PLMA-b-POEGMA Amphiphilic Block Copolymers as Nanocarriers for the Encapsulation of Magnetic Nanoparticles and Indomethacin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PLMA-b-POEGMA Block Copolymers Synthesis
2.3. Synthesis of Iron Oxide Nanoparticles
2.4. Preparation of PLMA-b-POEGMA Micelles Loaded with Magnetic Nanoparticles
2.5. Preparation of PLMA-b-POEGMA Micelles Loaded with Magnetic Nanoparticles and Indomethacin
2.6. Methods
3. Results and Discussion
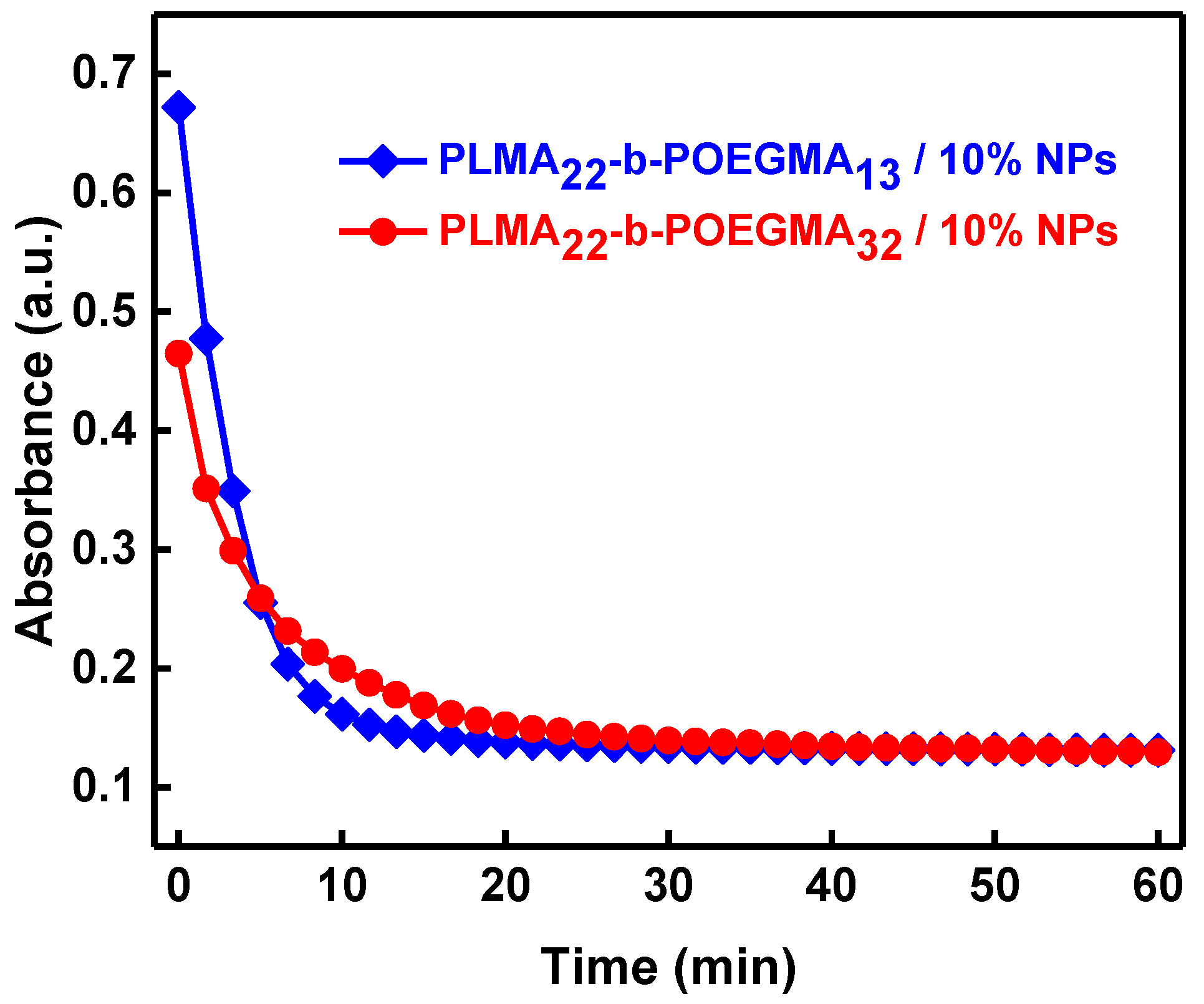
3.1. Encapsulation of Fe2O3 Nanoparticles in the PLMA-b-POEGMA Micelles
3.2. Encapsulation of Both Iron Oxide Nps and IND into PLMA-b-POEGMA Micelles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Nanotheranostics and Image-Guided Drug Delivery: Current Concepts and Future Directions. Mol. Pharm. 2010, 7, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconj. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Krasia-Christoforou, T.; Georgiou, T.K. Polymeric theranostics: Using polymer-based systems for simultaneous imaging and therapy. J. Mater. Chem. B 2013, 1, 3002–3025. [Google Scholar] [CrossRef]
- Talelli, M.; Rijcken, C.J.F.; Lammers, T.; Seevinck, P.R.; Storm, G.; van Nostrum, C.F.; Hennink, W.E. Superparamagnetic Iron Oxide Nanoparticles Encapsulated in Biodegradable Thermosensitive Polymeric Micelles: Toward a Targeted Nanomedicine Suitable for Image-Guided Drug Delivery. Langmuir 2009, 25, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Zahraei, M.; Marciello, M.; Lazaro-Carrillo, A.; Villanueva, A.; Herranz, F.; Talelli, M.; Costo, R.; Monshi, A.; Shahbazi-Gahrouei, D.; Amirnasr, M.; et al. Versatile theranostics agents designed by coating ferrite nanoparticles with biocompatible polymers. Nanotechnology 2016, 27, 255702. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Tritschler, U.; Pearce, S.; Gwyther, J.; Whittell, G.R.; Manners, I. 50th Anniversary Perspective: Functional Nanoparticles from the Solution Self-Assembly of Block Copolymers. Macromolecules 2017, 50, 3439–3463. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Gunatillake, P.A.; Meagher, L. Biomedical applications of polymers derived by reversible addition—Fragmentation chain-transfer (RAFT). Adv. Drug Deliv. Rev. 2015, 91 (Suppl. C), 141–152. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Christie, R.J.; Kataoka, K. Polymeric micelles for nano-scale drug delivery. React. Funct. Polym. 2011, 71, 227–234. [Google Scholar] [CrossRef]
