, 9(8), 395. https://doi.org/10.3390/cryst9080395