- Jones, M.-C.; Leroux, J.-C. Polymeric micelles—A new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999, 48, 101–111. [Google Scholar] [CrossRef]
- Pinto Reis, C.; Neufeld, R.J.; Ribeiro, J.A.; Veiga, F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine NBM 2006, 2, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Zhu, J. Encapsulation of inorganic nanoparticles into block copolymer micellar aggregates: Strategies and precise localization of nanoparticles. Polymer 2014, 55, 1079–1096. [Google Scholar] [CrossRef]
- Yuan, J.; Müller, A.H.E. One-dimensional organic–inorganic hybrid nanomaterials. Polymer 2010, 51, 4015–4036. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.E.; Lee, S.H.; Yu, J.; Lee, J.H.; Park, T.G.; Hyeon, T. Designed Fabrication of a Multifunctional Polymer Nanomedical Platform for Simultaneous Cancer—Targeted Imaging and Magnetically Guided Drug Delivery. Adv. Mater. 2008, 20, 478–483. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Piao, Y.; Hyeon, T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 2009, 38, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Yang, Q.; Dong, Z.; Zhang, J.; Zhang, W.; Wang, Q.; Tan, S.; Smyth, H.D.C. Magnetically triggered drug release from nanoparticles and its applications in anti-tumor treatment. Drug Deliv. 2017, 24, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hosseinkhani, H.; Hosseinkhani, M.; Boutry, S.; Simchi, A.; Journeay, W.S.; Subramani, K.; Laurent, S. Magnetic Resonance Imaging Tracking of Stem Cells in Vivo Using Iron Oxide Nanoparticles as a Tool for the Advancement of Clinical Regenerative Medicine. Chem. Rev. 2011, 111, 253–280. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, A.; Pispas, S. PLMA-b-POEGMA amphiphilic block copolymers: Synthesis and self-assembly in aqueous media. J. Polym. Sci. A 2017, 55, 155–163. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Pispas, S.; Hadjichristidis, N. Aggregation Behavior of Poly(butadiene-b-ethylene oxide) Block Copolymers in Dilute Aqueous Solutions: Effect of Concentration, Temperature, Ionic Strength, and Type of Surfactant. Langmuir 2003, 19, 48–54. [Google Scholar] [CrossRef]
- Raffa, P.; Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymeric Surfactants: Synthesis, Properties, and Links to Applications. Chem. Rev. 2015, 115, 8504–8563. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Szöllösi, L.; Kéki, S.; Faust, R.; Zsuga, M. Poly(vinyl alcohol)-based Amphiphilic Copolymer Aggregates as Drug Carrying Nanoparticles. J. Macromol. Sci. A 2009, 46, 331–338. [Google Scholar] [CrossRef]
- Zhao, Y.; Marcel, Y.L. Serum Albumin Is a Significant Intermediate in Cholesterol Transfer between Cells and Lipoproteins. Biochemistry 1996, 35, 7174–7180. [Google Scholar] [CrossRef] [PubMed]











| Block copolymers | NPs loaded | NPs and IND loaded | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Mw a (×104) (g/mol) | Mw/Mn a | wt % PLMA b | Dh c (nm) | PDI c | Dh c (nm) | PDI c | Dh c (nm) | PDI c |
| PLMA22-b-POEGMA58 | 3.3 | 1.30 | 16 | 162 | 0.28 | - | - | 166 | 0.33 |
| PLMA22-b-POEGMA32 | 2.07 | 1.31 | 27 | 122 | 0.35 | 198 | 0.37 | 194 | 0.39 |
| PLMA22-b-POEGMA13 | 1.17 | 1.18 | 52 | 140 | 0.31 | 200 | 0.35 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skandalis, A.; Sergides, A.; Bakandritsos, A.; Pispas, S. PLMA-b-POEGMA Amphiphilic Block Copolymers as Nanocarriers for the Encapsulation of Magnetic Nanoparticles and Indomethacin. Polymers 2018, 10, 14. https://doi.org/10.3390/polym10010014
Skandalis A, Sergides A, Bakandritsos A, Pispas S. PLMA-b-POEGMA Amphiphilic Block Copolymers as Nanocarriers for the Encapsulation of Magnetic Nanoparticles and Indomethacin. Polymers. 2018; 10(1):14. https://doi.org/10.3390/polym10010014
Chicago/Turabian StyleSkandalis, Athanasios, Andreas Sergides, Aristides Bakandritsos, and Stergios Pispas. 2018. "PLMA-b-POEGMA Amphiphilic Block Copolymers as Nanocarriers for the Encapsulation of Magnetic Nanoparticles and Indomethacin" Polymers 10, no. 1: 14. https://doi.org/10.3390/polym10010014
APA StyleSkandalis, A., Sergides, A., Bakandritsos, A., & Pispas, S. (2018). PLMA-b-POEGMA Amphiphilic Block Copolymers as Nanocarriers for the Encapsulation of Magnetic Nanoparticles and Indomethacin. Polymers, 10(1), 14. https://doi.org/10.3390/polym10010014







